The human microbiome is a complex ecosystem of bacteria residing in the body. It plays a crucial role in safeguarding the overall well-being of individuals while also making them more vulnerable to various diseases. The human microbiota, genetics, and health have a complex connection, which is significant for maintaining health and preventing infections. The microbiome has a role in several gastrointestinal, skin, dental, and systemic diseases, such as obesity, diabetes, and autoimmune disorders. Genetic variation and lifestyle and dietary choices modify the microbiome composition, thereby influencing the risk of developing severe infections. The microbiome impacts host gene expression and acts as a biomarker for several diseases. The gut microbiome and genes are linked in the pathogenesis of obesity and inflammatory bowel disease. Therefore, this review focuses on the relationship between the microbiome and genetics and elucidation of the complexity of this connection. Future research-based microbiome interventions to prevent diseases could lead to strategies for personalized medicine that enhance treatment efficacy and health outcomes.
Host-microbiome Interaction, Microbiome Variations, Host Genetic Background, Microbiota Adaptation, Healthy Bacteria, Factors Influencing Microbiome
The human microbiome consists of bacteria, viruses, microbial eukaryotes, and archaea that interact and inhabit the human body. The human body is estimated to comprise 5-724 × 1012 human cells and 30-400 × 1012 bacterial cells.1 These microbes belong to various taxonomic groups and are involved in several traits and diseases.2 They can colonize various locations in the human body, such as the respiratory tract, mucosa, skin, urogenital tract, mammary glands, and gastrointestinal tract, and have mutualistic, commensalistic, or pathogenic interactions.3,4 A significant symbiotic interaction between indigenous microbiota and human beings exists from childbirth and plays a pivotal role in general well-being and health.
The human body produces specific microbiota through coevolution and actively adjusts to their particular niches and habitats in different parts of the body.5 These organisms induce changes in various body parts and function as integral components of the body due to their biological activities from conception to death. The human microbiome is constantly evolving in the host due to hormonal changes, lifestyle, nutrition, age, and genetics. Therefore, as the human microbiota plays a vital role in well-being and illness, any alteration to it might have severe consequences.3 The gut has the largest concentration of microbes and plays a vital role in human health. Any changes in the microbial concentration might lead to life-threatening diseases such as inflammatory bowel disease (IBD), cardiovascular diseases, severe antibiotic resistance, bacterial infections, and cancer.6
Each individual’s microbiome exhibits genetic diversity, with individual cells containing different mutations. The human genome has around 20,000 protein-coding genes,7 which are intricately regulated in a tissue-specific manner by intrinsic host factors and environmental stimuli. Collectively, the microbial genome within the human body comprises 100 times more genes than the human genome and is often termed the “second genome” due to its expanded coding potential compared to that of the human genome.8 For instance, microbe-specific genes synthesize several essential compounds, such as biotin, Vitamin B12, and folic acid, which are required by humans while also facilitating microbial survival through transport systems and adhesion factors.9 Therefore, understanding the genetics of the human microbiome and its interactions with the human genome is crucial for understanding the physiological and biochemical relationships between these microbes and the human host.10 Moreover, many chronic conditions, such as hyperlipidemia, obesity, cancer, and high blood pressure, have genetic components along with nutritional constraints and are clustered in families due to genetic factors rather than environmental influence.9 Therefore, this review describes the role of the human microbiome in the development and cure of diseases and explores the role of genetics in the determination of microbial genes responsible for the onset of disease. Furthermore, the microbiome’s effects on host gene expression, challenges, limitations, and future interventions are briefly discussed. In order to achieve this goal, more than 150 reviews and research papers were explored using Pubmed, NCBI, and Google Scholar, and 65 papers were used to collect data for this review. Most papers were searched using keywords and those with similar or duplicated data were excluded from this review.
The human microbiome’s role in health and disease
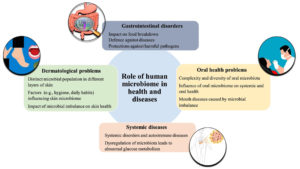
The human microbiome, which encompasses multiple physiological systems, has a significant effect on health and disease. Imbalances in the microbiome, known as dysbiosis, are associated with gastrointestinal disorders, dermatological conditions, oral health problems, and systemic diseases such as obesity and autoimmune conditions. The diversity of microbes in the gastrointestinal tract has an impact on the breakdown of food, the body’s ability to defend against diseases, and protection against harmful pathogens. Understanding dysbiosis is crucial for understanding several diseases and disease symptoms, such as inflammation, and is important for developing effective treatment strategies.11,12
Microbes are also used to treat diseases and microbiome-based treatments are more beneficial than intrusive procedures. Personalized therapeutics such as fecal transplants and probiotics are crucial for treating gastrointestinal infections.13 Thus, microbiomes have a role in disease development but also make a significant contribution to treating these diseases. Environmental factors can change the human microbiome; therefore, research on these microbes is necessary to treat gastrointestinal and other human infections.14 Environmental factors, such as daily habits, work, and personal routine, can also affect the microbiome in the skin layers and are responsible for several inflammatory skin diseases.15
Immunological responses and impairment of host cells’ antimicrobial peptide secretion are also attributes of several microbes, e.g., Staphylococcus epidermidis, residing in the skin.15,16 Although staphylococci such as Staphylococcus aureus can cause food poisoning, they also interact with other microbe species, prevent colonization by pathogenic microbes, and protect favorable lactobacilli.17 The microbiota in the oral cavity of humans is diverse, and consists of bacteria, viruses, and fungi. An imbalance in this microbe composition is responsible for several mouth diseases, such as oral thrush, dental caries, and periodontal diseases, in humans.18 Microbiome imbalance can also lead to systemic disorders, e.g., diabetes, obesity, and persistent inflammation in IBD and autoimmune disorders, such as multiple sclerosis and rheumatoid arthritis. Moreover, metabolic disorders, e.g., obesity and diabetes, also increase glucose metabolism and insulin resistance along with inflammation.19 Systemic and oral health can also be affected by the host’s immune system and the exchange of metabolic substances due to complex human biogeography.20 Therefore, it is critical to understand the diverse relationships between systemic disorders and the microbiome; such an understanding can potentially lead to the development of customized treatments and improvement of patient health (Figure 1).
Figure 1. The role of the human microbiome in health and disease. The microbiome inhabits different parts of the body and aids in digestion, protects against various gastrointestinal disorders, and influences oral health. There is bidirectional communication between the microbiome and the host’s immune system and metabolic processes. The microbiome present in the skin layers is influenced by daily habits and hygiene
Factors influencing microbiome composition
Each individual possesses their own personalized microbiome that shows a unique composition influenced by trajectories associated with different body sites. The skin also has a separate and unique microbiota influenced by factors such as the environment and skin features.21 Intestinal and oral microbes are two different categories that remain stable and separate from each other but external stimuli can cause temporary changes in the microbiota and their structure. The vaginal microbiome remains consistent, with distinct conditions that can be identified during disease, and is mostly controlled by Lactobacillus species.22 The gut microbiota is strongly influenced by diet, which is an effective method of controlling and modifying its composition. Short-term alteration of the diet can quickly change microbial profiles but long-term dietary habits have a major impact on microbial populations.23
Factors related to lifestyle, e.g., living with pets such as dogs, have a substantial impact on the composition of the microbiome. Owning pets and being exposed to livestock is associated with a lower risk of asthma, indicating that exposure to microbes could be a viable way to modulate the immune system.24 In addition, exercise decreases inflammation and causes subtle changes in the composition of microbial communities. The lack of sleep and stress are associated with changes in the composition of gut microbiota and related markers of inflammation.25 Antibiotics have a substantial influence on all microbiomes, especially the gut microbiome in adults. Exposure to antibiotics in early life is associated with lasting consequences that may contribute to the development of obesity, asthma, and IBD.26 The human microbiome shows unique variability in different bodily areas, which is determined by factors such as nutrition, lifestyle, antibiotic usage, and genetics. These factors can affect an individual’s health and susceptibility to disease (Figure 2).
Figure 2. Factors influencing the human microbiome. Various factors influence the human microbiome: nutritional elements such as types of food, meal timing, and probiotics; environmental factors such as pollution, hygiene, socioeconomic status, and physical activities; antibiotic usage, including treatment frequency and antibiotic resistance; and genetic influences, which encompass inherited predispositions, gene variants related to immune function, and epigenetic effects on gene expression
Genetic variation influences microbiome composition
Environmental factors have a significant effect on human microbial communities, but host genetics also plays a role in determining the composition of these microbiomes. A notable example of this relationship can be observed in the connection between single nucleotide polymorphisms (SNPs) and the Mediterranean fever (MEFV) gene due to alterations in the bacterial community structure in the human gut.27 Gut microbiota composition and genes linked to vulnerability to IBD are correlated.28 The energy metabolism of the gut microbiome can be affected by mutation in the fucosyltransferase 2 (FUT2) gene, which is linked to the development of Crohn’s disease.29 In persons with IBD, a correlation has been demonstrated between the quantity of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) alleles and increased frequency of microbial species in the Enterobacteriaceae inside the gut.28 These relationships and many others indicate the connection and complex relationship between the host’s genetic variation and microbial composition as different genes are affected by microbial populations inside the human body.
Genetic variation not only affects the gut microbiome but has an association with the entire host microbiome. A study of 416 pairs of twins by Goodrich et al. found that monozygotic twins (MZ) have hereditarily more similar microbial species compared to dizygotic twins (DZ), demonstrating the influence of genetic variation on the microbiome.30 Another study of MZ and DZ twins and environmental factors also showed the influence of genetic variation on microbial populations in both types of twins.31 Mouse models are valuable for determining this complex relationship. Quantitative trait locus (QTL)-mapping of these mouse models identified several genes that play a crucial role not only in generating immune responses but also in determining the microbial composition within the body.
Mitochondrial DNA of the host also has a role in determining the microbial population within cells; this enhances the understanding of the relationship between genetic variables and microbiome composition.32,33 Goodrich et al. demonstrated the effect of certain microbial families, such as the Christensenellaceae, on host genetic variation.10 However, environmental factors have a significant influence on microbial communities within the host as demonstrated by the reduced genetic effect in twin studies compared to the effects of environmental factors, highlighting their importance in shaping the host microbiome.
The microbial genome also has a significant influence on the microbiome composition in host cells and has a role in establishing the host’s immune system, especially during early colonization. Microbial colonization of the human body is affected by several factors; the most important include the method of delivery and microbial colonization of the mother during pregnancy.34 After colonization, the microbiome releases flagellin and compounds such as bacterial polysaccharides, which preserve the immunological balance and facilitates the development of the immune system of the host.35 More precisely, different types of immune cells, such as helper T cells, IgA-producing cells, and intra-epithelial lymphocytes, important for the development and effective functioning of the immune system, are affected by these microbial communities.36
The relationship between the microbiome and host genetics is important in shaping the host’s immune system and this complex interaction has a role in disease management and establishing specific treatment strategies for a wide range of microbial infections. Microbes residing in different regions of the human body produce several substances and interactions with these substances, as well as direct connection with host cells, have a significant effect on host cell genetics as evident from the example of SARS-CoV-2, which interacts with other microbes and produces several toxins by activating the expression of certain genes in different body regions. Studies using DNA microarray analysis to identify differences in intestinal biopsies between colonized mice and germ-free mice37 found an effect of microbial colonization on the host gene expression system, for example a change in gene expression in enterocytes after inoculating the host with a single stain of enteric virus. Likewise, the process of colonization by the entire microbial community results in significant changes in gene expression that are unique to many types of cells. These reactions are partially regulated by microbial sensing receptors of the innate immune system. Metagenomic profiles of the interactions of prokaryotic and eukaryotic organisms are also important for understanding gene expression in a species-specific manner in response to microbial colonization.38
Genetic factors influence health outcomes
Infectious diseases and syndromes have been widespread throughout human history, resulting in co-evolutionary interactions between pathogens and the human genome.39 The genetic variation of humans and the genomes of ancient and modern humans have been influenced by the continuous interaction between microbes and humans. The best example of this is the change in the shape and functions of red blood cells during malaria infections, when genetic variation due to Plasmodium plays a role.40,41
Although medical practices have improved the detection and diagnosis of infectious diseases, these diseases still pose a threat to the world health system. The emergence of COVID-19 due to SARS-CoV-2 is the latest example.42,43 The relationship between genetic variation and infectious diseases is also exemplified by the repeated emergence of diseases such as hepatitis, tuberculosis, malaria, and HIV. The effect of genetic variation on these infectious diseases44 and human genetic loci for vulnerability to these above-mentioned diseases is also important. These loci have been located by genome-wide association studies (GWAS).45 However, interaction between pathogens and humans is complex, and the case-controlled paradigm used by GWAS is not able to explain this relationship properly. On the other hand, next-generation sequencing and genotyping technology provides a deeper understanding of host defense against infection and the role of genetic variation in abnormal immune responses that ultimately lead to infections. Chromosomal variations and aberrations also have an important role in the development of disease conditions, and multi-omics approaches can be used to detect these abnormalities for treating infections.46 Ultimately, the overall health status and vulnerability of human beings are dependent on genetic variation, and understanding the genetics of complex host-pathogen relationships is important for identifying the causes of diseases and developing personalized treatment approaches to combat such infections and save lives.
How the microbiome affects gene expression
The microbiome plays a significant role in transforming the host transcriptome and many physiological systems and gene expression in different organs can be manipulated by changing the microbiome composition in the host. Germ-free models and probiotics can be used for this purpose. Mouse models have shown changes in drug-metabolizing enzymes in the liver affecting xenobiotic processing genes in the host.47 The metabolic functions of humans are strongly influenced by microbiome changes. As hepatic lipogenesis is reduced by the absorption of monosaccharides in the gut, the absence of short-chain fatty acids produced by microbes results in host energy metabolism by affecting the tricarboxylic acid cycle.48
Microbiomes also have a role in manipulating the host’s immune system as evidenced by germ-free and traditionally grown mouse models. These studies have shown a significant impact of microbiome disruption on the host’s immune system. More than 50% of the genes are affected in mice that lack innate immunity compared to wild-type mice, thus affecting both the host’s immune system and energy metabolism. Moreover, innate lymphoid cell composition, gene expression, and epigenetic profile are disrupted by the microbiome, thereby influencing the host immune system.49 Host cells strongly respond to microbial stimuli and these mouse studies suggest a complex relationship between the host’s microbiome, gene regulation, metabolism, and innate immunity, which indicates that the microbiome has an influence on host physiology.
In addition to mouse models, several other animal models such as zebrafish, Drosophila melanogaster, and Caenorhabditis elegans have also provided an understanding of this complex relationship. They have shown similar effects on gene regulation and metabolism and have indicated an evolutionary relationship between the microbiome and various host species.40 Therefore, a grasp of this complex relationship is necessary to understand these processes and reveal host and microbiome interactions and their impacts on health and disease. Humans have also been subjects in studies of the relationship between the microbiome and host. These investigations relied on collecting biopsies during gastric and colonic surgeries, as well as human cell cultures.50 Biopsies provide a true picture of the link between the microbiome at the interface of the intestinal lumen and host gene expression but the typical characteristics of the tissues cannot be retained due to their invasive nature. Therefore, there is a lack of understanding of healthy tissues as most of the samples came from unhealthy individuals suffering from a disease. Host-specific factors such as genetics and food metabolism can be studied by collecting inflamed tissues and diseased samples from patients and comparing them with healthy tissue to detect possible changes in the genome due to microbial communication.51
This comparative method was employed by Hasler et al. in their study of the pathophysiology of IBD; they observed a clear difference in microbiological association and metabolic pathway expression between IBD patients and healthy individuals.52 Another study analyzed regular colonoscopies and reported increased antimicrobial gene activity during IBD, indicating the effect of microbiome imbalance during disease conditions.53 Cell cultures are another cost-effective method of analyzing the host-microbiome interaction and co-culturing can also stimulate the host-microbiome environment.54 Despite the limitations of these experiments, co-culture methods are useful for understanding the host-microbiome interaction as they are effective in identifying differently controlled genes in the host and in microbes.
The role of microbiome-gene interactions in common diseases
Microbiome–gene interactions play a significant role in the development of common diseases. The human microbiota consists of diverse microorganisms, including bacteria, viruses, protozoa, Archaea, yeasts, and fungi, which create a complex ecosystem within the body. The microbiome composition varies according to genetic, metabolic, and physiological processes during an individual’s life.55 During the early stages of childhood, the microbiota becomes stable, leaving a distinct microbial “passport” for each person. The principal phyla observed are Bacteroidetes and Firmicutes. A functioning microbial core, essential for numerous interactions between the host and the microbiome, food metabolism, and immune modulation, consists of more than 50 bacterial species present in all individuals.56
The composition of the human microbiome has a substantial influence on health and diseases. Several disorders, e.g., IBD, obesity, and diabetes, are influenced by the interaction between host genes and microbial composition disrupting the energy balance and metabolic functions. Moreover, these microbes are involved in regulating inflammatory responses to autoimmune disorders such as multiple sclerosis and rheumatoid arthritis.57 Mental disorders, e.g., anxiety, Alzheimer’s disease, and Parkinson’s disease, also have a direct or indirect relationship with the microbiome composition of the host. Therefore, metagenomic advancements are crucial for understanding these relationships and developing novel treatment strategies to deal with such diseases.58 Research on non-human primates and animal models can play an important role in helping to grasp the connection between microbiome composition and host genetics and ultimately understand the vulnerability to diseases and devise effective treatment strategies.
Challenges and limitations in studying microbiome–genetics interactions
Recent progress in genotyping procedures and sequencing technologies has significantly transformed microbiology research, enabling the investigation of entire microbial communities, including holobiont systems. Current advancements in second and third generation sequencing technologies have made it possible to accurately measure the abundance of microorganisms in the human gut. Two primary techniques are commonly employed to measure the quantity of microorganisms: 16S rRNA gene sequencing (16S); and metagenomic sequencing (MGS).59 16S sequencing specifically focuses on the highly variable regions of the bacterial 16S rRNA gene, allowing very precise identification of bacteria and Archaea at the genus level. In contrast, MGS analyzes all genetic material in an environmental sample, allowing the identification of multiple microbial species and strains, as well as the investigation of the other taxonomic groups such as fungi, viruses, and protozoa. Moreover, MGS enables the estimation of the abundances of individual microbial genes and gene families, facilitating the examination of functional pathways and clusters. This also includes the investigation of biosynthetic routes for specific xenobiotics, as well as clusters of antibiotic resistance and virulence genes.60
Apart from microbiome-wide association studies (mbGWAS), the accuracy of microbial identification can be enhanced by specifically targeting 16S domains using the latest DNA separation methods and comparing these results with 16S amplicon databases.61 However, the reproducibility of heritability assessments can be affected by reference-based metagenomic techniques as they are general methods and do not offer a precise understanding of taxonomic annotation.62 Low replicon rates due to technical faults sometimes affect the identification but de novo metagenomic technology has the potential to identify microbes involved in various genetic changes, although they require a large number of reads per sample, which makes it difficult to assemble the genomes of microbes in low abundance.
Future research directions and implications for healthcare
The study of the genetic makeup of the gut microbiome is still in its early stages, similar to the initial genome-wide association studies of complex human traits. Insights gained from GWAS, including increasing sample sizes and promoting data sharing, are essential for progressing in microbiome genetics.63 Collaborative endeavors and meta-analyses can potentially expedite breakthroughs in studying host-microbiome interactions. While the impact of host genetics on the gut microbiome may not be significant, the further discovery of genetic variables that influence the microbiome, even if they have minor effects, might offer vital insights and help to tailor treatments to individuals.64
The integration of genetic and microbiome screening can improve personalized medicine and accurately predict medication responses.30 Moreover, it is crucial to apply systems genetics methods, which combine many layers of information, such as gene expression, proteomics, metabolomics, and other -omics data, to study both the human genome and the microbiome.65 Integrative techniques can also reveal complex connections and networks between the host’s genome and the microbiome’s composition and the host’s health condition. They help to understand the underlying causes of diseases and facilitate the development of specific treatments against multiple diseases.
The human microbiome has a substantial impact on both health and diseases by affecting a range of physiological processes in areas such as the stomach, skin, and oral cavity. It plays a pivotal role in both the onset and mitigation of diseases, particularly those involving inflammation and immune responses. Environmental factors such as diet, lifestyle, and antibiotic use critically modulate the microbiome. Genetic variation has an essential role in shaping the composition of the microbiome, which has an impact on an individual’s health consequences. Microbiome-based interventions can transform healthcare by providing personalized therapies and preventive methods, leading to enhanced patient outcomes. The use of integrated methods in future research has the potential to reveal the complex relationships between host genetics and the microbiome. The transition from microbiome research to clinical practice and its advancement of global public health will reflect the collaborative efforts of researchers, physicians, and policymakers.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Rosner JL. Ten Times More Microbial Cells than Body Cells in Humans. Microbe Magazine. 2014;9(2):47-47.
Crossref - Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260-70.
Crossref - Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract-a role beyond infection. Nat Rev Urol. 2015;12(2):81-90.
Crossref - Mina PR. Gut Microbiota: A Future Clinical Magic Bullet to Manifest Pathogenic Disease in the Current Future. J Pure Appl Microbiol. 2023;17(1):51-68.
Crossref - Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front Immunol. 2015;6:61.
Crossref - Pascal M, Perez-Gordo M, Caballero T, et al. Microbiome and Allergic Diseases. Front Immunol. 2018;9:1584.
Crossref - Salzberg SL. Open questions: How many genes do we have? BMC Biol. 2018;16(1):94.
https://doi.org/10.1186/s12915-018-0564-x - Grice EA, Segre JA. The Human Microbiome: Our Second Genome. Annu Rev Genomics Hum Genet. 2012;13:151-70.
Crossref - Nichols RG, Davenport ER. The relationship between the gut microbiome and host gene expression: a review. Hum Genet. 2021;140(5):747-760.
Crossref - Goodrich JK, Davenport ER, Clark AG, Ley RE. The Relationship Between the Human Genome and Microbiome Comes into View. Annu Rev Genet. 2017;51:413-433.
Crossref - Metwaly A, Reitmeier S, Haller D. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nat Rev Gastroenterol Hepatol. 2022;19(6):383-397.
Crossref - Zhao T, Wei Y, Zhu Y, et al. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front Immunol. 2022;13:1007165.
Crossref - Gubatan J, Boye TL, Temby M, et al. Gut Microbiome in Inflammatory Bowel Disease: Role in Pathogenesis, Dietary Modulation, and Colitis-Associated Colon Cancer. Microorganisms. 2022;10(7):1371.
Crossref - Rengarajan S, Vivio EE, Parkes M, et al. Dynamic immunoglobulin responses to gut bacteria during inflammatory bowel disease. Gut Microbes. 2020;11(3):405-420.
Crossref - Alkotob SS, Cannedy C, Harter K, et al. Advances and novel developments in environmental influences on the development of atopic diseases. Allergy. 2020;75(12):3077-3086.
Crossref - Lunjani N, Ahearn-Ford S, Dube FS, Hlela C, O’Mahony L. Mechanisms of microbe-immune system dialogue within the skin. Genes Immun. 2021;22(5-6):276-288.
Crossref - Nakatsuji T, Chen TH, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378):eaah4680.
Crossref - Baker JL, Mark Welch JL, Kauffman KM, McLean JS, He X. The oral microbiome: diversity, biogeography and human health. Nat Rev Microbiol. 2024;22(2):89-104.
Crossref - Pitocco D, Di Leo M, Tartaglione L, et al. The role of gut microbiota in mediating obesity and diabetes mellitus. Eur Rev Med Pharmacol Sci. 2020;24(3):1548-1562.
Crossref - Jaffe AL, Thomas AD, He C, et al. Patterns of Gene Content and Co-occurrence Constrain the Evolutionary Path toward Animal Association in Candidate Phyla Radiation Bacteria. mBio. 2021;12(4):e0052121.
Crossref - Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164(3):337-340.
Crossref - Ravel J, Brotman RM, Gajer P, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome. 2013;1(1):29.
Crossref - David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-63.
Crossref - Stein MM, Hrusch CL, Gozdz J, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375(5):411-421.
Crossref - Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol. 2017;312(6):G559-g571.
Crossref - Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212-4218.
Crossref - Khachatryan ZA, Ktsoyan ZA, Manukyan GP, Kelly D, Ghazaryan KA, Aminov RI. Predominant role of host genetics in controlling the composition of gut microbiota. PLoS One. 2008;3(8):e3064.
Crossref - Li E, Hamm CM, Gulati AS, et al. Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS One. 2012;7(6):e26284.
Crossref - Tong M, McHardy I, Ruegger P, et al. Reprograming of gut microbiome energy metabolism by the FUT2 Crohn’s disease risk polymorphism. ISME J. 2014;8(11):2193-2206.
Crossref - Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789-799.
Crossref - Kurilshikov A, Wijmenga C, Fu J, Zhernakova A. Host Genetics and Gut Microbiome: Challenges and Perspectives. Trends Immunol. 2017;38(9):633-647.
Crossref - McKnite AM, Perez-Munoz ME, Lu L, et al. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS One. 2012;7(6):e39191.
Crossref - Ma J, Coarfa C, Qin X, et al. mtDNA haplogroup and single nucleotide polymorphisms structure human microbiome communities. BMC Genomics. 2014;15:257.
Crossref - de Aguero MG, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296-1302.
Crossref - Fulde M, Sommer F, Chassaing B, et al. Neonatal selection by Toll-like receptor 5 influences long-term gut microbiota composition. Nature. 2018;560(7719):489-493.
Crossref - Hapfelmeier S, Lawson MAE, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328(5986):1705-1709.
Crossref - Bautista Balbas LA, Sandino Gomez R, Gil Conesa M, et al. Seroprevalence of SARS-CoV2 Infections in Health Care Personnel in a Long-Term Care Institution After the First Wave of the Pandemic: A Cross-Sectional Study. Workplace Health Saf. 2023;71(5):229-237.
Crossref - Sommer F, Nookaew I, Sommer N, Fogelstrand P, Bäckhed F. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol. 2015;16(1):62.
Crossref - Muhlemann B, Jones TC, Damgaard PdeB, et al. Ancient hepatitis B viruses from the Bronze Age to the Medieval period. Nature. 2018;557(7705):418-423.
Crossref - Quintana-Murci L. Human Immunology through the Lens of Evolutionary Genetics. Cell. 2019;177(1):184-199.
Crossref - Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat Rev Genet. 2014;15(6):379-393.
Crossref - Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923-1994.
Crossref - Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733.
Crossref - Sorensen TIA, Nielsen GG, Andersen PK, Teasdale TW. Genetic and environmental influences on premature death in adult adoptees. N Engl J Med. 1988;318(12):727-732.
Crossref - Chapman SJ, Hill AVS. Human genetic susceptibility to infectious disease. Nat Rev Genet. 2012;13(3):175-188.
Crossref - Casanova JL, Abel L. Lethal Infectious Diseases as Inborn Errors of Immunity: Toward a Synthesis of the Germ and Genetic Theories. Annu Rev Pathol. 2021;16:23-50.
Crossref - Fu ZD, Selwyn FP, Cui JY, Klaassen CD. RNA-Seq Profiling of Intestinal Expression of Xenobiotic Processing Genes in Germ-Free Mice. Drug Metab Dispos. 2017;45(12):1225-1238.
Crossref - Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517-526.
Crossref - Larsson E, Tremaroli V, Lee YS, et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61(8):1124-1131.
Crossref - Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A. 2016;113(1):E7-15.
Crossref - Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655-662.
Crossref - Hasler R, Sheibani-Tezerji R, Sinha A, et al. Uncoupling of mucosal gene regulation, mRNA splicing and adherent microbiota signatures in inflammatory bowel disease. Gut. 2017;66(12):2087-2097.
Crossref - Bennet SMP, Sundin J, Magnusson MK, et al. Altered intestinal antibacterial gene expression response profile in irritable bowel syndrome is linked to bacterial composition and immune activation. Neurogastroenterol Motil. 2018;30(12):e13468.
Crossref - Richards AL, Burns MB, Alazizi A, et al. Genetic and transcriptional analysis of human host response to healthy gut microbiota. mSystems. 2016;1(4):e00067.
Crossref - Kunstner A, Schilf P, Busch H, Ibrahim SM, Hirose M. Changes of Gut Microbiota by Natural mtDNA Variant Differences Augment Susceptibility to Metabolic Disease and Ageing. Int J Mol Sci. 2022;23(3):1056.
Crossref - Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227.
Crossref - Attaye I, Warmbrunn MV, Boot ANAF, et al. A Systematic Review and Meta-analysis of Dietary Interventions Modulating Gut Microbiota and Cardiometabolic Diseases-Striving for New Standards in Microbiome Studies. Gastroenterology. 2022;162(7):1911-1932.
Crossref - Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59-65.
Crossref - Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560-564.
Crossref - Kurilshikov A, Medina-Gomez C, Bacigalupe R, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53(2):156-165.
Crossref - Lopera-Maya EA, Kurilshikov A, van der Graaf A, et al. Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat Genet. 2022;54(2):143-151.
Crossref - Gacesa R, Kurilshikov A, Vich Vila A, et al. The Dutch Microbiome Project defines factors that shape the healthy gut microbiome. bioRxiv. 2020:1-33.
Crossref - Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210-215.
Crossref - Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565-569.
Crossref - Goodrich JK, Davenport ER, Beaumont M, et al. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe. 2016;19(5):731-43.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.