ISSN: 0973-7510
E-ISSN: 2581-690X
Ancient Natural Fertilizer (Jivamrit) is a traditional liquid natural mixture that undergoes fermentation and is frequently applied to enhance the soil microbiome. It is a great source of biomass with macro and micronutrients needed by crops, as well as increasing the organic carbon in the soil. With a thorough nutritional analysis that included bacterial isolation, biochemical tests, and bacterial identification by 16S rRNA sequencing, and nutrient analysis of Jivamrit, the current study concentrated on the isolation along with characterization of the major culturable bacteria from Jivamrit prepared using Gir cow native to Gujarat, India. Jivamrit yielded a total of fifteen major isolates, which were utilized for biochemical analysis and morphological evaluation. The biochemical analysis (catalase, indole synthesis, methyl red, VP test, urease, citrate, sucrose fermentation) and plant growth-promoting assays were performed on all isolated bacteria. The chemical analysis of Jivamrit revealed that it contains large amounts of macro and micronutrients and, importantly, contains Azotobacter, Zinc solubilizing bacteria, and total potash mobilizing bacteria. Molecular characterization of bacterial isolates was performed by amplifying the 16S rRNA gene using bacterial universal primers. PCR-amplified product was sequenced and was carried out, and its analysis revealed that the majority of isolates belong to the Bacillus and Priestia genera. A phylogenetic and evolutionary analysis imparts that the majority of isolates belonged to a different Bacillus species, while JW6 and JW9 belonged to Priestia megaterium. Thus, the present study revealed that major cultivable Bacillus and Priestia bacterial genus are present in Jivamrit. A literature survey revealed that the identified species immensely contribute to increasing soil fertility, plant growth, and yield. Therefore, Jivamrit acts as an alternative to chemical manure and works as a natural fertilizer, which minimises the hazardous effects of chemical farming practices on human well-being and the surroundings.
Jivamrit, Nutrient Analysis, Bacteria, Biochemical Test, Phylogenetic Analysis
Majorly farmers grow crops using chemical fertilizers for the production of various vegetables and different kinds of crops.1 Sustainable agriculture requires the implementation of environmentally friendly agricultural techniques on a global scale.2 In India, Subhash Palekar introduced natural farming in Maharashtra in 2006. This approach is chemical-free and climate-resilient. As farmers began to adopt these techniques, they became more widely known. Subsequently, several scientists and experts claimed that natural farming is a good substitute for chemical farming, which has a favourable impact on sustainable development either directly or indirectly.3 Natural farming is an eco-friendly approach that is gaining popularity worldwide as a substitute for traditional farming to increase sustainability.4 Natural farming is an exclusive form of agriculture where the cost of procuring the necessary materials such as seeds, fertilizers and chemicals for crop protection is not incurred in the market.5 Farmers are seriously adopting natural farming despite its early beginnings as it has more favourable outcomes.6 Farmers even mention that labour and production expenses have dropped by 14-45%.6 It has long been used in impoverished nations like India, and by boosting the microbial variety of the soil, it also improves crop yields and production quality. To boost yield per unit of land, global farming systems rely heavily on various chemicals, such as pesticides, herbicides, and fertilizers.1 However, excessive use of chemical fertilizers has resulted in various issues, including major pollution of the soil, water, and air, resulting in downfall of input efficiency, food quality, development of resistance in various weeds, pathogens, and pests, lowering of soil quality, lack of micronutrient in the soil, toxicity seen in various beneficial living organisms present above and below the soil surface, decreased revenue from production, etc.7 Instead, they should use more organic amendments like manures, which not only give plants the nutrients they need to thrive but also maintain the high quality of soil for their next crop.4 One of the key elements in crop protection and productivity is soil fertility. Earthworms increase soil fertility by breaking down organic matter and improving soil porosity, which promotes high root penetration and water infiltration capability. This makes nutrients in the soil more readily available to the plants. Even if inorganic pesticides are used, the use of suitable natural fertilizers can significantly improve the number of earthworms and thus promote sustainable agriculture.7
Ancient Natural Fertilizer (Jivamrit) is a traditional fermented, liquid manure that is often used to improve the soil microbiome.8 It contains an abundance of actinomycetes, fungi and bacteria, as well as other helpful microorganisms. Palekar’s technique for Jivamrit, which is made from cow dung, cow urine, pulse flour, and jaggery, is one among the four pillars of low-budget natural farming. The soil is useful in combating plant diseases. Farmers use non-standard organic compositions like Jivamrit, Beejamrut and Panchgavya in their fields.9 Jivamrit is a combination of two terms Jeevan and Amrut. Jeevan signifies life, whereas Amrut denotes medicinal remedy. Jivamrit is offered in two forms: liquid and solid (Ghan Jivamrit). It is rich supplier of natural carbon, phosphorus, potassium, nitrogen and necessary micronutrients, which are commonly used as natural manure.10 Jivamrit is a part of crop production and is essential to all aspects of crop management, including integrated disease, integrated pest and integrated soil fertility management.9
The extensive use of pesticides, herbicides, and insecticides in the agricultural sectors poses serious health hazards to humans and animals. Numerous toxicological effects, including as neurotoxicity, carcinogenicity, endocrine disruption, and reproductive toxicity, are exhibited by these agrochemicals.11 Due to processes like cholinesterase inhibition and the creation of oxidative stress, acute exposure to these chemicals frequently causes symptoms including headaches, dizziness, respiratory distress, and gastrointestinal
abnormalities.12,13 Epidemiological research has linked chronic exposure, even at sub-lethal levels, to a number of cancers, including leukaemia and non-Hodgkin lymphoma, cognitive decline, and neurological illnesses including Parkinson’s disease, cognitive impairments, and various malignancies like non-Hodgkin lymphoma and leukemia.14,15 Certain insecticides have endocrine-disrupting qualities that can cause developmental abnormalities and reproductive dysfunctions, such as decreased fertility and congenital deformities. Similar negative effects are experienced by animals from these toxicants, and their impacts are amplified throughout trophic levels through bioaccumulation and biomagnification, leading to a substantial loss of biodiversity and disturbances to the ecosystem.16 In order to reduce these significant health and environmental concerns, strong regulatory measures and the development of safer, sustainable alternatives are desperately needed. These chemicals’ persistence and environmental mobility only serve to worsen their negative effects.17,18
The biochemical properties of Jivamrit are numerous. Jivamrit contains important macronutrients like nitrogen, potassium, phosphorus and necessary micronutrients such as zinc, iron, copper and manganese for the development of plants. It constituents carbohydrates, enzymes, amino acids, vitamins, antioxidants, immune boosters, growth regulators (auxins, gibberellins) and important microbes (e.g. Bacillus spp., Streptomyces and Micrococcus etc). Therefore, the current study focused on the isolation and characterization of major culturable bacteria from Jivamrit prepared using Gir cow native to Gujarat, India, with a comprehensive nutritional analysis that included bacterial isolation, biochemical tests, bacterial identification by 16S rRNA sequencing and nutrient analysis of Jivamrit, and phylogenetic analysis.
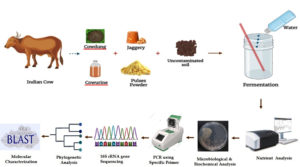
Inputs needed for Jivamrit Preparation
The Jivamrit was prepared according to the following composition. 10 kg of freshly collected Gir cow dung, 5-10 litres Gir cow urine, 2000 g of jaggery, 2000 g pulse flour, 1000 g of uncontaminated soil and 200 liters of water.9,10
Preparation of Jivamrit
The all above materials were to be mixed in 200 litres of water and it should be stirred well. The mixture should then be kept aside to ferment for 48 hours in the shade. It should be at least stir two times a day, preferably in morning and evening. This process should be repeated for 5-7 days (Figure 1).9,10
Chemical analysis of Jivamrit
After 5 days of incubation, the chemical analysis was performed at the food testing laboratory in Junagadh, Gujarat, India. The details of the methodology used in the analysis are mentioned in Table 1.
Table (1):
Methods and results of chemical and microbial analysis of Jivamrit
No. |
Parameters |
Result |
Methods |
|---|---|---|---|
1. |
Yeast & Mould Count |
3.15 X 104 CFU/ml |
IS:5403-1999 |
2. |
Azotobacter |
6.0 X 103 CFU/ml |
FCO 1985 (Amendment in 2012) |
3. |
Zinc Solubilizing Bacteria |
9.5 X 103 CFU/ml |
FCO 1985 (Amendment in 2012) |
4. |
Total Potash Mobilizing Bacteria |
9.5 X 103 CFU/ml |
FCO 1985 (Amendment in 2012) |
5. |
Nitrogen |
910 ppm |
Micro Kjeldahal Method |
6. |
Potassium |
18.8 ppm |
Flame Photo Meter |
7. |
Phosphorus |
40.48 ppm |
Nitro Vando Method |
8. |
EC |
7.03 ppm (Ds/m) |
EC Meter |
9. |
pH |
4.0 |
pH Meter |
10. |
Copper |
0.074 ppm |
MP-AES Digestion |
11. |
Iron |
1.974 ppm |
MP-AES Digestion |
12. |
Manganese |
0.241 ppm |
MP-AES Digestion |
13. |
Zinc |
0.079 ppm |
MP-AES Digestion |
14. |
Organic Carbon (%) |
1.33 |
– |
Enumeration of Jivamrit bacteria
A microbiological count carried out using the serial dilution method from Jivamrit was employed for the isolation of bacteria. Jivamrit measured 100 ml, and added into
0.9 l of autoclaved distilled water.19 Homogenization of solution is done, followed by decimally diluted (10-1 to 10-5), and aliquots of the resultant solution (10-5) were utilized to plate on suitable growth media (nutrient agar) using pour plate method. The colony-forming units were counted following incubation at 37 ± 0.5 °C. Bacterial count was determined from the original sample.20,21
Isolation of pure culture bacteria
Total fifteen different and district bacterial colonies were selected from above mentioned procedure. Afterword’s streaking of the colonies on nutrient agar plates was done and incubated at 37 °C for 24 hours. All isolates were labelled as JW1, JW2, JW3, JW4, JW5, JW6, JW7, JW8, JW9, JW10, JW11, JW12, JW13, JW14, and JW16. The fifteen bacterial cultures were selected for further identification and preserved on nutrient agar slant for future use.
Morphological characterization of Jivamrit bacteria
The isolated bacteria were further examined, followed by sub-culturing on nutrient agar, and then gram staining was carried out to visualize and screen motility. A loop of slant agar pure culture of isolate was immersed in nutrient broth and incubated at 37 °C for 24 hours. Post overnight incubation, the culture was distributed on nutrient agar plates and incubated for 24 hours at 37 °C. Post incubation, colony parameters like cell size, shape, texture, opacity, elevation, motility, and margin were recorded (Table 2). The motility of the bacteria was observed by the hanging drop method.
.
Table (2):
Morphological analysis of isolated bacteria
| Colony Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Code | Size | Shape | Texture | Elevation | Opacity | Gram Staining | Motility |
| 1 | JW1 | Small | Round | Dry | Flat | Translucent | + | + |
| 2 | JW2 | Small | Irregular | Dry | Raised | Opaque | + | + |
| 3 | JW3 | Small | Irregular | Dry | Flat | Translucent | + | + |
| 4 | JW4 | Moderate | Round | Dry | Raised | Opaque | + | + |
| 5 | JW5 | Small | Round | Dry | Flat | Translucent | + | + |
| 6 | JW6 | Small | Irregular | Moist | Flat | Opaque | + | + |
| 7 | JW07 | Small | irregular | Dry | Flat | Opaque | – | + |
| 8 | JW08 | Small | Round | Dry | Flat | Opaque | + | + |
| 9 | JW09 | Moderate | Round | Dry | Flat | Opaque | + | + |
| 10 | JW10 | Moderate | Round | Dry | Flat | Opaque | + | + |
| 15 | JW11 | Small | Round | Dry | Flat | Opaque | + | + |
| 12 | JW12 | Small | Round | Dry | Flat | Opaque | + | + |
| 13 | JW13 | Small | Irregular | Moist | Flat | Opaque | + | + |
| 14 | JW14 | Small | Irregular | Dry | Flat | Opaque | + | + |
| 15 | JW16 | Small | Irregular | Moist | Flat | Opaque | + | + |
Biochemical characterization of Jivamrit bacteria
All morphologically identified bacteria were further tested for biochemical tests viz. catalase, indole production, methyl red, VP test, urease, citrate, sucrose fermentation. Other biochemical tests such as starch hydrolysis, citrate utilization and H2S production performed for the identification of bacteria.
Extraction of bacterial DNA
Jivamrit bacterial isolates along with their genomic DNA extraction was performed using the procedure mentioned in Sambrook et al.22 and some modifications were done. Cultivation of all bacterial isolates were done in nutrient broth medium and incubated overnight at 37 °C.
Post incubation, cells were collected by centrifugation at 10,000 rpm for 5 minutes. The resulting pellet was resuspended in lysis buffer-1 (containing Tris, EDTA, and SDS at pH 4.0) and subjected to centrifugation for 10 minutes at 10,000 rpm. Following this, 500 µL of a chloroform: isoamyl alcohol mixture (24:1) was added, mixed, and centrifuged again at 10,000 rpm for 10 minutes. To the supernatant, 2.5 volumes of chilled 100% ethanol and 1/10th volume of sodium acetate were added. The mixture was incubated at -20 °C overnight. The next day, the samples were centrifuged for 10 minutes at 10,000 rpm and washed with 70% ethanol. After centrifugation, the pellets were air-dried for 3 hours, and the DNA was then dissolved in 20 µL of distilled water.
Amplification of 16S rDNA
To identify all bacterial isolates, full-length 16S rRNA primers (4032-47/50 8F 5′-AGAGTTTGATCCTGGCTC-3’, 4032-48/50 1492R 5’-CGGTTACCTTGTTACGAC-3’), were used to amplify (approx. 1500 bps), the PCR was performed under the following conditions: an initial denaturation step at 95 °C for 3 minutes, followed by cyclic denaturation at 95 °C for 30 seconds, annealing at 58 °C for 30 seconds, and cyclic extension at 72 °C for 1 minute. The process concluded with a final extension at 72 °C for 10 minutes.
Electrophoresis of PCR products
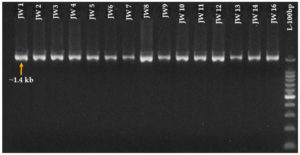
Amplified products were separated using a 1% agarose gel prepared as follows: 1 gram of agarose was dissolved in 100 mL of 1X TAE buffer (prepared with sterile distilled water) and heated until boiling. The solution was then cooled to 50 °C, and 2 µL of ethidium bromide (10 µg/100 mL stock) was mixed. This mixture was poured into the gel casting unit (Tarson). The products for analysis were mixed with 6X loading dye (Bromophenol blue), and the sample was loaded in wells. Electrophoresis was run at 60-80 V for duration of 1-1.5 hr. The results were analysed on a Gel Documentation system (Bio Rad) (Figure 2).
Figure 2. An agarose gel image displaying the PCR bands were amplified with universal 16S rRNA primers (4032-47/50 8F / 4032-48/50 1492R). JW1 to JW14 and JW16 are isolates isolated from the Jivamrit. A 100 bp size marker was used
PCR products sequencing
Amplified products of few representative samples of Jivamrit isolate were eluted from the gel elution method. The samples were sequenced at Sanger sequencing facility from Eurofins Genomics India Pvt. Ltd., Bangalore, with BigDye V3.1 technology, Genetic Analyzer (ABI3730XL, ABI, USA). The ABI Sequencing Analysis Software 5.3.1 is used to process the raw sequence files and create FASTA files. To ensure high-quality sequencing results, we selected sequences with a QV20+ value greater than 600.
Phylogenetic analysis with Sequence
Sequence similarity searches available on GenBank sequences were performed using BLAST (Basic local alignment search tool) algorithm of National Centre for Biotechnology Information (NCBI) and visual FASTA result tool of European Molecular Biology laboratory, European Bioinformatics Institute (EMBL-EBI). Analysis of phylogeny was carried out using the Maximum Likelihood (ML) technique using MEGA5 software. The 16S rRNA gene sequences of isolates for investigation were used for build phylogenetic tree, which was then compared to previously published bacterial isolates. Phylogenetic tree constructed with the help of iTOL tool.
Morphological characteristics
Diluted samples 10-3, 10-4, and 10-5 produce isolated and distinct colonies on the nutrient agar. These isolated colonies were selected for the isolation of the pure culture of the bacteria. Fifteen bacterial isolates were isolated namely JW1, JW2, JW3, JW4, JW5, JW6, JW7, JW8, JW9, JW10, JW11, JW12, JW13, JW14, and JW16. Morphological analysis revealed that JW1, JW2, JW3, JW5, JW6, JW7, JW8, JW11, JW12, JW13, JW14, and JW16 are tiny in size, while JW 4, JW9 and JW10 are intermediate in size. JW1, JW4, JW5, JW8, JW11, JW12, have round colonies and JW2, JW3, JW6, JW7, JW13, JW14 and JW16 have irregular forms. The texture of the colonies is both dry and wet. The height of colonies ranged from elevated to flat, and the majority of colonies were transparent or opaque. The details characteristics are mentioned in Table 2.
Chemical Analysis of Jivamrit
The detailed biological chemical analysis of the Jivamrit is mentioned in Table 1. The values mentioned below suggest that Jiwaamrit contain very high number of Yeast moulds, Azotobacter, Zinc solubilising bacteria, Total Potash Mobilizing Bacteria and other important major macro & micronutrients.
Biochemical characterization
All Jivamrit isolates did not display a red ring at the top of the tube, indicating that all isolates cannot generate indole amino acids. For the Voges-Proskauer test JW1, JW2, JW3, JW4, JW5, JW6, JW8, JW9, JW10, JW11, JW14, JW16 positive and turned red in test tubes. In the citrate production test JW1, JW11, JW13, and JW14 were positive, converting green to blue colour formation. In the sucrose fermentation test, all isolates showed positive results; Durham tubes are placed in test tubes, and they turn yellow in colour. JW1 and JW12 isolates show positive results in the starch hydrolysis test. Except for JW5 and JW6, which showed yellow colour, all isolates were urease-positive pink in colour. All Jivamrit isolates are catalase-positive (Table 3). The morphology and biochemical properties of all isolates were compared with Bergys manual of bacteriology and confirms that the isolates belong to the Bacillus and Pristia genus (Table 4).
Table (3):
Biochemical analysis of isolated bacteria
| Biochemical Test | PCR Amplification | Accession Number | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Code | Indole | MR | VP Test | Citrate | SFT | SH Test | Urease | Catalase | ||
| 1 | JW1 | – | – | + | + | + | + | + | + | + | NA |
| 2 | JW2 | – | + | + | – | + | – | + | + | + | PP754450 |
| 3 | JW3 | – | – | + | – | + | – | + | + | + | NA |
| 4 | JW4 | – | + | + | – | + | – | + | + | + | PP733981 |
| 5 | JW5 | – | + | + | – | + | – | – | + | + | PP733838 |
| 6 | JW6 | – | – | + | – | + | – | – | + | + | PP734095 |
| 7 | JW7 | – | – | – | – | + | – | + | + | + | NA |
| 8 | JW8 | – | – | + | – | + | – | + | + | + | PP754264 |
| 9 | JW9 | – | + | + | – | + | – | + | + | + | PP754340 |
| 10 | JW10 | + | + | – | + | – | + | + | + | PP754342 | |
| 12 | JW12 | – | + | – | – | + | + | + | + | + | PP754356 |
| 13 | JW13 | – | + | – | + | + | – | + | + | + | PP754382 |
| 14 | JW 14 | – | + | + | + | + | – | + | + | + | NA |
| 15 | JW16 | – | – | + | – | + | – | + | + | + | NA |
Table (4):
Basic Local Alignment Search Tool (BLAST) Evaluation and molecular identification through sequence of 16S rRNA gene
No. |
Isolate code |
Identified Microorganism |
Accession Number |
Agriculture Applications |
Reference |
|---|---|---|---|---|---|
1. |
JW2, JW5, JW10 |
Bacillus subtilis |
PP754450, PP733838, PP754342 |
• Plant growth promoting Rhizobacterium. • Bioremediation, biofilm formation, lipopeptide synthesis. • Dissolve soil phosphorus, boost nitrogen fixation, and generate siderophores. • Enhance fruits and grains yield. |
23,24,25,26 |
2. |
JW4, JW8, JW12, JW13, JW11 |
Bacillus sp. |
PP733981, PP754264, PP754356, PP754382, PP754353 |
• Able to improve crop tolerance to multiple abiotic stresses. •High amounts of the auxin hormone, indole acetic acid. • Plant-beneficial Bacillus spp. related to roots or rhizospheres. • Seed germination and plant growth. Plants takes phosphorus (P) and nitrogen (N) through root. |
27,28,29 |
3. |
JW6, JW9 |
Priestia megaterium |
PP734095, PP754340 |
• Promote plant development and enhance resilience to environmental stress. • Decrease in drought stress, cellular division, cell growth, photosynthesis, respiration, plant hormone synthesis, and nutrient metabolism. |
24,30 |
Phylogenetic analysis
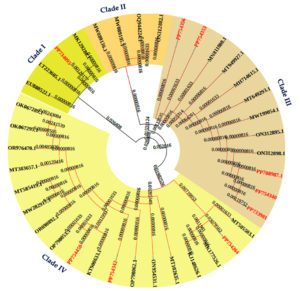
A total of 10 isolates out of 15 collected from the Jivamrit were further subjected to 16S rRNA sequence analysis. Phylogenetic analysis carried out Maximum Likelihood (ML) technique using MEGA5 software revealed that, isolates used in present study were placed in the four distinct clades. Isolate JW6 place in the clade I and shared the clade with MK129280, LT223605, and EU 88522 and PP734095. Five isolates (PP754356, PP754353, PP708987, PP754340, and PP733981) were found to be similar to MN81108, MT949927, MH714615, MT649293, MW199054, and ON312895. There are eighteen distinct isolates in the light-yellow colour. PP754264, PP754342, and PP754450 had similarities with MT505503, MK177526, KJ148626, MT102635, ON954531, OP798062, KT900633, OP798053, OP690892, MW282917, MT585416, MT38657, OR976470, OK067295, and OK067289. A phylogenetic tree of identified bacterial isolates, including their closest relatives are shown in Figure 3.
Evolutionary test and analysis of nucleotide sequence of 16S rRNA gene of bacterial isolates by Maximum Likelihood method. Total 38 sequences were used for the construction of the tree in which 10 sequences were used from the present study (Highlighted by the red colour). Evolutionary history deduced with the help of Maximum Likelihood approach and the Tamura-Nei model. This review involves 38 nucleotide sequences. Evolutionary test were performed in MEGA X. iTOL Tool was used for annotation of the tree (Figure 3).
The best-known application of Jivamrit is to increase the productivity of crops and improve the texture and fertility of soil. It also increases the beneficial microflora of soil, increased palatability, improves the pH of soil, an excellent source of NPK and other essential micronutrients.31-33 It is also proven that it increases the nutrient value in crops. As it is prepared from natural resources it does not contain harmful chemicals.34 Apart from this, it also increases the earthworm count in soil which enhances soil aeration and increases its water retention capacity.35,36 The detailed understanding of microbe-related, biochemical, macronutrient, and micronutrient analysis of Jivamrit helps it to establish as a well-known fertilizer, selective crop application and improved the content of beneficial microorganisms. Most powerful living part of the soil is thought to be bacteria.37 Many of them function as decomposers and their counterparts assist in nitrogen assimilation in soil, phosphate solubilization, antibiotic and siderophore products and cycling of nutrients. Increased numbers of beneficial soil bacteria improve soil quality and promote plant growth by lowering plant disease outbreaks.38,39 During preparation of Jivamrit, it needs to be kept under cover and in the shade at all times. It needs to be made sure that no insect lays or falls into the mixture, always keep the container covered with a plastic cover or wire mesh to prevent this. There is a need to carry out all precautionary measures as contamination of other organisms leads to damage the quality of Jivamrit.
In the present study, chemical and biological analysis was carried out different test methods (Table 1). Abundant amount of total Yeast & Mould (3.15 X 104 CFU/ml) found in the Jivamrit. These fungi may be responsible for the soil fertility and decomposition of the waste material present in the soil. However, it can be beneficial for organic matter breakdown and nutrient recycling in soil. Devakumar et al.40 previously reported that the genus., Fusarium sp., Trichoderma sp., Penicillium sp., Aspergillus sp., were found in the Jivamrit. Azotobactor spp. are free living nitrogen-fixing bacteria and its presence supports atmospheric nitrogen fixation, enhancing soil nitrogen which is crucial for plant growth. Very high amount of Azotobacter (6.0 X 103 CFU/ml) found in the Jivamrit. Similarly, Zinc solubilizing bacteria (9.5 X 103 CFU/ml) are vital for converting insoluble zinc compounds into forms accessible to plants and it is the potential to improve zinc bioavailability in the soil. The essential micronutrient potassium facilitates the solubilization and mobilization. Nitrogen plays a crucial role for the vegetative growth and metabolic functions in plants. Potassium and Phosphorus is also beneficial to crops and it is necessary for enzyme activation and osmoregulation of plants, process of photosynthesis and genetic material synthesis in plants. Electrical conductivity suggests salinity stress, requiring careful management in agricultural applications. The pH value denotes an acidic environment, this may show due to the organic acids present in the Jivamrit. Soil pH influences nutrient availability and microbial activity; thus, the acidic nature might limit certain nutrient uptake and microbial growth depending on crop requirements. Trace elements copper is essential for enzymatic functions and photosynthesis in plants. Iron is crucial for chlorophyll synthesis and electron transport within plant cells. Manganese is one of the important elements for photosynthesis, respiration and nitrogen assimilation. Zinc is a critical component of many enzymes and proteins, influencing growth hormone production and internode elongation. In the majority of the parameters, Jivamrit recorded the highest values of the nutrient along with diverse beneficial microorganisms. For natural crops, Jivamrit is a useful biofertilizer that boosts the nutritional value of the crops while also increasing growth and yield.41
Present study results show that, in Jivamrit the Bacillus spp. are the most cultivable genus present along with Priestia megaterium. Jivamrit is a complex natural fertilizer that contains millions of bacteria of several different genus. These bacterial species have been shown to modify the soil microbial population and be crucial to the nitrogen cycling, organic matter breakdown, and carbon cycle.42 Enhancing soil structure, adding more organic matter, and improving water retention all had positive environmental effects that minimized ecological harm and promoted sustainable land use.43 The 16S rRNA sequence of isolates was performed in NCBI – BLAST (National Centre for Biotechnology Information- Basic Local Alignment Search Tool), which revealed that isolate code JW2, JW5, JW10, JW4, JW8, JW11, JW12, JW13 belongs to different bacillus species whereas JW6 and JW9 belong to Priestia megaterium. The different application related to soil quality and crop yield is mentioned in table 4.
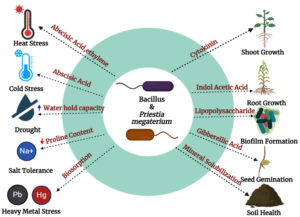
Microorganisms like bacteria and fungi identified from fields practicing low-budget natural farming have exhibited significant plant-growth-promoting effects, suggesting their part to enhancing soil properties. Additionally, the functional annotation of the reads revealed, compared with stored information and processed cluster, the cellular processing and signalling cluster, and metabolism cluster, the metabolism cluster was the most abundant. This shows how the soil’s microorganisms are interacting. Increased in the abundance of relative genes linked to metabolism, like ligase, hydrolase, and isomerase, suggested that these enzymes could aid in the breakdown, growth, inhibition of pathogens, and recycling of nutrients, as well as aid soil microbes in enhancing soil fertility.44-47 Improved root development, higher biomass, and better resistance to pests and diseases were all seen in plants treated with Jivamrit (Figure 4).48
Figure 4. Mechanism of different species Bacillus and Pristia megaterium on the plant overall development
As a chemical-free and climate-resilient alternative to farming, natural farming is becoming more and more popular around the world. It’s high time for everyone to acknowledge the harmful impacts of overusing chemical fertilizers and to take actions to reduce there application.49
The bacteria isolated in the present study were characterized using the morphological, biochemical and molecular level. The morphology was observed based on the Shape, Texture, elevation, opacity, gram staining and motility.
In an agricultural field, these remedies when used properly, it will give necessary nutrients, growth enhancers, and biological agents to unquestionably boost crop yields. Previously, researchers described that, during the fifth and sixth days of preparation, Jivamrit is an affordable liquid natural manure that is a great source of natural carbon, important microorganisms like bacteria that fix nitrogen and dissolve phosphate, macro and micronutrients. To enhance soil fertility and promote sustainable crop yields, quality, economic viability, and nutrient utilization efficiency, and resource use efficiency, Jivamrit serves as an excellent natural fertilizer, offering a viable alternative to chemical fertilizers and acting as an effective bioenhancer.
Low-budget natural farming is an age-old method employed by small to medium-scale farmers in India. Despite having a long history, it has failed to gain widespread acceptance in the scientific community due to insufficient supporting evidence. The present study concentrated on isolating and characterizing the primary culturable bacteria from Jivamrit prepared using Gir cows native to Gujarat, India, with a comprehensive nutritional analysis which included bacterial isolation, biochemical tests, and bacterial identification by 16S rRNA sequencing and nutrient analysis of Jivamrit, and phylogenetic analysis. This study provides that Jivamrit prepared from the Gir Cow contains important agricultural beneficial bacteria and essential macro and micronutrients. The current study revealed that Bacillus spp. are the most cultivable genus present along with Priestia megaterium in the Jivamrut. Both organisms are responsible for the soil fertility and increases the plant growth as well as plant growth promoting bacteria. Adopting such eco-friendly techniques globally could help preserve ecosystems and mitigate the negative impacts of agrochemicals. Nonetheless, further evidence is needed to support the widespread adoption of these natural practices.
ACKNOWLEDGMENTS
The authors would like to thank Gujarat State Biotechnology Mission, Govt. of Gujarat, for providing funding for a research project on natural farming.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AW conceptualized and designed the study. GB and JT performed data collection, literature survey and drafted the manuscript. VB and BAC reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This work was supported by Gujarat State Biotechnology Mission (GSBTM), Govt. of Gujarat vide grant number: GSBTM/JD (R&D)/661/2022-23/00172644 dated 06.02.2023.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Chavan PD, Umesha C, Embadwar P. Effect of Organic Manure on Growth and Yield of Chickpea (Cicer arietinum L.) Varieties. International Journal of Environment and Climate Change. 2023;13(10):2977-2983.
Crossref - Eyhorn F, Muller A, Reganold JP, et al. Sustainability in global agriculture driven by organic farming. Nat Sustain. 2019;2:253-255.
Crossref - Singh JS, Koushal S, Kumar A, Vimal SR, Gupta VK. Book Review: Microbial Inoculants in Sustainable Agricultural Productivity- Vol. II: Functional Application. Front Microbiol. 2016;7:2105.
Crossref - Madhusudhan L. Organic farming – ecofriendly agriculture. Journal of Ecosystem and Ecography. 2016;6:209.
Crossref - Swarup A. Lessons from long-term fertilizer experiments in improving fertilizer use efficiency and crop yields. Fertilizer News. 2001;46:59-66. https://iiss.icar.gov.in/Rti%20Appl/RTI%2080.pdf
- Chandel V, Sharma S, Bhatt K. Assessment of plant growth-promoting potential and biocontrol traits of bacterial isolates from the rhizosphere of traditional Himalayan medicinal plants. Arch Microbiol. 2021;203(8):4607-4620.
- Rutting T, Huygens D, Jorgensen BB. Nitrogen cycling in interconnected freshwater wetlands: A review. J Geophys Res Biogeosci. 2018;123(5):1406-1425.
Crossref - Singh A, Purohit SS, Purohit AK. Introduction to Plant Physiology. Agrobios (India). 2008. https://hau.ac.in/storage/app/uploads/xeiwCo42pWOJsE5a6O629tgrKrrs6HrPqp0gfetc.pdf
- NITI Aayog. Guidelines for Organic Farming Practices (Publication No. XYZ123). NITI Aayog. 2021. https://naturalfarming.niti.gov.in/
- Palekar S. Zero Budget Natural Farming: Five Layer Palekar’s Model. Subhash Palekar Natural Farming. 2016. https://nampannai.blogspot.com/2016/12/zero-budget-natural-farming-indias-best.html
- Aakanksha T, Saravanan R. Natural farming: A game changer in the era of social, economic and ecological crisis. Discussion Paper 11, MANAGE – Centre for Agricultural Extension Innovations, Reforms and Agripreneurship, National Institute of Agriculture Extension and Management (MANAGE), Hyderabad, India. 2020. https://www.manage.gov.in/publications/discussion%20papers/discussionpapers.asp
- Pathak VM, Verma VK, Rawat BS, et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front Microbiol. 2022;13:962619.
Crossref - Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B. Effect of endocrine disruptor pesticides: A review. Int J Environ Res Public Health. 2011;8(6):2265-2303.
Crossref - Naughton SX, Terry AV Jr. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology. 2018;408:101-112.
Crossref - Alavanja MCR, Ross MK, Bonner MR. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J Clin. 2013;63(2):120-142.
Crossref - Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268(2):157-177.
Crossref - Aulakh C, Singh H, Walia S, Phutela R, Singh G. Effect of farmyard manure and Jeevamrit in maize-wheat organic production system in Punjab. Agricultural Research Journal. 2018;55:485-489.
Crossref - Sánchez-Bayo F, Wyckhuys. Worldwide decline of the entomofauna: A review of its drivers. Biological Conservation. 2019;232:8-27.
Crossref - Damalas CA, Koutroubas SD. Current status and recent developments in biopesticide use. Agriculture. 2018;8(1):13.
Crossref - Somasegaran P, Hoben HJ. Handbook for rhizobia methods in legume-rhizobium technology.Springer, Heidelberg, New York. 1994.
Crossref - Kaeberlein T, Lewis K, Epstein SS. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science. 2002;296(5570):1127-1129.
Crossref - Sambrook J, Fristchi EF, Maniatis T. Molecular cloning: a laboratory manual, Cold Sproing Harbor Laboratory Press, New York. 1989.
- Mahapatra S, Yadav R, Ramakrishna W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J Appl Microbiol. 2022;132(5):3543-3562.
Crossref - Hwang HH, Chien PR, Huang FC, et al. A plant endophytic bacterium Priestia megaterium strain BP-R2 isolated from the halophyte Bolboschoenus planiculmis enhances plant growth under salt and drought stresses. Microorganisms, 2022;10(10), 2047.
Crossref - Kilian M, Steiner U, Krebs B, Junge H, Schmiedeknecht G, Hain R. FZB24® Bacillus subtilis – mode of action of a microbial agent enhancing plant vitality. Pflanzenschutz-Nachrichten Bayeer. 2000;1:72-93.
- Dursun A, Ekinci M, Donmez MF. Effects of foliar application of plant growth promoting bacterium on chemical contents, yield and growth of tomato (Lycopersicon esculentum L.) and cucumber (Cucumis sativus L.). Pak J Bot. 2010;42(5):3349-3356.
- Hassan E, Byoung RJ, Glick BR. Potential use of Bacillus spp. as an effective biostimulant against abiotic stresses in crops-A review. Curr Res Biotechnol. 2023;5:100128.
Crossref - Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci U S A. 2013;110(17):E1621-E1630.
Crossref - Dihazi A, Jaiti F, Taktak W, et al. Use of two bacteria for biological control of bayoud disease caused by Fusarium oxysporum in date palm (Phoenix dactylifera L) seedlings. Plant Physiol Biochem. 2012;55:7-15.
Crossref - Mondaca P, Celis-Diez JL, Díaz-Siefer P, et al. Effects of sustainable agricultural practices on soil microbial diversity, composition, and functions. Agriculture, Ecosystems & Environment. 2024;370:109053.
Crossref - Singh DP, Singh HB, Prabha R (Eds.). Microbial inoculants in sustainable agricultural productivity. 2016;2:308. New Delhi: Springer.
- Kumar A, Avasthe RK, Babu S, et al. Jeevamrut: A low-cost organic liquid manure in organic farming for sustainable crop production. Int J Curr Microbiol Appl Sci. 2021;9:32-34.
- Moyo E, Mhango M, Moyo P, Dzinamarira T, Chitungo I, Murewanhema G. Emerging infectious disease outbreaks in Sub-Saharan Africa: Learning from the past and present to be better prepared for future outbreaks. Front Public Health. 2023;11:1049986.
Crossref - Tiwari S, Rai S, Adhikari J, Bista S. Organic Farming: A Reliable Strategy For Sustainable Agriculture In Nepal. Science, 2023;7(2):91-103.
- Mishra VK, Kumar S, Pandey VK. Effect of organic manure and bio-fertilizers on growth, yield and quality of brinjal (Solanum melongena L.). Int. J. Pure App. Biosci. 2017;6(1):704-707.
Crossref - Yashvardhan V, Singh R, Singh D, Singh T. Comparative Study of Organic Fertilizers on Growth, Development and Economics of Brinjal (Solanum melongena var. Azad B-3) with Reference to Temperature. 2023;
Crossref - Song JE, Ko HJ, Kim AS. Comparison of the Efficacy of Anti-Obesity Medications in Real-World Practice. Drug Des Devel Ther. 2024;18:845-858.
Crossref - Fierer N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017;15(10):579-590.
Crossref - Kannaiyan K. Biofertilizers: Key factors in organic farming. The Hind Survey of Indian International Journal of Modern Plant and Animal Sciences. 2000;1(2):82-95. https://www.thepharmajournal.com/archives/2023/vol12issue6/PartBD/12-6-549-988.pdf
- Warghane A, Bhatt V, Chopade BA, et al. Jivamrit as a Sustainable Approach: A Review of Natural Farming and Future Agriculture. Recent Adv Food Nutr Agric. 2024.
Crossref - Bhadu K. Effect of Jeevamrut on growth, yield and quality of organic wheat (Triticum aestivum L.). (M.Sc. Thesis, Department of Agronomy, Rajasthan College of Agriculture, MPUAT, Udaipur, Rajasthan, India). 2019. http://krishikosh.egranth.ac.in/handle/1/5810149373
- Shang L, Wan L, Zhou X, Li S, Li X. Effects of organic fertilizer on soil nutrient status, enzyme activity, and bacterialcommunity diversity in Leymus chinensis steppe in Inner Mongolia, China. PLoS ONE. 2020;15:(10)e0240559.
Crossref - Sahu H, Kumar U, Mariappan S, Mishra AP, Kumar S. Impact of organic and inorganic farming on soil quality and crop productivity for agricultural fields: A comparative assessment. Environmental Challenges. 2024;15.
Crossref - Maduka CM, Udensi C. Comparative analysis of the effect of some organic manure on soil microorganisms. Bionatura. 2019:4:922-925.
Crossref - Reinhold-Hurek B, Bunger W, Burbano CS, Sabale M, Hurek T. Roots Shaping Their Microbiome: Global Hotspots for Microbial Activity. Annu Rev Phytopathol. 2015:53:403-424.
Crossref - Sharma N, Kumar J, Abedin MM, et al. Metagenomics revealing molecular profiling of community structure and metabolic pathways in natural hot springs of the Sikkim Himalaya. BMC Microbiol. 2020;20(1):246.
Crossref - Yan H, Zhu L, Wang YJ, et al. Comparative metagenomics analysis of the rhizosphere microbiota influence on Radix Angelica sinensis in different growth soil environments in China. Food Sci Technol. 2021;41(Suppl 2):775-784.
Crossref - Tahat MM, Alananbeh KM, Othman YA, Leskovar DI. Soil Health and Sustainable Agriculture. Sustainability. 2020; 12(12):4859.
Crossref - Tiwari A, Raj S. Natural Farming: A Game Changer in the Era of Social, Economic and Ecological Crisis. Discussion Paper 11, MANAGE – Centre for Agricultural Extension Innovations, Reforms and Agripreneurship, National Institute of Agriculture Extension and Management (MANAGE):Hyderabad, India. 2020. https://www.manage.gov.in/managecia/DiscussionPapers.aspx
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.