ISSN: 0973-7510
E-ISSN: 2581-690X
The study investigated Trichoderma-mediated morphological and biochemical responses in drought-susceptible and tolerant rice cultivars, namely IR-64 and DRR-44, under drought-stressed and normal conditions. Various morphological and biochemical parameters were recorded 30, 60, and 90 days after transplanting. The shoot length was insignificant, while the root length was significant in drought-susceptible DRR-44 compared to non-stressed plants. The number of roots was also significant in Trichoderma BHU-1 treated plants of both cultivars. Proline content was more substantial in drought susceptible cultivars than tolerant and similarly, lignin, TPC, PAL, and PO activities were higher in Trichoderma BHU-1 treated drought-stressed plants than in normal ones. The result revealed that Trichoderma BHU-1 treatment modulates an increase in root length, shoot length, and the total number of tillers and roots under drought conditions. It also maintained the level of phenolics in plants by upregulating the pathway thereby helping the plant to sustain drought.
Drought Tolerance, Oxidative Stress, Peroxidase, Rhizospheric Microorganisms
Rice (Oryza sativa) forms a principal constituent of the diet for more than half of the global population from the Poaceae (Gramineae) family, originating about 130 million years ago. It is also a means of fodder, such as husk and straw, for animals in many countries.1 It has been documented to be simultaneously domesticated in Southern China and North-Eastern India around 8000 years ago.2 India comes first in the area under cultivation and second in rice production. Among Indian states, West Bengal, Punjab, and Uttar Pradesh are the highest producers, with Punjab also having the highest productivity. Rice cultivation depends heavily on irrigation facilities; hence, it is carried out primarily in well-puddled lands. Various abiotic stresses hamper rice production potential, and drought is the most threatening problem. It has progressed severely in many areas worldwide.3 Rainfall uncertainty, groundwater depletion, and increased soil salinity have only increased the stress magnitude. Rice is invulnerable to drought at the reproductive growth stage, where even moderate stress can cause extreme yield decline.4 Drought can cause yield loss of 15-50% in rice, based on the period and magnitude of stress.5 Thus, one of the objectives of rice development programs has been to increase the ability to survive under long-day drought conditions.
Trichoderma spp. is a cosmopolitan fungus inhabiting many soil types and possesses root-colonizing ability in various plants in different ecosystems. It is a symbiotic avirulent microbe that can induce defence mechanisms and growth in host plants under substandard conditions.6 For many years, the genus has been used as a biocontrol agent, but over the current period, it has become common as a plant growth promoter.7 Various Trichoderma spp. are reported to improve the growth of plants under drought conditions in crops like wheat,8 tomato,9 Arabidopsis,10 and rice.11 The primary underlying mechanisms in imparting drought tolerance are root modulation, phytohormone stimulation,12 siderophore production,13 phosphate solubilization, upregulation of defence-related proteins,14 and enhanced plant antioxidant system.15 Root colonization of rice plants by Trichoderma spp. also changes net photosynthesis, stomatal conductance, and leaf greenness to alleviate drought stress.13 The present investigation aims at the comparative analyses of a drought-tolerant Trichoderma spp., BHU-1 isolate on two rice cultivars, IR-64 (drought susceptible) and DRR-44 (drought tolerant).
Biological material
Seeds of rice cultivars IR-64 and DRR-44 were procured from the Department of Genetics and Plant Breeding, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, India. The pure culture of Trichoderma spp. was obtained by isolating the microbe from the agricultural soil samples collected from drought-stressed regions of Uttar Pradesh, India, namely Banda, Bundelkhand (25.4530°N, 78.6098°E), Mirzapur (25.1337°N, 82.5644°E), and Chandauli (25.1794°N, 83.2934°E). Rhizospheric soil samples from agricultural fields of these regions were collected and stored in aseptic polybags. Single spores purified cultures of Trichoderma spp. were isolated using the method developed by Elad et al.,16 and were preserved at 4 °C during the investigation period.
In vitro selection of effective Trichoderma isolates against drought
The drought tolerance potential of Trichoderma isolates was measured using the protocol described by Aujla and Paulitz.17 Potato dextrose agar (PDA), amended with 10% polyethene glycol (PEG), was used to study all the isolates’ growth parameters for seven days. The mycelial growth of all isolates was evaluated after the incubation period, and those with the highest growth were selected for the pot experiment.
Experimental setup
The pot experiments were carried out in Kharif by placing them in a well-ventilated net house in a natural setting. The maximum and minimum temperatures during the total growth period of rice ranged from 31.0 °C to 41.0 °C and 15.8 °C to 33.5 °C, respectively. All experiments were conducted in a completely randomized block design with four replications. Seeds of rice cultivars IR-64 and DRR-44 were sown in different pots filled with sterilized puddled soil for raising the seedlings. Four-week-old seedlings were treated with spore suspension of Trichoderma isolate BHU-1 @ 1.6 × 106 CFU/mL-1 (colony forming unit)18 for one hour. The untreated seedlings of both cultivars served as control. The untreated and treated seedlings were transplanted in plastic pots filled with sterilized puddled soil and grown in a net house under the natural conditions described earlier. Plants were subjected to drought stress by withholding irrigation for five days and then re-irrigation on the sixth day.19
Evaluation of morphological and biochemical parameters
Various morphological parameters were recorded at different time intervals after the transplanting. The shoot length of the plants was measured employing a meter scale at 30, 60, and 90 days after transplanting (DAT). The root length and total number of roots were measured at 30 and 60 DAT. The total number of tillers and leaves was counted at 60 DAT, while the total number of panicles was counted at 90 DAT. The biochemical parameters, namely phenylalanine ammonia-lyase (PAL), proline content, total phenolic content (TPC), and peroxidase (PO), were estimated for all the samples at the time of panicle initiation, while chlorophyll and lignin content were estimated at 30 and 60 DAT. PAL activity in samples was estimated and expressed in terms of 1 M TCA g-1 fresh weight (FW).20 Proline content was estimated by Bates et al.,21 and TPC as per Zheng and Shetty.22 PO enzyme activity of the samples was estimated according to Zia et al.23 The chlorophyll and lignin content of the samples was estimated by Arnon24 and Zhang et al.,25 respectively.
Statistical analysis
All experimental data was analyzed with SPSS (v16.0). The differences among the treatments were calculated by applying one-way ANOVA.
Selection of potential isolates of Trichoderma spp. for drought tolerance
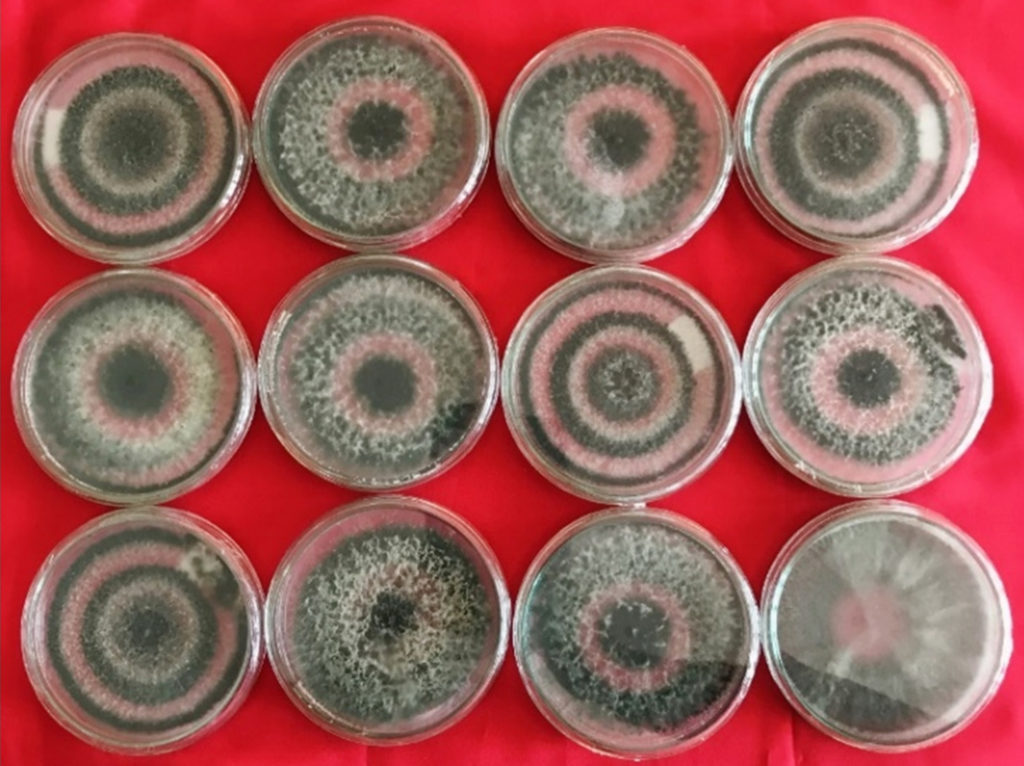
All the isolates of Trichoderma covered the 10 cm viable growth space in Petri plates within seven days of incubation on the PDA medium (Figure 1). However, restricted mycelial growth of all the isolates was observed on the PDA medium amended with 10% PEG. BHU-1 isolate exhibited better colony diameters than all the other tested isolates.
Morphological parameters
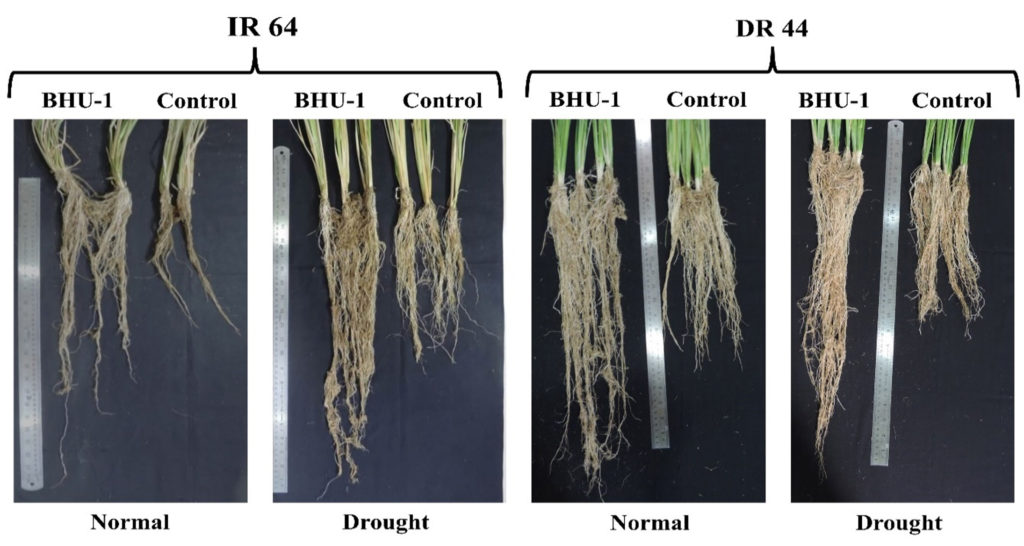
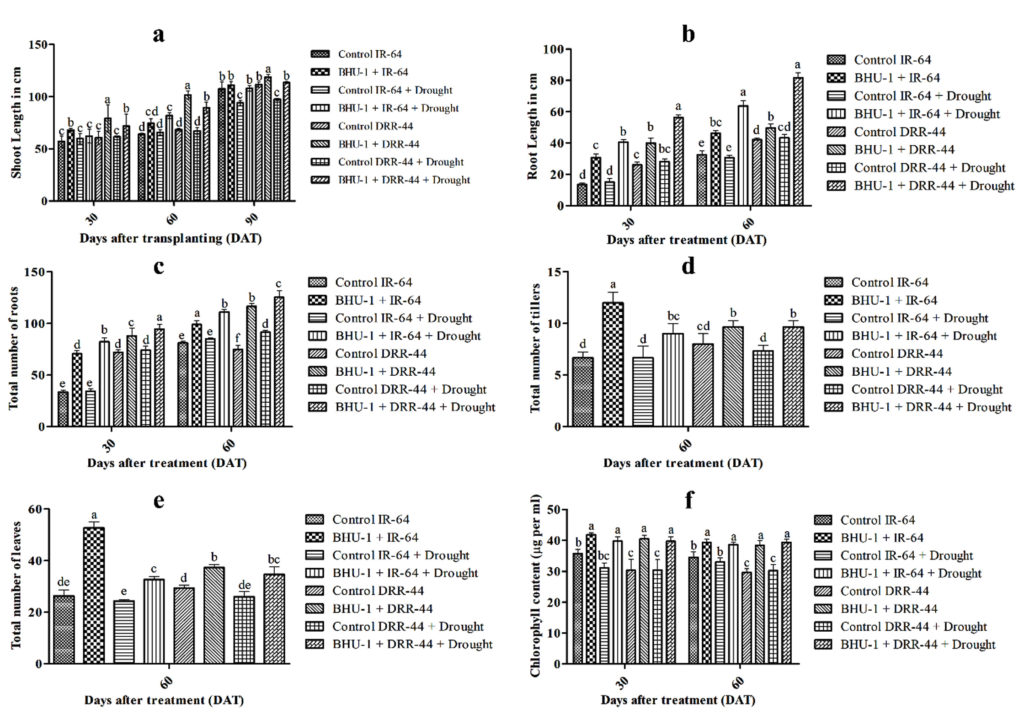
BHU-1 treated seedlings of both IR-64 and DRR-44 showed higher shoot lengths than untreated ones under irrigated conditions (Figure 2). There was, however, an insignificant difference in the shoot lengths of treated and untreated IR-64 seedlings under drought conditions. In contrast, BHU-1-treated seedlings of DRR-44 showed higher shoot lengths than the untreated ones under drought conditions (Figure 3a). The shoot length was significantly minimal for the untreated IR-64 seedlings under stress. The root lengths and the total number of roots were significantly higher in BHU-1 treated seedlings of both the cultivars under normal and drought conditions than the untreated ones. Among the cultivars, the seedlings of DRR-44 had higher root lengths (Figure 3b) and a higher total number of roots (Figure 3c) compared to the seedlings of IR-64 for each treatment, both at 30 and 60 DAT. The total number of tillers per hill and the total number of leaves per hill were both highest in BHU-1-treated IR-64 seedlings under normal conditions. BHU-1 treated seedlings of IR-64 and DRR-64 also had higher tillers (Figure 3d) and the number of leaves (Figure 3e) per hill compared to untreated ones under stress. Interestingly, the difference was insignificant in treated and untreated DRR-44 seedlings under drought conditions. The trend was similar for leaf chlorophyll content in both the cultivars at 30 and 60 DAT (Figure 3f).
Figure 2. Root growth of IR-64 and DRR-44 cultivars treated with Trichoderma BHU-1 isolate and untreated plants under normal and drought conditions
Figure 3. (a) Shoot length of different treatments at 30, 60 and 90 DAT; (b) root length of different treatments at 30 and 60 DAT; (c) total number of roots of different treatments at 30, 60 and 90 DAT; (d) total number of tillers of different treatments at 60 DAT; (e) total number of leaves of different treatments at 60 DAT; and (f) chlorophyll content of different treatments at 30 and 60 DAT. The letters on vertical bars indicate the significance level in the mean standard deviation according to DMRT at p ≤ 0.05.
Biochemical parameters
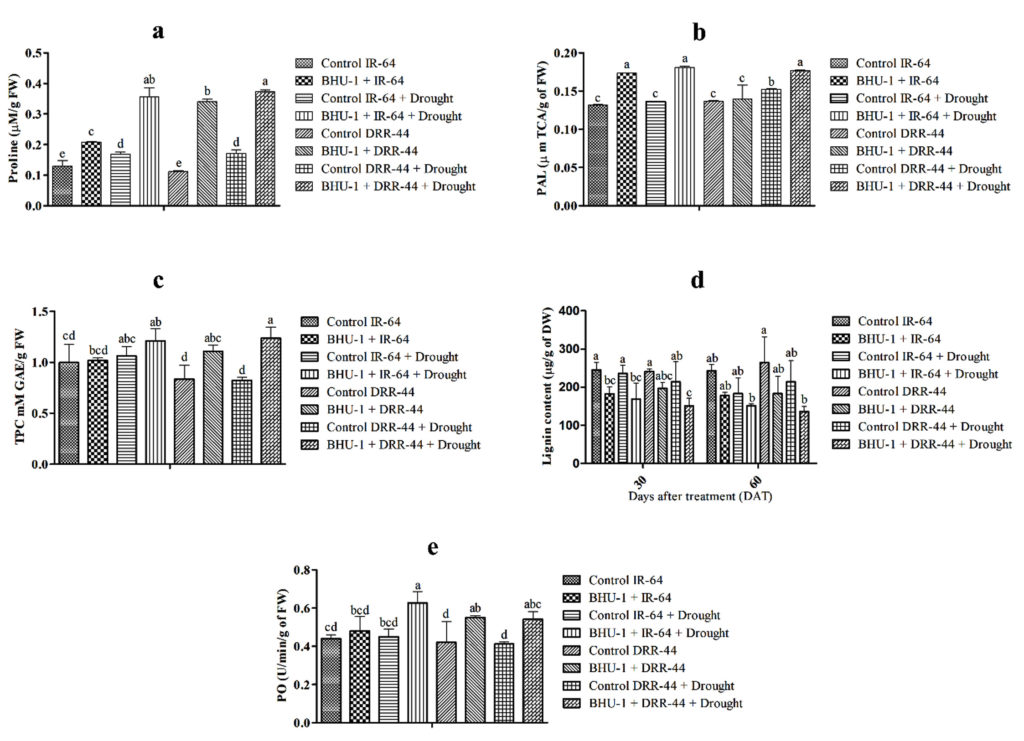
Proline was recorded significantly higher in BHU-1 treated seedlings of IR-64 and DRR-44 than in untreated ones under drought stress. BHU-1 treated seedlings of both cultivars also showed a higher accumulation of proline under stress than in normal conditions (Figure 4a). PAL activity was higher in BHU-1 treated seedlings of both cultivars under drought stress. There was, however, an insignificant difference in the PAL activity of BHU-1 treated IR-64 seedlings under normal and drought stress. In contrast, BHU-1 treated DRR-44 seedlings have significantly lower PAL activity under normal conditions than drought stress (Figure 4b). The estimated TPC was also higher in BHU-1 treated seedlings of both cultivars under normal and drought conditions. Additionally, the BHU-1 treated seedlings of both cultivars have higher TPC under drought stress than normal ones (Figure 4c). Lignin was observed to be lower rice plants under drought stress in both the cultivars for BHU-1-treated and untreated seedlings. Among the cultivars, BHU-1-treated DRR-44 control plants had higher lignin than IR-64 control plants, while the opposite was for drought-stressed plants (Figure 4d). There was a higher peroxide (PO) activity in BHU-1-treated seedlings of both cultivars than untreated ones under both conditions. Higher activity was also observed in drought-stressed plants than normal (Figure 4e).
Figure 4. (a) Proline content of different treatments at panicle initiation; (b) PAL activity of different treatments at panicle initiation; (c) TPC of different treatments at panicle initiation; (d) lignin content of different treatments at 30 and 60 DAT; and (e) PO activity of different treatments at panicle initiation. The letters on vertical bars indicate the significance level in mean standard deviation according to DMRT at p ≤ 0.05
Potential of Trichoderma spp. for imparting drought tolerance
PEG is the most common salt used for studying the effect of water potential on fungal mycelial growth.17,26 Various researchers have used the same in vitro culture method for the selection of novel Trichoderma isolates tolerant to drought13,27,28 and different soil microbes.29,30 Microbes from environmentally diverse sites are considered reservoirs of traits that have the potential to benefit a wide range of host plants to abiotic stresses.30,31 Plants and microbes in mutualism have adapted themselves through the co-evolution of heritable genetic variation, which promotes colonization and abiotic stress responses.32 Trichoderma spp. are “environmental generalists and opportunists” capable of rapid evolution for surviving new or stressful environmental conditions as a plant symbiont or a free-living microbe.33 It is mainly attributed to the production of asexual conidiospores and chlamydospores, which can survive in harsh environmental conditions. Hence, it is very plausible that the BHU-1 isolate of Trichoderma spp. in the present investigation can potentially alleviate drought stress in rice as it evolved in the drier region of Uttar Pradesh.
Effects of drought on morphological parameters in the presence of Trichoderma spp.
Drought is a primary constraint in rice production, and developing drought-tolerant rice cultivars is very difficult since quantitative traits control them. Due to this stress, there are many morphological effects on rice plants, such as reduced plant height, rolling of leaves, leaf senescence, stomatal closure, low biomass, etc.34 Moreover, the prolonged water deficit leads to wilting, oxidative damage, and lower photosynthesis and metabolic reactions. Many inherent tolerance mechanisms in rice plants help them overcome these stresses, such as stomatal closure adjustment and alteration of turgor pressure for hydraulic conductivity in leaves and roots. However, these adjustments are insufficient, and thus, the application of Trichoderma spp. is proposed for combating the stress as they can alter plant response for enhanced drought tolerance by several mechanisms.9
Many Trichoderma spp. have been identified as drought-escape microbes in crops by inducing higher relative water content and induction of lateral roots.35-37 The most universal mode of action of Trichoderma spp. is promoting plant growth by enhancing the development of root and shoot. The improved growth of roots serves the dual purpose of providing niches for the growth of fungi and increasing mineral and water uptake.38 Plant root modifications by Trichoderma spp. is the most studied mechanism as it is hypothesized that thicker and deeper roots (Figure 4) might be an imminent mode of drought stress alleviation.39 However, the trend is not the same every time since roots’ response to water deficit is highly correlated to crop genotype and period and drought intensity.40,41 Additionally, with an increase in root length, there is increased water conductance owing to the increased area of root contact with soil moisture, which enhances plant productivity under water stress.42
Genus Trichoderma has been described to upregulate the production of several proteins related to photosynthesis and carbohydrate metabolisms in plants they interact with.43,44 It directly reflects the prowess of Trichoderma spp. in inducing plant growth and energy metabolism. Simultaneously, the secondary metabolite concoction of Trichoderma spp. is shown to have several auxin-like metabolites possessing the ability to induce plant growth and development.12,45 Concurrently, Trichoderma spp. also has mineral solubilizing ability, indirectly aiding plant growth promotion by increasing the nutrient uptake in plant-available forms.46,47 Thus, the root colonization of Trichoderma spp. in plants undoubtedly promotes the development of roots and conjointly imparts several benefits.
The levels of proline increase in leaves during abiotic stresses,19 and the same was observed in the present investigation. However, the increase was comparatively higher in Trichoderma-treated seedlings than the untreated ones under drought conditions. Since metabolic processes are protected by proline during the stresses, elevated proline levels become imperative in stabilizing crucial cellular structures.48 Thus, it is evident that BHU-1 isolate increases the proline accumulation to combat the stress in both cultivars. Additionally, proline also has an essential role in scavenging reactive oxygen species (ROS), maintaining protein and membrane structure, and improving energy generation,49,50 thus increasing growth in Trichoderma-treated plants.
Trichoderma-inoculation enhances PAL activity, and TPC in rice leaves in the present investigation as microbial inoculation is proven to influence the accumulation of polyphenolics and activation of PAL enzyme activity.51,52 As PAL is a defence-related enzyme and polyphenols are antioxidants, a higher PAL and TPC in leaves are deemed to enhance the tolerance of rice under drought conditions. The phenylpropanoid pathway gets activated during stresses, where PAL is a crucial enzyme that aids in plant structural and physiological protection.53 TPC accumulation has been concluded to increase in plant cells under different stresses,54,55 aligning with the results of our present investigation. Priming of plants with Trichoderma spp. also increases TPC accumulation under stressed conditions.56 Thus, it is evident that Trichoderma spp. stimulate the accumulation of PAL and TPC during the stressed environments, thereby imparting tolerance. A simultaneous growth improvement in Trichoderma-treated rice plants under drought conditions results from oxidative stress protection by higher PAL and TPC.13 PAL and TPC accumulations are directly related to antioxidative activity and protecting the plants by scavenging ROS and stimulating cell wall formation.49,50
The present investigation also shows a positive influence of Trichoderma BHU-1 isolate on PAL and TPC, significantly increasing stressed plants compared to the control. The increase could result from the upregulation in defence-related enzyme production in plants stimulated by Trichoderma spp. through root colonization.39,57 Chitinases, peroxidases, hydroperoxide lyase, and β-1,3-glucanase lipoxygenase are the major plant enzymes associated with it. There can also be an accumulation of antimicrobial molecules like phytoalexins resulting from modulated plant metabolism, which impart tolerance against stresses. Lignin deposition of plant tissues strengthens their tolerance to stress as the level of lignification modulates the hydraulic conductivity of vascular tissues during drought.58 It also serves as plants’ mechanical support, decreasing evaporation and water leakage from plant cells.59 Trichoderma spp. are concluded to trigger the production of detoxification proteins in response to ROS production in plants under abiotic stresses. These detoxification proteins serve as ROS scavengers and shield the cells from oxidative damage.13 The ROS scavenging ability is also evident by lower lipid peroxide concentration in Trichoderma-treated plants than the untreated ones.15 All these results confirm the role of Trichoderma spp. in stress alleviation by managing the oxidative damage of ROS.
The results revealed that the selected isolates could tolerate in vitro drought stress up to 1% polyethene glycol. Seeds and seedlings treatment with Trichoderma isolate BHU-1 showed a significant increase in dry weight, root length, flag leaf length, and the total number of tillers under drought-challenged conditions. Moreover, Trichoderma’s application efficiently responded to various antioxidative enzymes like PO. It also maintained the level of phenolics in plants by increasing the activity of PAL and TPC, thereby helping the plant sustain itself during abiotic stress. An optimum level of TPC was also observed in leaf extracts, which lower the stress level in plants; similarly, an increased level of lignin was also observed in the roots of rice plants under drought-challenged conditions. Thus, the application of a native drought-tolerant Trichoderma isolate is a potential method of alleviating abiotic stresses through the modulation of both morphological and biochemical parameters of plants.
ACKNOWLEDGMENTS
The authors are highly thankful to the International Rice Research Institute, India, for funding this work, and the Department of Mycology and Plant Pathology, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, India, for providing necessities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
HBS conceptualized and visualized the study. RY and RNY performed fieldwork. NWZ, PB and HBS applied methodology. PB and SKY performed statistical analyses. RNY wrote the original draft. MMR wrote the manuscript. SKY and MMR reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was funded by the International Rice Research Institute (IRRI), India.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Goodman BA. Utilization of waste straw and husks from rice production: A review. J Bioresour Bioprod. 2020;5(3):143-162.
Crossref - Sang T, Ge S. The puzzle of rice domestication. J Integr Plant Biol. 2007;49(6):760-768.
Crossref - Passioura J. The drought environment: physical, biological and agricultural perspectives. J Exp Bot. 2006;58(2):113-117.
Crossref - Arshad MS, Farooq M, Asch F, Krishna JSV, Prasad PVV, Siddique KHM. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol Biochem. 2017;115:57-72.
Crossref - Guo H, Wang R, Garfin GM, et al. Rice drought risk assessment under climate change: Based on physical vulnerability a quantitative assessment method. Sci Total Environ. 2020;751:141481.
Crossref - Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species – opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2(1):43-56.
Crossref - Hermosa R, Viterbo A, Chet I, Monte E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology. 2011;158(1):17-25.
Crossref - Donoso EP, Bustamante RO, Caru M, Niemeyer HM. Water Deficit as a Driver of the Mutualistic relationship between the fungus Trichoderma harzianum and two wheat genotypes. Appl Environ Microbiol. 2008;74(5):1412-1417.
Crossref - Rawal R, Scheerens JC, Fenstemaker SM, Francis DM, Miller SA, Benitez MS. Novel Trichoderma isolates alleviate water deficit stress in susceptible tomato genotypes. Front Plant Sci. 2022;13:896090.
Crossref - Senizza B, Araniti F, Lewin S, Wende S, Kolb S, Lucini L. Trichoderma spp.-mediated mitigation of heat, drought, and their combination on the Arabidopsis thaliana holobiont: a metabolomics and metabarcoding approach. Front Plant Sci. 2023;14:1190304.
Crossref - Bashyal BM, Parmar P, Zaidi NW, Aggarwal R. Molecular programming of drought-challenged Trichoderma harzianum-bioprimed rice (Oryza sativa L.). Front Microbiol. 2021;12:655165.
Crossref - Contreras-Cornejo HA, Macias-RodriiGuez L, Corteis-Penagos C, Loipez-Bucio J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009;149(3):1579-1592.
Crossref - Shukla N, Awasthi RP, Rawat L, Kumar J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol Biochem. 2012;54:78-88.
Crossref - Saravanakumar K, Arasu VS, Kathiresan K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat Bot. 2012;104:101-105.
Crossref - Mastouri F, Bjorkman T, Harman GE. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol Plant-Microbe Interact. 2012;25(9):1264-1271.
Crossref - Elad Y, Chet I, Henis Y. A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica. 1981;9(1):59-67.
Crossref - Aujla IS, Paulitz TC. An improved method for establishing accurate water potential levels at different temperatures in growth media. Front Microbiol. 2017;8:1497.
Crossref - Meshram S, Patel JS, Yadav SK, et al. Trichoderma mediate early and enhanced lignifications in chickpea during Fusarium oxysporum f. sp. ciceris infection. J Basic Microbiol. 2018;59(1):74-86.
Crossref - Pandey V, Ansari MW, Tula S, et al. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta. 2016;243(5):1251-1264.
Crossref - Brueske CH. Phenylalanine ammonia lyase activity in tomato roots infected and resistant to the root-knot nematode, Meloidogyne incognita. Physiol Plant Pathol. 1980;16(3):409-414.
Crossref - Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205-207.
Crossref - Zheng Z, Shetty K. Solid-state bioconversion of phenolics from cranberry pomace and role of Lentinus edodes β-glucosidase. J Agric Food Chem. 2000;48(3):895-900.
Crossref - Zia MA, Kousar M, Ahmed I, Iqbal HMN, Abbas RZ. Comparative study of peroxidase purification from apple and orange seeds. Afr J Biotechnol. 2011;10(33):6300-6303.
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949;24(1):1-15.
Crossref - Zhang S, Yang Q, Ma R. Erwinia carotovora ssp. carotovora infection induced “Defense Lignin” accumulation and lignin biosynthetic gene expression in chinese cabbage (Brassica rapa L. ssp. pekinensis). J Integr Plant Biol. 2007;49(7):993-1002.
Crossref - Jin X, Harman GE, Taylor AG. Conidial biomass and desiccation tolerance of Trichoderma harzianum produced at different medium water potentials. Biol Control. 1991;1(3):237-243.
Crossref - Poosapati S, Ravulapalli PD, Tippirishetty N, Vishwanathaswamy DK, Chunduri S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. Springerplus. 2014;3(1):641.
Crossref - Khoshmanzar E, Aliasgharzad N, Neyshabouri MR, Khoshru B, Arzanlou M, Lajayer BA. Effects of Trichoderma isolates on tomato growth and inducing its tolerance to water-deficit stress. Int J Environ Sci Technol. 2019;17(2):869-878.
Crossref - Mhadhbi H, Chihaoui S, Mhamdi R, Mnasri B, Jebara M, Mhamdi R. A highly osmotolerant rhizobial strain confers a better tolerance of nitrogen fixation and enhances protective activities to nodules of Phaseolus vulgaris under drought stress. Afr J Biotechnol. 2011;10(22):4555-4563.
- Subramanian P, Kim K, Krishnamoorthy R, Mageswari A, Selvakumar G, Sa T. Cold Stress tolerance in psychrotolerant soil bacteria and their conferred chilling resistance in tomato (Solanum lycopersicum Mill.) under low temperatures. PLoS ONE. 2016;11(8):e0161592.
Crossref - Giauque H, Connor EW, Hawkes CV. Endophyte traits relevant to stress tolerance, resource use and habitat of origin predict effects on host plants. New Phytol. 2018;221(4):2239-2249.
Crossref - Hoeksema JD. Ongoing coevolution in mycorrhizal interactions. New Phytol. 2010;187(2):286-300.
Crossref - Kubicek CP, Steindorff AS, Chenthamara K, et al. Evolution and comparative genomics of the most common Trichoderma species. BMC Genom. 2019;20(1):485.
Crossref - Dietz KJ, Zorb C, Geilfus CM. Drought and crop yield. Plant Biol. 2021;23(6):881-893.
Crossref - Shin R, Burch AY, Huppert KA, et al. The Arabidopsis Transcription Factor MYB77 modulates auxin signal transduction. Plant Cell. 2007;19(8):2440-2453.
Crossref - Sun J, Xu Y, Ye S, et al. Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell. 2009;21(5):1495-1511.
Crossref - Racic G, Vukelic I, Prokic L, et al. The influence of Trichoderma brevicompactum treatment and drought on physiological parameters, abscisic acid content and signalling pathway marker gene expression in leaves and roots of tomato. Ann Appl Biol. 2018;173(3):213-221.
Crossref - Quazi SAJ, Ferdous J, Shozib HB, Khaton A, Zaidi NW. Role of Trichoderma asperelloides and Trichoderma brevicompactum in improving drought tolerance in rice. Plant Stress. 2024;12:100457.
Crossref - Shukla N, Awasthi RP, Rawat L, Kumar J. Seed biopriming with drought tolerant isolates of Trichoderma harzianum promote growth and drought tolerance in Triticum aestivum. Ann Appl Biol. 2014;166(2):171-182.
Crossref - Sigari TA, Yamauchi A, Kamoshita A, Wade LJ. Genotypic variation in response of rainfed lowland rice to drought and rewatering. Plant Prod Sci. 2000;3(2):180-188.
Crossref - Xu W, Cui K, Xu A, Nie L, Huang J, Peng S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant. 2015;37(2):9.
Crossref - Kim Y, Chung YS, Lee E, Tripathi P, Heo S, Kim K-H. Root response to drought stress in rice (Oryza sativa L.). Int J Mol Sci. 2020;21(4):1513.
Crossref - Zin NA, Badaluddin NA. Biological functions of Trichoderma spp. for agriculture applications. Ann Agric Sci. 2020;65(2):168-178.
Crossref - Dutta P, Mahanta M, Singh SB, et al. Molecular interaction between plants and Trichoderma species against soil-borne plant pathogens. Front Plant Sci. 2023;14:1145715.
Crossref - Nieto-Jacobo MF, Steyaert JM, Salazar-Badillo FB, et al. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front Plant Sci. 2017;8:102.
Crossref - Song M, Wang X, Xu H, Zhou X, Mu C. Effect of Trichoderma viride on insoluble phosphorus absorption ability and growth of Melilotus officinalis. Sci Rep. 2023;13(1):12345.
Crossref - Asghar W, Craven KD, Kataoka R, et al. The application of Trichoderma spp., an old but new useful fungus, in sustainable soil health intensification: A comprehensive strategy for addressing challenges. Plant Stress. 2024;12:100455.
Crossref - Mona SA, Hashem A, Abd_Allah EF, et al. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J Integr Agric. 2017;16(8):1751-1757.
Crossref - Ahanger MA, Agarwal RM, Tomar NS, Shrivastava M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L cultivar Kent). J Plant Interact. 2015;10(1):211-223.
Crossref - Hashem A, Abd_Allah EF, Alqarawi AA, Egamberdieva D. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J Biol Sci. 2015;23(1):39-47.
Crossref - Wada KC, Mizuuchi K, Koshio A, Kaneko K, Mitsui T, Takeno K. Stress enhances the gene expression and enzyme activity of phenylalanine ammonia-lyase and the endogenous content of salicylic acid to induce flowering in Pharbitis. J Plant Physiol. 2014;171(11):895-902.
Crossref - Singh DP, Singh V, Gupta VK, et al. Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci Rep. 2020;10(1).
Crossref - Akula R, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720-1731.
Crossref - Quan NT, Anh LH, Khang DT, et al. Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture. 2016;6(2):23.
Crossref - Gnanasekaran N, Kalavathy S. Drought stress signal promote the synthesis of more reduced phenolic compounds (chloroform insoluble fraction) in Tridax procumbens. Free Rad Antiox. 2016;7(1):128-136.
Crossref - Imran M, Abo-Elyousr KAM, Mousa MAA, Saad MM. Use of Trichoderma culture filtrates as a sustainable approach to mitigate early blight disease of tomato and their influence on plant biomarkers and antioxidants production. Front Plant Sci. 2023;14.
Crossref - Dixon DP, Lapthorn A, Edwards R. Plant glutathione transferases. Genome Biol. 2002;3(3):reviews3004.1.
Crossref - Zhou Y, Zhang Y, Wang X, et al. Root specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in Populus. New Phytol. 2020;227(2):407-426.
Crossref - Bang SW, Choi S, Jin X, et al. Transcriptional activation of rice CINNAMOYL-CoA REDUCTASE 10 by OsNAC5, contributes to drought tolerance by modulating lignin accumulation in roots. Plant Biotech J. 2021;20(4):736-747.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.