ISSN: 0973-7510
E-ISSN: 2581-690X
The current study outlines an environmentally friendly procedure to synthesis the extracellular secondary metabolite-mediated copper oxide nanoparticles (CuONPs) using Bioinspired green chemistry method with Pseudomonas aeruginosa isolated from a marine water sample. The in vitro bio-reduction of Copper sulphate to CuONPs in the presence of secondary metabolites was confirmed using various analytical techniques, including UV-visible spectroscopy, FTIR spectroscopy, particle size analysis, scanning electron microscopy (SEM) and X-ray diffraction (XRD). In the UV-visible spectroscopy the synthesised nanoparticles was characterized with the maximum absorbance at 570 nm. SEM analysis showed the nanoparticles to be nearly cuboidal in shape with the size range of 108.0 nm to 252.2 nm at the 1,00,000x magnification. X-ray diffraction revealed particle sizes ranging from 4.19 nm to 26.95 nm with diffraction peaks at 38.05° and 31.67°, corresponding to lattice planes [6.56] and [100], indicating a polycrystalline wurtzite structure. The synthesized CuONPs exhibited significant antimicrobial activity against clinical pathogenic bacteria such as Escherichia coli, Bacillus cereus, Staphylococcus aureus and Proteus mirabilis, as well as fungicidal activity against phytopathogenic fungi including Alternaria solani, Rhizoctonia solani and Colletotrichum capsici. Additionally the photocatalytic activity of the Pseudomonas aeruginosa mediated CuONPs was estimated through the degradation of the triarylmethane dye-malachite Green.
Secondary Metabolite, Copper Oxide Nanoparticles, Antimicrobial Activity, Photocatalytic Activity, Malachite Green Dye
Marine microorganism such as marine bacteria inhabit oceanic environments and possess unique metabolic capabilities due to their adaptation to extreme conditions such as high salinity, pressure, and variable temperatures. These microorganisms have gained a lot of interest for their potential applications in the field of biotechnology, particularly in the synthesis of nanoparticles.1 One such innovative approach involves the infusion of marine bacteria with copper oxide nanoparticles are well-known for their potent antimicrobial and photocatalytic properties. The incorporation of Copper oxide nanoparticles with marine bacteria provides a dual advantage it not only harnesses the intrinsic biological activity of the marine bacteria but also amplifies their effectiveness through the enhanced physicochemical properties of the nanoparticles. This synergy can lead to improvising the novel antimicrobial drugs and photocatalytic materials, which are crucial for the applications such as medical therapies, water purification, and environmental remediation. The characteristics of the antimicrobial activity of CuONPs is attributed due to its ability to generate reactive oxygen species (ROS), which can damage microbial cell membranes, proteins, and DNA, ultimately leading to apoptosis.2 Furthermore, the photocatalytic activity of these nanocomposites under visible light exposure enables the degradation of organic pollutants, making it highly effective in environmental applications. In this study, we explore the synthesis, characterization, and application of marine bacteria infused with copper oxide nanoparticles, focusing on their antimicrobial and photocatalytic activities.3 This innovative approach not only provides insights into the development of advanced nanocomposites but also paves the way for sustainable solutions in healthcare and environmental management.
Microorganisms exposed to toxic metal environments often develop mechanisms to detoxify and survive under such harsh conditions. One such mechanism involves in the conversion of hazardous metal ions into less toxic forms, such as sulphides or metal oxides. This detoxification process is an area of significant interest, as it not only helps in understanding microbial resilience but also provides insights into developing new methods for nanoparticle synthesis. According to a comprehensive review by Chen et al.,4 CuONPs have gained prominence due to their diverse applications, including antimicrobial agents, batteries, coating on textiles, high-temperature superconductors, solar energy conversion tools, gas sensors, and electronic devices.4 Research conducted by Cioffi et al.,5 also further supports the antibacterial potential of copper nanoparticles, highlighting their efficacy in preventing the proliferation of harmful bacteria. The application of nanoparticles synthesized by such microbes holds great promises for their potential use in biocontrol strategies against various plant pathogens.
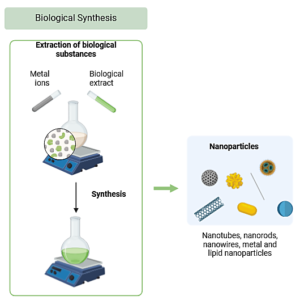
In the past, the majority of biogenic nanoparticle synthesis research has focused on terrestrial bacteria and fungi. While these studies have advanced the field using marine bacteria which offer novel properties due to the extremophilic conditions. In our study, we synthesized CuONPs using secondary metabolites from Pseudomonas aeruginosa isolated from a marine environment by using Bio-inspired green chemistry method with graphical representation in Figure 1.6 A study by Iravani et al.,7 states that Pseudomonas stutzeri and Pseudomonas aeruginosa have developed the ability to resort to specific defence mechanisms to quell stresses like toxicity of heavy metal ions or metals, they can even survive and grow at high metal ion concentrations. Pseudomonas aeruginosa is a versatile bacterium known for its unique adaptation to high salt concentrations, pressure, varying nutrient conditions, genetic diversity and metabolic flexibility, which enables these organisms to produce biomolecules with distinct properties, which in turn can facilitate the creation of nanoparticles with novel characteristics, stability, and bioactivity, makes it an excellent candidate for nanoparticle synthesis. Previous Studies also indicated that nanoparticles synthesized from marine Pseudomonas sp. may exhibit a broader and more potent range of antimicrobial activities than those synthesized by terrestrial strains, Using Pseudomonas aeruginosa as a reducing agent to synthesize copper nanoparticles offers several distinct advantages over traditional chemical and physical synthesis methods, in traditional methods toxic and hazardous waste were produced but Pseudomonas aeruginosa mediated synthesis is inherently eco-friendly. The process relies on biological secondary metabolites that reduce copper ions naturally, making it a sustainable and safe alternative by eliminating the need for potentially explosive or carcinogenic reducing agents, such as hydrazine, which are common in traditional synthesis. When it comes to Biocompatibility and Stability, the Nanoparticles produced using Pseudomonas aeruginosa often exhibit enhanced biocompatibility due to biological capping agents like protein or polysaccharides on the surface. These natural capping agents can improve nanoparticle stability and make them better suited for biomedical applications. Even Though traditional chemical synthesis often provides precise control over size and shape by adjusting reaction conditions, biological synthesis is evolving to achieve similar control, aided by understanding how different Pseudomonas strains and growth conditions influence nanoparticle morphology. So, using Pseudomonas as a reducing agent is a green, safe, and cost-effective alternative to traditional synthesis. Although traditional methods might offer precise control over particle characteristics, ongoing research is advancing green methods to meet similar standards of control and scalability while offering a more sustainable pathway for nanoparticle production.
Figure 1. Graphical representation of Bio-inspired green chemistry method designed using BioRender software
The CuONPs synthesized in this study demonstrated potent biocontrol activity against several phytopathogens, highlighting its possibility as a substitute for conservative chemical pesticides. These nanoparticles were also effective in the degradation of dyes commonly used in industrial applications.8
Copper oxide nanoparticles synthesized from Pseudomonas aeruginosa have shown unique characteristics compared to other nanoparticles, particularly due to the specific biological pathways involved in their synthesis. For the enhanced photocatalytic activity biosynthesized copper oxide nanoparticles must have a high surface area-to-volume ratio, stability and biocompatibility due to their unique shapes and smaller sizes the Pseudomonas secondary metabolite synthesised nanoparticle is beneficial for photocatalytic applications. Copper oxide nanoparticles are effective at generating ROS under light irradiation, which is beneficial for applications like photodegradation of pollutants. Pseudomonas derived nanoparticles are particularly effective in degrading organic pollutants and dyes due to their high photocatalytic efficiency. While TiO2‚ is widely used for photocatalysis, it is limited by its need for UV light activation due to its larger bandgap (~3.2 eV). Copper oxide nanoparticles, with a narrower bandgap (~1.2-2.1 eV for CuO), can be activated under visible light, making them more suitable for energy-efficient applications even ZnO nanoparticles are also efficient photocatalyst, but they can suffer from photo corrosion under prolonged light exposure. Copper oxide nanoparticles, particularly those stabilized by biosynthesis from Pseudomonas, often show enhanced durability and reduced photo corrosion, making them suitable for longer-term applications.
Malachite Green is widely used but notoriously difficult to degrade due to its complex triphenylmethane structure with aromatic rings, so the copper nanoparticles catalysing ability in degradations of the Malachite Green underscores their potential utility in waste water management and other eco-friendly remediation efforts.9 The synthesized nanoparticles exhibited desirable characteristics, such as significant antimicrobial and photocatalytic activities, which is used in many applications. Further research is about optimizing the synthesis process, exploring the mechanistic aspects of nanoparticle formation, and expanding the scope of applications for CuONPs in agriculture, medicine, and environmental remediation.
Collection of phytopathogens
The phytopathogens Alternaria solani, Rhizoctonia solani and Rhizopus stolonifer, and Colletotrichum capsica were collected from N.G. Ranga Agricultural University, Tirupati, Andhra Pradesh. Morphological characteristics of the fungi were observed. The fungal cultures are inoculated at the edges of the agar block, covered with a coverslip, and incubated at 37 °C. After 72 hours of incubation, the coverslip containing the fungal growth is carefully placed on an aseptic slide with lactophenol cotton blue stain drop to preserve the morphology and prevent air bubble formation. The slide is then examined under a phase-contrast microscope to observe their growth.10
Collection of bacterial pathogens
Clinical isolates of drug-resistant bacterial pathogens, such as Gram-positive methicillin-resistant, Staphylococcus aureus (MRSA), Bacillus cereus including Gram-negative pathogens Extended-spectrum b-lactamase (ESBL)-producing Escherichia coli, and Proteus mirabilis, were collected from the Department of Microbiology at SVIMS, Tirupati, and Andhra Pradesh. For further use these isolates were preserved on agar slants and stored at 4 °C.11
Sub-culturing of marine bacteria
The marine bacterial strain MO1 (Pseudomonas aeruginosa), known for its potent antimicrobial activity, was sub-cultured using Zobell’s marine broth medium (HIMEDIA 2216) and nutrient broth. The cultures were maintained as pure cultures until further use. The isolate MO1 (Pseudomonas aeruginosa) was inoculated into the respective media and incubated for 2 days at 35 °C until dark green pigmentation was observed, indicating the production of secondary metabolite. This marine isolate was subsequently used in the bio-synthesis of CuO nanoparticles.12
Identification of marine bacteria
The identification of marine bacteria with potent antimicrobial activity was performed using molecular techniques, including DNA extraction, DNA sequencing, and phylogenetic analysis. Bacterial DNA was extracted according to the method Clarridge JE,13 using the Hi-Media spin column kit. The bacterial 16S rRNA gene was amplified with 1500 base pairs in length, using polymerase chain reaction (PCR) in a thermal cycler by following Darby et al., method.14 NCBI-BLAST algorithms were utilized to determine the evolutionary and functional relationships and identify members of gene families. The BLAST nucleotide program was specifically employed to search for and identify closely related strains. Data alignment was carried out to verify the similarity values and difference in the query sequences of the database. The top 10 hits observed in the BLAST search results were reported for the marine isolates.
Phylogenetic analysis
The evolutionary analyses of strain MO1 were conducted using MEGA11 software. The phylogenetic tree, with the highest log likelihood value (-5375.58), was constructed using the method Tamura-Nei model.15 The initial tree was constructed using the BioNJ algorithms and the Neighbour-Join method. A total of 10 nucleotide sequences were used in this analysis, with 1175 positions.
GenBank submission
The sequence data of the marine isolate was submitted to GenBank using the BankIt tool provided by NCBI.16 The submission process resulted in the assignment of accession numbers for the sequences obtained from the marine isolate.
Biosynthesis and characterization of metal oxide nanoparticles (CuONPs) using marine bacteria MO1
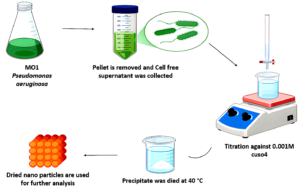
The Bio-inspired green chemistry method based biosynthesis of nanoparticles using marine bacteria offers an environmentally friendly alternative method to chemical methods. This biological approach avoids the use of toxic chemicals and high-energy processes, sustainable methods that avoid harmful chemicals and reduce energy consumption. In the Biological Synthesis pseudomonas strains have strains have natural mechanisms for reducing metal ions and can act as reducing and capping agents. The bacterial cells or their secondary metabolites are exposed to CuSO4 solution. The metabolites in Pseudomonas can reduce Cu²+ ions to CuO, forming copper nanoparticles. We carried out the experiment at room temperature and neutral pH, minimizing energy usage. The process avoids all hazardous chemicals, using only the copper precursor, bacteria, and water, leading to non-toxic, by-products. The advantage of this green approach is the by-products are typically less toxic and biodegradable. The process has minimal waste generation due to the direct reduction of metal ions by the bacteria, Green synthesis with Pseudomonas aeruginosa is scalable for large-scale production due to its simplicity. The marine isolate MO1, identified as Pseudomonas aeruginosa, was inoculated into the medium Zobell Marine (ZM) broth. The culture was incubated with a rotation speed of 130 rpm for 2 days at 30 °C in orbital shaker, to obtain the dark green pigmentation, which indicates the production of secondary metabolites.17 Following incubation, the ZM broth culture was centrifuged to separate the bacterial cells from the supernatant at 10,000 rpm for 15 minutes at a cool temperature of 4 °C. The collected supernatant was then used for the biosynthesis of metal oxide nanoparticles.18,19 For the synthesis, 100 ml of the supernatant was titrated with a 0.001 M of Sigma-Aldrich Copper sulphate (CAS 7758-98-7) anhydrous powder solution in a 1:1 ratio until a colour change and precipitate formation were observed. The precipitate was completely dried and used for further analysis. The copper oxide nanoparticle was characterized by the following UV-spectral analysis, particle size analysis, FTIR, XRD and FE-SEM analysis.20
UV spectral analysis
UV spectroscopy is a widely used analytical technique for the quantitative identification of biological macromolecules, transition metal ions and highly conjugated organic compounds. During the nanoparticle synthesis, a colour change in the culture supernatant was observed within an hour of adding copper sulphate, while the control sample maintained a dark green colour. After 24 hours of incubation, to determine the characteristic properties of the copper oxide nanoparticles, the precipitate of nanoparticles was subjected to UV spectral analysis using a UV-1800 [Shimadzu] spectrometer with a wave length between 400 nm to 665 nm.21,22
Particle size analysis
Particle size analysis is crucial for understanding the changes in physical properties such as size, microstructure, charge, surface properties, mechanical characteristics, and shape of nanoparticles. A particle size analyser (Horiba SZ-100) was employed to measure and detect the particle size distribution of the synthesized nanoparticles. This analysis is essential for quality control and for developing efficient processes to achieve high-quality end products.23 Additionally, the zeta potential measurement provided insights into the dispersion stability of the nanoparticles. A high zeta potential indicates a more stable dispersion, which is vital for the development of stable formulations, suspensions, dispersions, or emulsions.
FTIR spectroscopy of synthesized CuO nanoparticles
The bio-reduction of copper ions to form copper nanoparticles by Pseudomonas aeruginosa involves several possible mechanisms that are facilitated by the bacteria’s enzymes, metabolites, and cellular components. These mechanisms allow the reduction of copper ions (Cu²+) to elemental copper (Cup), resulting in the formation of copper nanoparticles. For the biological synthesis we used secondary organic metabolites as reducing agent, proteins and polysaccharides secreted by Pseudomonas aeruginosa act as reducing and stabilizing agents. These biomolecules contain functional groups (e.g., amino, hydroxyl, and carboxyl groups) that can donate electrons to copper ions, reducing them to Cup. These organic compounds can also stabilize the formed nanoparticles by capping them, preventing aggregation. This pathway provide a natural and efficient way to produce copper nanoparticles while minimizing the need for harsh chemicals. For the identification of biomolecule functional group we used FTIR analysis. Fourier-transform infrared (FTIR) spectroscopy is to determine the stretching and bending frequencies of functional groups attached to the surface of copper nanoparticles. In this study, FTIR spectroscopy is employed to analyse the spectra of copper oxide nanoparticles (CuONPs) at various laser fluences.24The resulting spectra display multiple absorption peaks at infrared frequencies, which correspond to different modes of interatomic bond variations, such as stretching or bending vibrations and hydrogen bond interactions. The KBr pellet technique was used for solid samples, where approximately 0.125 grams of the crude sample was mixed thoroughly with 0.25 to 0.50 grams of potassium bromide using a mortar and pestle. The mixed sample was then placed at the bottom of a pellet die, covered completely using a spatula, and pressed at a pressure of 5,000 to 10,000 psi. The die was carefully removed to produce a transparent and properly formed disc for analysis.
X-ray diffraction (XRD) analysis
X-Ray Diffraction (XRD) is used to determine the internal structure and mineral composition of both naturally occurring and artificially manufactured crystalline materials. This technique operates on the principle of constructive interference of monochromatic X-rays, producing a unique fingerprint for each crystalline material present in a sample.25 In this study, CuONPs were characterized by XRD using an instrument Malvern Panalytical X’Pert³ Powder X-ray diffractometer. The diffraction peaks were recorded and interpreted according to the standards set by the Joint Committee on Powder Diffraction Standards (JCPDS).26
Field emission scanning electron microscopy (FE-SEM)
FE-SEM is used in various fields, including biology, nanotechnology, microstructural imaging, compositional analysis of materials, and materials science to know the mean particle morphology and size of copper oxide nanoparticles.27 In this study, CuO nanoparticles were sent to the Vellore Institute of Technology (VIT) for analysis using a Thermo-Fisher Scientific FEI Quanta 250 FEG model. This equipment is utilized to examine the detailed morphology of the material.
Antimicrobial activity
The antimicrobial activity of copper oxide nanoparticles (CuONPs) was evaluated against clinical bacterial pathogens and phytopathogens using the disc diffusion method. Freshly prepared bacterial and phytopathogenic cultures were uniformly spread onto the surface of the respective media plates using a sterile cotton swab. Subsequently, 10 µl of copper oxide nanoparticles synthesized from the MO1 strain were impregnated onto sterile Whattmans 6 mm discs. The impregnated discs were then placed on the inoculated agar plates and incubated under appropriate conditions to allow for microbial growth and diffusion of the nanoparticles. Zones of inhibition around the discs were measured to determine the antimicrobial efficacy of the CuONPs against the tested pathogens.27
Photocatalytic activity
The photocatalytic degradation activity of the MO1 strain of Pseudomonas aeruginosa and the synthesized copper oxide nanoparticles (CuONPs) was assessed through the degradation of the dye Malachite Green. The dye was prepared with a concentration of 10 ppm in 50 ml of modified minimal media.28,29 A total of 10 mg of CuONPs was added to the dye solution, and 3 ml of inoculum was introduced into two separate conical flasks, one flask was kept in a static condition, while the other was placed on an orbital shaker set at 120 rpm for 12 hours to facilitate adsorption. After the incubation period, the supernatants from the abiotic control, static condition, shaking condition, and CuONPs-treated samples were collected following centrifugation at 7000 rpm for 15 minutes. These supernatants were then subjected to analysis using a UV-visible spectrophotometer to determine the extent of dye degradation, indicating the photocatalytic efficiency of the nanoparticles.
Collection of phytopathogens and its identification
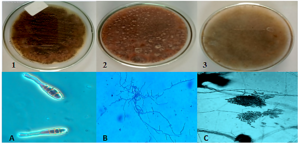
The pathogens Alternaria solani, Rhizoctonia solani and Colletotrichum capsici were cultured on Potato Dextrose Agar (PDA) media (HIMEDIA M096). The fungal staining was performed, and the morphological characteristics were observed to identify the pathogens under the phase contrast microscope, the morphology of each pathogen is presented in Figure 2.
Figure 2. Isolation of fungal pathogens from infected vegetables on PDA media plates and microscopic observation of fungal staining under phase contrast microscope 1. Alternaria solani (A), 2. Rhizoctonia solani (B), 3. Colletotrichum capsici (C)
Phylogenetic tree
The phylogenetic tree of strain MO1, constructed with bootstrap values above 50%, demonstrates that the strain has a close genetic relationship with Pseudomonas aeruginosa. The strain MO1 is particularly closely related to the type strain KX946966. Only Good quality sequence data was used for comparison and Phylogenetic analysis which is shown in Figure 3.
Figure 3. Phylogenetic tree of MO1 (along with bootstrap values above 50%), the phylogeny of the strain MO1 showed close homology with Pseudomonas aeruginosa close to strain type KX946966
GenBank submission
The genomic 16S rRNA nucleotide sequence of the marine strain Pseudomonas aeruginosa was deposited to GenBank database. The strain was assigned the accession number ON032863 by GenBank.
Biosynthesis and characterization of (CuONPs) metal oxide nanoparticles using marine bacteria MO1
The strain MO1, identified as Pseudomonas aeruginosa and assigned the GenBank accession number ON032863, has demonstrated potent antimicrobial activity. The strain was subcultured in Zobells marine broth medium (HIMEDIA 2216). After incubation, the broth culture was centrifuged at 10,000 rpm for 15 minutes in cooling centrifuge. The supernatant was carefully collected and used for the biosynthesis of metal oxide nanoparticles. For the synthesis, 100 mL of the supernatant was titrated with a 0.001 M copper sulphate solution in a 1:1 ratio. The reaction was monitored until a colour change and the formation of a precipitate which indicates the synthesis of copper metal oxide nanoparticles. The precipitate was then dried at 40 °C. The dried sample was collected and stored for subsequent analysis.
Figure 4 shows the image of Biosynthesis of copper metal oxide Nanoparticle using Marine bacteria MO1 (Pseudomonas aeruginosa).
UV spectral analysis
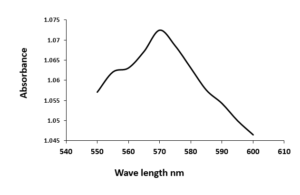
The optical properties of the copper oxide nanoparticles (CuONPs) were evaluated using UV-visible spectrum analysis. A 0.001 M copper sulphate solution was used as a control for baseline correction. The CuONPs exhibited the maximum peak of absorbance in the region of 540 nm to 600 nm, as shown in Figure 5. This range is characteristic of copper nanoparticles. The highest peak observed at 570 nm confirms the presence of copper oxide nanoparticles. The position of the Surface Plasmon resonance (SPR) band may be different due to the following factors such as particle shape, size, and the capping agent. This variation in the SPR band position is indicative of different physio-chemical properties of the synthesized nanoparticles.
Particle size analysis and zeta potential
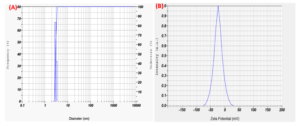
The size of the metal oxide nanoparticles was analysed using a particle size analyser (HORIBA SZ-100). The stability of the nanoparticles was assessed by measuring their zeta potential value. The particle size measurements were conducted at a scattering angle of 173 °C, maintaining a temperature of 25 °C. The transmission percentage (T%) before the mean measurement was 3, and the viscosity of the dispersion medium was found to be 0.896 mPa·s. The particle distribution was found to be standard, with the representation of the MO1 CuONPs scattering light intensity at a count rate of 4567 Kcps (kilo counts per second). The results of the calculations are presented in Table 1, and the corresponding graph is shown in Figure 6A.
Table (1):
Calculation results of MO1 CuONPs Size Analysis
Peak no. |
S.P. Area Ratio |
Mean |
S.D |
Mode |
|---|---|---|---|---|
1 |
1.00 |
3.0 nm |
0.2 nm |
3.0 nm |
2 |
0 |
0 |
0 |
0 |
Total |
1.00 |
3.0 nm |
0.2 nm |
3.0 nm |
The Zeta potential of the sample was measured at a temperature of 25 °C. The mean Zeta potential of the MO1 sample was calculated to be -23 mV, and the mean electrophoretic mobility was -0.00018 cm²/V·s, as shown in Figure 6B.
Figure 6. (A). MO1 CuONPs particle size analysis showing peak at 3 nm; (B). MO1 CuONPs Zeta potential at -23 m
FTIR (Fourier transform infrared spectroscopy analysis)
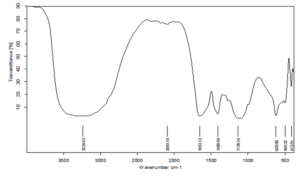
The analysis aims to identify the efficient stabilization of bio-reduced CuO nanoparticles (CuONPs), potential biomolecules involved in their reduction, and the functional groups of biomolecules and biomasses bound to the external surface of CuONPs.30 The graphical image of IR spectra of the sample MO1 CuONPs are depicted in Figure 7. The peaks with reference to the presence of functional groups are shown in the Table 2.
Table (2):
List of obtained frequency region in infrared spectroscopy and possible functional group of the synthesised MO1 CuONPs
Group frequencies, wave number (cm-1) |
Size and shape of the peak |
Functional group |
|---|---|---|
3234.63 |
strong, broad O-H stretching |
alcohols |
2000-1650 |
weak C-H bending |
Aromatic compound |
1440-1395 |
medium O-H stretching |
carboxylic acids |
1409.58 |
C-F stretching |
Fluoro compounds |
1138.34 |
strong C-O bending |
aliphatic ethers |
620.50 |
strong C-I stretching |
halo compounds |
413.51 |
Sharp |
nanostructure formation of CuO |
XRD (X-Ray diffraction)
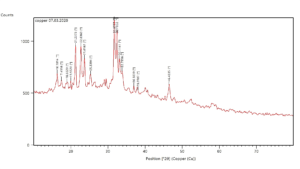
The wavelength of X-Rays ranges from 0.01 nm to 10 nm, which allows them to penetrate deep into materials, including those with crystalline structures. This penetrating capability makes X-ray spectroscopy a powerful tool for analysing the properties of various materials. Figure 8 illustrates the images of X-ray diffraction (XRD) pattern of biosynthesized nanoparticles produced from the MO1 marine isolate, which formed a powdered X-ray diffraction pattern with copper sulphate (CuSO4) crystals. The X-ray diffraction (XRD) pattern of biosynthesised nanoparticles shows several peaks that indicate crystalline phase. However, there appears to be a noticeable amount of background noise. It’s because of the impurities in the sample; if the copper nanoparticles (CuONPs) were synthesized using biological methods via Pseudomonas aeruginosa, residual organic compounds or secondary metabolites might be present. These materials do not form crystalline phases and could contribute to background noise. This is common in green synthesis, where biological molecules are used as reducing and capping agents. This method often lead to partially amorphous or non-crystalline phases due to incomplete crystallization, which can produce a diffuse background signal rather than sharp crystalline peaks but that do not affect the primary crystalline structure of CuONPs. The CuSO4 crystals exhibit a cubic structure with a specific space group. The XRD analysis shows that the nanoparticles are composed of nanoscale particles, as indicated by the distinct line extensions of the XRD peaks.31 From this pattern, we conclude the peak position, intensity and full width at half maximum (FWHM) values. The peaks at 36.9239°, 38.0582°, 46.4335°, suggest that copper oxide is present in our sample with reference to the 2θ values of the peak positions are listed in Table 3 and closely match the reference data for powdered CuO from the International Centre for Diffraction Data (ICDD). This similarity confirms the formation of a crystalline cubic phase with a cuprite structure, and a peak value of 38.0582 corresponding to the (6.56) plane, which aligns with the reported values for the monoclinic phase of CuO in the literature.
Table (3):
The peak positions with 2θ values determine peak intensity, position and width, at half-maximum (FWHM) data in full width
Pos. [°2θ] |
Height [cts] |
FWHM Left [°2θ] |
d-spacing [Å] |
Rel. Int. [%] |
|---|---|---|---|---|
16.3094 |
137.55 |
0.1863 |
5.43053 |
19.79 |
17.4356 |
64.93 |
0.2908 |
5.08221 |
9.34 |
18.9220 |
46.25 |
0.5050 |
4.68620 |
6.65 |
19.9520 |
58.54 |
0.1179 |
4.44654 |
8.42 |
21.2375 |
398.89 |
0.1930 |
4.18020 |
57.38 |
22.6960 |
403.42 |
0.3694 |
3.91477 |
58.03 |
23.6365 |
285.65 |
0.2271 |
3.76108 |
41.09 |
25.3094 |
135.58 |
0.7985 |
3.51615 |
19.50 |
31.6777 |
695.18 |
0.3201 |
2.82230 |
100.00 |
32.4052 |
590.06 |
0.2620 |
2.76058 |
84.88 |
33.1141 |
326.74 |
0.3944 |
2.70309 |
47.00 |
33.7956 |
208.05 |
0.5582 |
2.65012 |
29.93 |
36.9239 |
77.27 |
0.1145 |
2.43246 |
11.11 |
38.0582 |
45.59 |
2.1517 |
2.36253 |
6.56 |
46.4335 |
145.75 |
0.4586 |
1.95403 |
20.97 |
Figure 8. XRD (X-ray diffraction) image of the biosynthesised CuONPs produced by MO1 culture (Pseudomonas aeruginosa)
Additionally, the XRD pattern shows copper nanoparticle diffraction peaks at 31.6777, corresponding to the lattice plane (100), indicating a polycrystalline wurtzite structure. The particle size or standard particle dimensions were calculated using the Debye-Scherrer formula:
D = (0.94 l) / (β Cosθ)
Where D is Average Crystallite size, β is X-Ray wavelength, θ is Bragg angle, l is Line broadening in radians (which is full width half maximum intensity value) Using this formula, the size of the copper nanoparticles with a diffraction peak at 31.6777 was calculated to be approximately 26.95 nm for the (100) lattice plane. In contrast, the particle size corresponding to the XRD line at 38.0582 was identified as 4.19 nm.
FE-SEM
The SEM image of copper sulphate synthesized by the Pseudomonas aeruginosa MO1 strain, presented as a crystalline powder, reveals the surface morphology at magnifications ranging from 50,000x to 100,000x, with an operating voltage of 20.00 kV, as shown in Figure 9(a). The SEM analysis illustrates that the surface of the MO1 CuO nanoparticles aggregates is rough and clustered. This observation suggests that the crystalline structures are predominantly cuboidal in shape, with a tendency to aggregate, even after extended ultrasonic vibration.32 The particle size, determined using the SEM scale at a magnification of 100,000x, ranges from 108.0 nm to 252.2 nm. Furthermore, the Energy-dispersive X-ray spectroscopy analysis, depicted in Figure 9(b), confirms the presence of copper nanoparticles, with no detectable impurities.
Figure 9. SEM image of the biosynthesised copper Nanoparticles by (a) High magnification view (b) EDX Spectrum
Antimicrobial activity
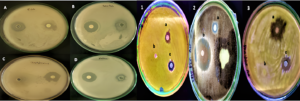
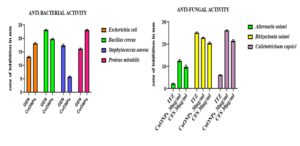
The antimicrobial activity of biosynthesized CuONPs was evaluated using a standard drug, gentamicin (30 µg), and 30 µg/ml of synthesized CuONPs against various clinical pathogens. According to the results presented in Figure 10, all tested pathogens, including Bacillus cereus, Escherichia coli, Staphylococcus aureus and Proteus mirabilis, were sensitive to both the standard drug and the biosynthesized CuONPs.33,34 For antifungal activity, the antifungal agent Itraconazole (30 µg/ml) was used as the standard. The phytopathogens Alternaria solani and Colletotrichum capsici initially showed minimal inhibition but developed resistance to the antifungal agent after two days. However, for Rhizoctonia solani, the standard antifungal agent showed maximum inhibition, as indicated in Figure 11.
- Escherichia coli, the inhibition zone around the gentamicin disk was 13.5 (±0.28) mm, whereas it was 18.1 (±0.44) mm for CuONPs.
- Bacillus cereus, the inhibition zone with gentamicin was 23.3 (±0.33) mm, compared to 20.5 (±0.28) mm with CuONPs.
- Staphylococcus aureus, the inhibition zone with gentamicin was 17.5 (±0.28) mm, while it was 5.6 (±0.33) mm with CuONPs.
- Proteus mirabilis, the inhibition zone around the gentamicin disk was 17 (±0.57) mm, and 22.5 (±0.28) mm for CuONPs.
- Alternaria solani, the inhibition zone with Itraconazole was 2.5 (±0.28) mm, 12.1 (±0.44) mm with CuONPs, and 13.8 (±0.44) mm with cell-free supernatant.
- Rhizoctonia solani, Itraconazole showed an inhibition zone of 25.5 (±0.44) mm, 16.1 (±0.44) mm with CuONPs, and 21 (±0.28) mm with cell-free supernatant.
- Colletotrichum capsici, the inhibition zone with the standard antifungal agent was 6.1 (±0.44) mm, 25.16 (±0.44) mm with CuONPs, and 21.8 (±0.44) mm with cell-free supernatant.
These experimental findings demonstrate that the biosynthesized copper nanoparticles (CuONPs) and the cultural supernatant exhibit antimicrobial activity against all tested phytopathogens, suggesting that the synthesized bioactive compound, CuONPs, is a potent antimicrobial agent.
Figure 10. The Antibacterial activity of biosynthesised CuONPs against the clinical pathogens A) Escherichia coli, B) Bacillus cereus, C) Staphylococcus aureus, D) Proteus mirabilis and Antifungal activity against phytopathogens 1) Alternaria solani, 2) Rhizoctonia solani, 3) Colletotrichum capsici
Figure 11. Graphical representation of Antimicrobial activity of Biosynthesised Nanoparticles against clinical and phytopathogens were measured by zone of inhibition in mm. Here, 30 µg/ml standard drugs (gentamicin for bacteria, ITZ-Itraconazole for fungi) and copper nanoparticles were used to determine the test. (A) Antibacterial activity, (B) Antifungal activity
Photocatalytic activity
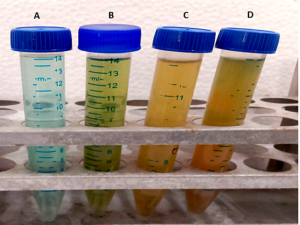
The dye degradation activity of the marine culture MO1 (Pseudomonas aeruginosa) and the synthesized CuONPs was studied using UV-visible spectroscopy. Figure 12 visually depicts the colour variation of the samples, indicating the reduction of malachite green dye.
Figure 12. Dye degradation of collected supernatant of A) Abiotic sample, B) MO1 sample placed in static condition, C) MO1 sample shaking condition, D) CuONPs
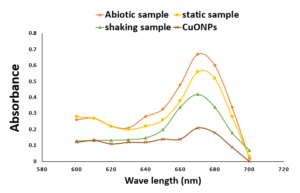
The graphical representation of different conditions (abiotic, static, shaking) involving Pseudomonas aeruginosa and synthesized CuONPs was shown in Figure 13. The graph measures absorbance in the range of 600 nm to 700 nm wavelength, with the highest absorption peak observed at 670 nm. The decrease in absorption peaks suggests a rapid reduction of malachite green by the copper nanoparticles.
Figure 13. Comparative degradation study of Malachite Green dye with abiotic sample, MO1 static sample, MO1 shaking sample and CuONPs
Summary
This study presents a sustainable method for synthesizing (CuONPs) copper oxide nanoparticles using extracellular secondary metabolites from Pseudomonas aeruginosa isolated from marine water. The bio-reduction of copper sulphate to CuONPs was confirmed through particle size analysis, FTIR spectroscopy, XRD, UV-visible spectroscopy, and SEM. UV-visible spectroscopy showed a characteristic absorption peak at 570 nm, confirming the formation of CuONPs. XRD analysis indicated a polycrystalline wurtzite structure with particle sizes ranging from 4.19 nm to 26.95 nm, while SEM images showed 108.0 nm to 252.2 nm at a magnification of 100,000x and nearly cuboidal shape. The CuONPs exhibited significant antimicrobial activity against clinical bacteria (Bacillus cereus, Escherichia coli, Staphylococcus aureus, and Proteus mirabilis) and fungicidal effects against phytopathogenic fungi (Alternaria solani, Rhizoctonia solani, and Colletotrichum capsici). Additionally, the nanoparticles showed effective photocatalytic activity, degrading Malachite Green dye. FTIR analysis revealed the presence of functional groups associated with copper oxide, confirming the purity of the nanoparticles.
Nanoparticles synthesized from marine Pseudomonas species present significant advantages, including enhanced antimicrobial and photocatalytic activity, eco-friendly synthesis processes, and unique structural properties. These attributes underscore the potential of marine bacteria as valuable resources in the development of novel nanomaterials for various applications. The green synthesis of copper oxide nanoparticles (CuONPs) using Pseudomonas aeruginosa aligns with the principles of green chemistry by employing eco-friendly, sustainable, and efficient methods. This approach utilizes the bacterium’s natural metabolic processes to reduce copper ions into nanoparticles, eliminating the need for toxic chemicals and harsh conditions. Overall, copper oxide nanoparticles synthesized from Pseudomonas offer several advantages over chemically synthesized counterparts, including eco-friendly production, enhanced photocatalytic activity, and unique biological capping. These properties make them highly attractive for applications in environmental remediation, antimicrobial treatments, and dye degradation.
ACKNOWLEDGMENTS
The authors would like to thank the DST-FIST- Department of Applied Microbiology, DST-CURIE, SPMVV and Instrumentation Facility (SIF) at VIT for providing the necessary research facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
TST and PSD designed the study. TST performed experiments and data analysis. PSD supervised the study. TST wrote the manuscript. PSD performed critical revisions. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available in the GenBank repository under the Accession number ON032863.
ETHICS STATEMENT
Not applicable.
- Chakraborty N, Banerjee J, Chakraborty P, et al. Green synthesis of copper/copper oxide nano particles and their applications: a review. Green Chem Lett Rev. 2022;15(1):187-215.
Crossref - Camacho-Flores BA, Martinez-Alvarez O, Arenas-Arrocena MC, et al. Copper: Synthesis techniques in nanoscale and powerful application as an antimicrobial agent. J Nanomater. 2015;1:415238.
Crossref - Chen P, Zhang P, Cui Y, Fu X, Wang Y. Recent progress in copper-based inorganic nanostructure photocatalysts:properties, synthesis and photocatalysis applications. Materials Today Sustainability. 2023;21: 100276
Crossref - Chen Y, Wang D, Zhu X, Zheng X, Feng L. Long-term effects of copper nanoparticles on wastewater biological nutrient removal and N2O generation in the activated sludge process. Environ Sci Technol. 2012;46(22):12452-12458.
Crossref - Cioffi N, Torsi L, Ditaranto N, et al. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater. 2005;17(21):5255-5262.
Crossref - Shantkriti S, Rani P. Biological synthesis of copper nanoparticles using Pseudomonas fluorescens. Int J Curr Microbiol Appl Sci. 2014;3(9):374-383
- Iravani S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int Sch Res Notices. 2014;2014(1):359316.
Crossref - Waris A, Din M, Ali A, et al. A Comprehensive Review of Green Synthesis of Copper Oxide Nanoparticles and Their Diverse Biomedical Applications. Inorganic Chemistry Communications. 2020.
Crossref - Bhavyasree PG, Xavier TS. Green synthesised copper and copper oxide based nanomaterials using plant extracts and their application in antimicrobial activity: Review. Current Research in Green and Sustainable Chemistry. 2022;5;100249.
Crossref - Venkateswarlu N, Sireesha O, Aishwayra S, Vijaya T, Sriramulu A. Isolation screening of rhizosphere fungi antagonistic to rice stem rot disease pathogen sclerotium orizae catt. Asian J Pharm Clin Res., 2015;8(5):54-57
- Isnansetyo A, Kamei Y. Pseudoalteromonas phenolica sp. nov., a novel marine bacterium that produces phenolic anti-methicillin-resistant Staphylococcus aureus substances. Int J Syst Evol Microbiol. 2003;53(2):583-588.
Crossref - Mahalakshmi D, Thanikachalam PV, Elumalai K. The potential of copper oxide nanoparticles in nanomedicine: A comprehensive review, Biotechnol Notes. 2024;5:80-99.
Crossref - Clarridge JE 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;;17(4):840-862
Crossref - Darby AC, Chandler SM, Welburn, SC, Douglas AE. Aphid-symbiotic bacteria cultured in insect cell lines. Appl Environ Microbiol. 2005;71(8):4833-4839.
Crossref - Tamura K, Stecher G and Kumar S. MEGA 11: Molecular Evolutionary Genetics Analysis version 11. Mol Biol Evol. 2021;38(7):3022-3027.
Crossref - National Library of Medicine. Pseudomonas aeruginosa strain KX946966 16S ribosomal RNA gene, partial sequence. https://www.ncbi.nlm.nih.gov/nuccore/ON032863. Accessed 22 November 2019.
- Crisan MC, Teodora M, Lucian M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl Sci. 2022;12(1):141.
Crossref - Koteeswari P, Suresh S, Fatimah I, et al. Green synthesis and characterization of copper oxide nanoparticles and their photocatalytic activity. Inorg Chem Commun. 2022;144:109851.
Crossref - Lanje AS, Sharma SJ, Pode RB, Ningthoujam RS. Synthesis and optical characterization of copper oxide nanoparticles. Adv Appl Sci Res. 2010;1(2):36-40.
- Shah MA, Al-Ghamdi MS. Preparation of copper (Cu) and copper oxide (Cu2O) nanoparticles under supercritical conditions. Mater Sci Appl. 2011;2(8):977- 980.
Crossref - Ali SG, Haseen U, Jalal M, Khan RA, Alsalme A, Ahmad H, Khan HM. Green Synthesis of Copper Oxide Nanoparticles from the Leaves of Aegle marmelos and Their Antimicrobial Activity and Photocatalytic Activities. Molecules. 2023;28(22):7499.
Crossref - Wang G, Zhao K, Gao C, et al. Green synthesis of copper nanoparticles using green coffee bean and their applications for efficient reduction of organic dyes. J Environ Chem Eng.2021;9(4):105331
Crossref - Karthik AD, Kannappan G. Synthesis of copper precursor, copper and its oxide nanoparticles by green chemical reduction method and its antimicrobial activity. J Appl Pharm. Sci 2013;3(5):016-021.
- Elango M, Deepa M, Subramanian R, Musthafa AM. Synthesis, Characterization, and Antibacterial Activity of Polyindole/Ag-Cuo Nanocomposites by Reflux Condensation Method. Polym Plast Technol Eng. 2018;57(14):1-12.
Crossref - Jeyaraman R, Kadarkaraithangam J, Arumugam M, Govindasamy R, Rahnuman AA. Synthesis and antimicrobial activity of copper nanoparticles. Mater Lett. 2012;71:114-116.
Crossref - Fatma S, Kalainila P, Ravindran E, Renganathan S. Green synthesis of copper nanoparticle from Passiflora foetida leaf extract and its antibacterial activity. Asian J Pharm Clin Res. 2017;10(4):79-83.
Crossref - Wong-Ng W, McMurdie HF, Hubbard CR, Mighell AD. JCPDS-ICDD Research Associateship (Cooperative Program with NBS/NIST). J Res Natl Inst Stand Technol. 2001;106(6):1013-28.
Crossref - Naz S, Gul A, Zia M, Javed R. Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Appl Microbiol Biotechnol. 2023;107(4):1039-1061.
Crossref - Dehaj MS, Mohiabadi MZ. Experimental study of water-based CuO nanofluid flow in heat pipe solar collector. J Thermal Analysis Calorim. 2019;137(1-2):2061-2072.
Crossref - Zhang S, Lin L, Huang X, Lu Y, Zheng D, Feng Y. Antimicrobial properties of metal nanoparticles and their oxide materials and their applications in oral biology. J Nanomate. 2022;2022(1):2063265.
Crossref - Meng Y. A Sustainable approach to fabricating Ag nanoparticles/PVA hybrid nanofiber and its catalytic activity. Nanomaterials. 2015;5(2):1124-1135.
Crossref - Ogunyemi SO, Abdallah Y, Zhang M, et al. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. Oryzae. Artif Cells Nanomed Biotechn. 2019;47(1):341-352.
Crossref - Joshi NC, Chaudhary N, Rai N. Medicinal plant leaves extract based synthesis, characterisations and antimicrobial activities of ZrO2 nanoparticles (ZrO2 NPs). BioNanoSci. 2021;11(2):497-505.
Crossref - Das B, Dash SK, Mandal D, et al. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arabian J Chem. 2017;10(6):862-876.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.