ISSN: 0973-7510
E-ISSN: 2581-690X
Phytoestrogen-rich diet alters the composition of gut microbiota by enhancing the growth of beneficial bacteria and decreasing the microbial load of pathogenic organisms. Drosophila is an invertebrate model system used for research studies, as it shares 70% genetic homology with humans. The present study aimed to analyse microbiological profile of phytoestrogen rich supplement and its impact on gut-microbiome composition in Drosophila melanogaster. The phytoestrogen rich supplement was mixed with formula 424 plain and flies were exposed to it. Gut of flies was dissected and cell suspension was prepared. Bacterial colonies were developed by streaking method. Gram staining was performed to differentiate the bacterial cells and further gut microbiome composition (Acetobacteraceae and Lactobacillales taxa) was analysed by 16S rRNA sequencing. The microbiological analysis was carried out to ascertain the microbial load of the developed product for consumption. The total bacterial count and coliform counts of the phytoestrogen rich supplement were <10 CFU/g. Also, the developed supplement exhibited minimal yeast and mold growth (<1 CFU/g). Gram staining showed gram positive (Bacilli and cocci). 16S rRNA sequencing showed significance with mild variation in similarity. It confirmed the presence of Bacillus paramycoids. The developed supplement has showed improved gut microbiome composition in the Drosophila. In future, studies can be extended to humans to analyse the efficacy of the supplement in the gut microbiome composition.
Drosophila melanogaster, Phytoestrogen Supplement, Gut Microbiome
Phytoestrogens are plant-derived substances with a structural similarity to 17b-estradiol. Among the categorised phytoestrogens, four phenolic compounds are prominent: isoflavones, stilbene, coumestan, and lignan.1-3 Isoflavones, present in soybeans and legumes such as red clover, include genistein, daidzein, glycitein, formononetin, and biochanin. Achieving health effects from isoflavones generally requires a daily dosage of 40-70 mg, averaging at 50 mg.1,4 Asian populations typically consume 15-50 mg daily, whereas Western countries have an average intake of about 2 mg per day.1 Phytoestrogens in the diet are initially inactive and bound to glycidic molecules. Once ingested, they undergo partial hydrolysis in the small intestine and further breakdown by colonic bacteria, forming primary metabolites called aglycones. These aglycones can be further transformed into secondary metabolites and bacterial end-products. Both primary and secondary metabolites undergo conjugation in the intestines and liver prior to entering the bloodstream for circulation. They are eventually excreted in urine or bile.5,6 Factors such as dose, bioavailability, sex differences, intestinal transit duration, and intestinal gut microbiota composition impact their uptake, levels in the bloodstream, and elimination through urine.5,6 The gut microbiota can modify the activity and enhance the bioavailability of phytoestrogens through enzymatic reactions.5,7 Diets enriched with phytoestrogens impact the composition of gut microbiota. The bioavailability of phytoestrogens is significantly influenced by gut bacteria diversity, ranging from 13% to 35% in the general population and reaching up to 40% in Asians and vegetarians.4 In this study, phytoestrogen rich supplement was formulated from underutilised ingredients such flaxseed, fenugreek seed, and nicker nuts. The review studies examined the attributes of fenugreek seeds including their potential as an anti-diabetic, antibacterial, gastric stimulant, for anorexia, anti-diabetic agent, galactagogue, hepatoprotective effect and anti-cancer.8-10 Review studies conducted ‘hitherto’ concluded that flaxseeds have demonstrated favourable impacts on lipid profiles, inflammatory cytokines, and anthropometric indices in individual with dyslipidemia-related conditions. Whole flaxseed and lignans are key contributors to reducing blood lipid levels. Notably, this effect was more pronounced in patients with poor sugar level control.11 Sharma et al. in their study investigated the effects of Nicker nuts seed extracts on rats, revealing hypoglycemic, antihyperglycemic and hypolipidemic activities. Additionally, it exhibited anti-hypercholesterolemic and anti hypertriglyceridemic effects.12-14 The Drosophila genome exhibits around 70% similarities to the human genome, contains fewer redundant genes, and roughly 75% of genes associated with human illnesses have counterparts in flies.15 Flies are a reliable model to include in studies of the gut microbiome because of their simple microbiome and simplicity of growing gnotobiotic animals.16-18 Microbial contamination can take place in any step of product development. Phytoestrogen rich supplement have the potential to affect gut microbiome composition, our hypothesis posited that phytoestrogen rich supplement would induce notable changes in the gut microbiome of Drosophila melanogaster. Hence, the current study aimed to analyse the microbiological profile of developed phytoestrogen rich supplement and to check the effectiveness of phytoestrogen rich supplement on the gut microbiome of Drosophila melanogaster. Thus, the study aimed to analyse microbiological profile of phytoestrogen rich supplement and its impact on gut-microbiome composition in Drosophila melanogaster.
Experimental study design was implemented for this study. The period of the study was for 6 months. Phytoestrogen rich supplement was prepared at the Department of Clinical Nutrition – Food Science Lab. Further experimental work was carried out at the Department of Biomedical Sciences – Drosophila Lab. Institutional ethics committee clearance (CSP/21/AUG/98/465) was obtained from Sri Ramachandra Institute of Higher Education and Research.
Phytoestrogen rich supplement were prepared using seeds and pulses. The ingredients such as flax seeds, fenugreek seeds and nicker nuts were chosen in the ratio of 20:10:5. They were procured with the help of a botanical expert to ensure accurate identification of specific plant species. Dhals and other ingredients such a red gram dal (Cajanus cajan), Bengal gram dal (Cicer arietinum), black gram dal (Phaseolus mungo), green gram dal (Vigna radiate), black pepper (Piper nigrum), sesame seeds (Sesamum indicum) and salt were obtained from the nearby department store in Chennai. All the dry ingredients including dhals, flaxseeds, fenugreek seeds, nickernuts and black pepper were roasted at 120°C for 10-15 minutes and subsequently ground into a fine powder using an electronic blender. The powdered supplement was stored in sealed laminated aluminium packets.
Drosophila (Canton – S) was bred in normal culture conditions in corn meal agar at 21°C in a BOD incubator with normal light and dark cycles. Formula 424 plain (Instant food) was prepared by weighing 4 g portions of instant food per test experiment. The beaker, glass vials, glass rod, instant food, the phytoestrogen rich supplement was mixed with formula 424 plain (instant food) was sterilised under UV radiation for 20 minutes. 20 g of phytoestrogen supplement was measured, and diluted in 20 µl of distilled water, with 5 ml of the sample then centrifuged for 10 minutes at 1000 rpm. In a beaker, instant food was blended with distilled water, and 5 glass vials marked as negative control, T1, T2, T3, T4 were prepared (Figure 1). Each vial, sealed with cotton, was placed in an incubator for 18 hours.
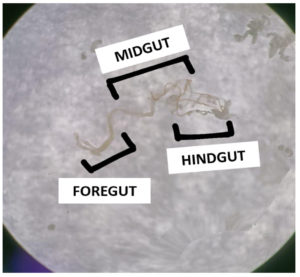
Larval transfer was done after an 18-hour incubation period, glass vials were retrieved. Drosophila larvae scooped from the instant food, were transferred to petri dish with phosphate buffer saline. Each glass vial received 25 larvae in the 2nd and 3rd instar stages. Prior to introducing the larvae, 20 µl of distilled water was added along the sides of each glass vial using a micro-pipette. The glass vials, containing the larvae, were returned to the incubator for 48-hours. Followed by larval transfer, gut of Drosophila was dissected by using a pair of forceps; the abdomen of the fly was firmly held, while simultaneously gripping the thorax with another pair. This facilitated the separation of the thorax from the abdomen, exposing the visible gut with the foregut/crop likely connected to the thorax. The gut was carefully extracted from the thorax, with a focus on unfolding it by grabbing the crop and pulling it slightly anteriorly. Employing forceps, the posterior end of the abdomen and the edge of the anterior open cuticle were seized, gently breaking the cuticle and gradually pulling the posterior end until the entire gut emerged from the abdominal cavity. Subsequently, the foregut, malpighian tubules, hindgut, and ovaries were excised, leaving only the bare midgut (Figure 2), which was then transferred to an Eppendorf tube containing Luria Broth and incubated for 48 hours.
Figure 2. Showing the dissected gut of larvae (Foregut, Midgut and Hindgut) where midgut was taken for further analysis
Bacterial colonies were developed by streaking method. Nutrient agars, Sabouraud Dextrose (SD) Agar, Lactobacillus agar were prepared prior to the streaking process. The suspension obtained from the dissected gut was used to inoculate Petri plates filled with nutrient agar, designated as Negative control (NC), T1, T2, T3, and T4. The plates were placed in an incubator at 37°C for the duration of 48 hours. After incubation, 5 test tubes were designated and labeled as Positive control (PC), T1, T2, T3, and T4. Each test tube was with 5 ml of Luria Broth. Subsequently, the individual colonies from the nutrient agar were transferred into their designated test tubes with caution. Each test tube was subjected to further incubation at 37°C for additional 48 hours. Following this, quadrant streaking was performed on nutrient agar plates, followed by transferring individual colonies into Luria Broth. This process was repeated after 48 hours. Petri plates with SD Agar, and quadrant streaking was conducted. The same procedure was applied to Lactobacillus agar.
Gram staining was performed to differentiate bacterial cells. Using a sterile inoculating loop, a single colony was moved from the agar plate to a sterile glass slide. The slide underwent a sequence of staining steps. Crystal violet was applied for 60 seconds, followed by a water wash. Iodine application for 60 seconds and subsequent washing were performed. A quick treatment with 95% ethyl alcohol for 5 to 10 seconds was carried out, followed by another water wash. Finally, safranin was applied for 45 seconds, and the slide was washed with water, concluding the staining procedure. The glass slides, containing bacterial samples from (NC), T1, T2, T3, and T4 in both nutrient agar and SD agar were scrutinized using a microscope to detect the presence of bacteria.
16S rRNA sequencing was done to analyse the gut microbiome composition. The MagNA Pure instrument and its accompanying software were utilised for DNA extraction, following the protocol outlines.19,20 A volume of 100 µL supernatant underwent processing and then the sample was eluted in a 100 µL of elution buffer. Later, PCR amplification was carried out to target the V1-2 region of the bacterial 16S rRNA gene, utilizing F27 (AGAGTTTGATCCTGGCTCAG) and R357 (CTGCTGCCTYCCGTA) primers, along with the FastStart High Fidelity PCR System and dNTPack. Paired-end reads were merged employing the fast-join tool, and primer removal was executed with Cutadapt 1.6. Reference-based chimera detection was carried out using USEARCH 6.1 and sequence classification was achieved through blasting in the NCBI database. Clustering was accomplished by UCLUST, applying 97% sequence similarly threshold, and a phylogenetic tree was generated using FastTree. For alpha diversity analyses, PD whole tree and Shannon indices were calculated using QIIME 1.9, while beta diversity was assessed through the Calypso Biomarker Discovery pipeline with QIIME 1.9 outputs. In order to maintain the quality of the data, Operational Taxonomic Units (OTUs) underwent filtration based on their abundance in at least two samples, with a collective minimum abundance threshold of 10.
Nutrient analysis of phytoestrogen rich powder
Nutrient composition of phytoestrogen rich powder is represented in Table 1.
Table (1):
Nutrient Composition of Phytoestrogen rich supplement
Parameters |
Results |
|---|---|
Energy (kcal) |
422.06 |
Carbohydrate (g) |
57.2 |
Protein (g) |
22.5 |
Fat (g) |
10.2 |
Fiber(g) |
4.4 |
Moisture (g) |
2.98 |
Omega – 3 fatty acid (mg) |
217 |
Vitamin A (IU) |
386 |
Vitamin E (mg) |
2.4 |
Polyphenols (mg) |
123 |
Flavonoids (mg) |
56 |
Iron (mg) |
5.9 |
Calcium (mg) |
0.36 |
Selenium (mg) |
9.5 |
Nutrient composition of developed supplement from Table 1 reveals that the supplement was dense in energy and boasted notable levels of omega 3 fatty acid, calcium, vitamin A, polyphenols and flavonoids. It also contained a moderate quantity of selenium, protein, and carbohydrates, along with fiber and iron. A minimal amount of moisture and vitamin E was detected during the nutrient analysis.
Microbiological Profile Examination
Microbial analysis of developed supplements was provided in Table 2.
Table (2):
Microbiological Profile Examination of Phytoestrogen rich supplement
Pathogens |
Phytoestrogen rich supplement (CFU/g) |
|---|---|
Yeast and Mold counts |
|
Total bacterial count |
|
Coliform text |
The microbiological examination of the phytoestrogen rich supplement is given in the Table 2. The microbiological assessment was conducted to ensure the safety of the supplement for consumption. The total bacterial count and coliform counts of the phytoestrogen rich supplement were <10 CFU/g and also the developed supplement exhibited minimal yeast and mold growth (<1 CFU/g). These results indicate that the developed supplement was safe for consumption.
Gram staining
The result of the Gram staining is presented in Table 3.
Table (3):
Gram Staining
| Culture Media | Samples | Interpretation | Organism |
|---|---|---|---|
| Nutrient Agar | NC | Gram-positive | Bacilli |
| T1 | Gram-positive | Bacilli | |
| T2 | Gram-positive | Bacilli | |
| T3 | Gram-positive | Bacilli | |
| T4 | Gram-positive | Bacilli | |
| Sabouraud Dextrose Agar | NC | Gram-positive | Cocci |
| T1 | Gram-positive | Bacilli | |
| T2 | Gram-positive | Cocci | |
| T3 | Gram-positive | Bacilli | |
| T4 | Gram-positive | Bacilli |
*NC- Negative control
Gram straining results revealed that all the samples are found to be gram-positive and organisms found were bacilli and cocci.
16S rRNA sequencing
The results of the 16S rRNA sequencing depict the bacterial strain identified within the samples (Table 4).
Table (4):
Bacterial strain present in the samples
Samples |
Bacterial Strains |
Similarity (%) |
|---|---|---|
NC |
Bacillus paramycoids |
99.09 |
T1 |
Bacillus paramycoids |
99.62 |
T2 |
Bacillus paramycoids |
99.73 |
T3 |
Bacillus paramycoids |
99.86 |
T4 |
Bacillus paramycoids |
99.73 |
NC- Negative control
16S rRNA sequencing result were found to be significant. It reveals that bacterial species present in the samples were Bacillus paramycoids. It showed 99.09% similarity for negative control, 99.62% similarity for T1, 99.73% similarity for T2, 99.86% similarity for T3, 99.73% similarity for T4.
Developed phytoestrogen rich supplement has impacted the gut microbiome composition of Drosophila. The supplement is rich in flavonoids and polyphenols. Flavonoids are sub-class of polyphenols. Flavonoids serve as metabolic substrates for beneficial bacteria such as Bifidobacterium and Lactobacillus species, aiding their growth and proliferation. This helps maintain a stable and beneficial gut community. Flavonoids and their metabolites possess the potential to inhibit the progression of harmful pathogens while endorsing the growth of useful bacteria such as Bifidobacterium and Lactobacillus. In our study, exposure of Drosophila melanogaster to the phytoestrogen rich supplement resulted in the presence of Bacillus paramycoids in the gut of the flies. This modulation could lead to improved gut health through reductions in endotoxin production, enhanced conversion of primary bile acids to secondary forms, maintenance of immune homeostasis in the gut, and promotion of nutrient absorption.21,22 In this study, the supplement was formulated using ingredients abundant in phytoestrogens such as flaxseed, fenugreek seed and nicker nuts which were underutilized thus far.
The phytoconstituent found in the phytoestrogen rich supplement was acknowledged for its capability to manage irregular menstrual cycles, increase insulin secretion and lower blood glucose levels. A prior investigation also noted significant level of phytochemicals such as alkaloids, glycosides, reducing sugars, flavonoids, as well as substantial amounts of carbohydrates and proteins in nicker nuts.23 Phytoestrogen rich supplement contained a significant amount omega-3 fatty acids and fiber, which were believed to reduce blood cholesterol and triglyceride levels and also enhances high-density lipoprotein levels.24 Previous studies have demonstrated that flaxseed is a rich source of alpha-linolenic acid, lignan, and fiber.25 Analysis of fenugreek’s nutritional composition unveiled the presence of various phytochemicals, including flavonoids, phytoestrogens, alkaloids, tannins, and saponins.26,27 Phytoestrogens, resembling natural 17β-estradiol and binding to estrogen receptors (alpha and beta), regulate cellular functions and gene expression, potentially influencing estrogen-related processes.28 Likewise, the phytoestrogen rich supplement was discovered to be abundant in calories and protein. Analysis revealed that the developed supplement was rich in vitamin A, iron and selenium, suggesting its antioxidant properties. Additionally, its notable iron content suggests potential use in treating anemia and menstrual irregularities.24 Previous studies have noted black sesame seeds as a valuable source of certain minerals, notably calcium, phosphorus, and iron.29
Several studies have reported that incorporation of flaxseed oil into the diet resulted in the reduction in the number of pathogenic bacteria Spirochaetes while increasing the presence of phylum Actinobacteria as well as the genera Blautia and Bifidobacterium in colonic digesta. It also improves the intestinal function, alters the gut microbiota and mucosal fatty acid profile. Additionally, it has been observed to enhance intestinal functionality, induce changes in gut microbiota composition, and modify the fatty acid profile of the mucosal layer.30 Another study evidenced that flaxseed fibers positively influenced the gut bacteria and metabolism in mice. They boosted beneficial bacteria like Bifidobacterium and Akkermansia while reducing harmful ones such as Prevotella and Allobaculum. Additionally, flaxseed fibers restored levels of beneficial short-chain fatty acids like butyrate and lactate. These changes led to improved metabolic outcomes, protecting mice from diet-induced obesity and enhancing overall metabolic health.31 Black sesame seeds, rich in fiber, antioxidants, minerals such as iron and calcium, could contribute to digestive health benefits.32 Fenugreek seed supplementation found to have therapeutic effects. Studies indicated that fenugreek supplementation can effectively modify the composition of intestinal microbiota, consequently enhancing host physiology.33 A prior investigation employing metabolomic and microbiome analyses revealed that microbiota originating from the Lachnospiraceae and Runinococcacea families within the Firmicutes phylum contributes to heightened coprostanol production in healthy individuals. This, in turn, leads to decreased blood cholesterol levels by facilitating increased excretion of fecal cholesterol.34 Sesame seeds was also used in the preparation of phytoestrogen rich supplement. Studies have shown that sesame seeds found to impacts the gut microbiota. High-fat diets disrupt gut microbiota, as evidenced by increased levels of harmful bacteria like Helicobacter, Faecalibaculum, Lachnoclostridium, Allobaculum, Odoribacter, and Mucispirillum. However, treatment with sesamolin reverses this trend, elevating the levels of beneficial bacteria such as Bacteroides, Parabacteroides, Alistepes, Prevotellaceae_UCG_001, and Alloprevotella.35
From the current study, it is observed that developed phytoestrogen rich supplement found to influence the gut microbiome composition in Drosophila. From the literatures, the underutilised ingredients used for the preparation of the supplement were found to have beneficial effects in metabolic health. Hence, the supplement can be further used to analyze gut microbiome composition to check the efficacy of supplements in humans as well as can be used to analyse the efficacy of supplements where conditions involve dysbiosis of the gut microbiota.
Strengths
Drosophila served as a model for studying the gut microbiome composition owing to genetic homology with humans. It’s rapid reproduction rate and ease of maintenance further facilitated its use in research. Additionally, maintaining Drosophila was cost effective compared to other model organisms.
Limitations
The dosage of supplement administered to Drosophila cannot be equivalent to human consumption levels. Drosophila studies mainly focuses on physiological and genetic responses, overlooking behavioral changes, especially those pertinent to physiological changes in the gut and human health.
Future recommendations
Future studies could extend its application to human subjects to assess the efficacy of the supplement in improving gut microbiome composition. This could be particularly beneficial for women with polycystic ovary syndrome (PCOS) as it may aid in regulating menstrual irregularities, metabolic activities, improving intestinal function and reducing blood cholesterol levels.
Phytoestrogens are polyphenolic compounds that mimic the structure and function of estrogen. Phytoestrogen rich supplement contains flavonoids and polyphenols which are found to have an impact on the gut microbiota. The developed phytoestrogen rich supplement has exhibited a positive potential impact on gut microbiome composition in Drosophila melanogaster. These findings highlight the impact of phytoestrogen-rich supplement in enhancing gut microbiome composition and suggest their possible use in dietary interventions for optimizing the gut microbiota balance.
ACKNOWLEDGMENTS
The authors would like to express their heartfelt appreciation for the funding provided through the Chancellor’s Summer Research Fellowship by the institution. The authors
also extend their sincere gratitude to the Department of Biomedical Sciences for generously providing access to the Drosophila lab.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by the Sri Ramachandra Institute of Higher Education and Research under Chancellor’s Summer research fellowship from June 2021 to December 2021.
DATA AVAILABILITY
All data generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Sri Ramachandra Institute of Higher Education and Research, Chennai, India, with reference number CSP/21/AUG/98/465.
- Desmawati D, Sulastri D. A Phytoestrogens and their Health Effect. Open Access Maced J Med Sci. 2019;7(3):495-499.
Crossref - Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65(8):995-1016.
Crossref - Sirotkin AV, Harrath AH. Phytoestrogens and their effects. Eur J Pharmacol. 2014;741:230-236.
Crossref - Eisenbrand G. Isoflavones as phytoestrogens in food supplements and dietary foods for special medical purposes. Mol Nutr Food Res. 2007;51(10):1305-1312.
Crossref - Viggiani MT, Polimeno L, Di Leo A, Barone M. Phytoestrogens: Dietary Intake, Bioavailability, and Protective Mechanisms against Colorectal Neoproliferative Lesions. Nutrients. 2019;11(8):1709.
Crossref - Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr. 2003;133(3):956S-964S.
Crossref - Farhat EK, Sher EK, Dzidic-Krivic A, Banjari I, Sher F. Functional biotransformation of phytoestrogens by gut microbiota with impact on cancer treatment. J Nutr Biochem. 2023;118:109368.
Crossref - Meghwal M, Goswami TK. A review on the functional properties, nutritional content, medicinal utilization and potential application of fenugreek. J Food Process Technol. 2012;3(9):1-10.
Crossref - Nagulapalli Venkata KC, Swaroop A, Bagchi D, Bishayee A. A small plant with big benefits: Fenugreek (Trigonella Foenum graecum Linn.) for disease prevention and health promotion. Mol Nutr Food Res. 2017;61(6).
Crossref - Knott E.J, Richard AJ, Mynatt RL, Ribnicky D, Stephens JM, Bruce-Keller A. Fenugreek supplementation during high-fat feeding improves specific markers of metabolic health. Sci Rep. 2017;7(1):12770.
Crossref - Yang C, Xia H, Wan M, et al. Comparisons of the effects of different flaxseed products consumption on lipid profiles, inflammatory cytokines and anthropometric indices in patients with dyslipidemia related diseases: systematic review and a dose-response meta-analysis of randomized controlled trials. Nutr Metab. 2021;18(1):91.
Crossref - Sharma SR, Dwivedi SK, Swarup D. Hypoglycaemic, antihyperglycaemic and hypolipidemic activities of Caesalpinia bonducella seeds in rats. J Ethnopharmacol. 1997;58(1):39-44.
Crossref - Manikandaselvi S, Vadivel V, Brindha P. Caesalpinia bonducella L.: A nutraceutical plant. J Chem Res. 2015;7(12):137-142.
- Nazeerullah K, Sunil K, Pal SR, Neelam D. A Pharmacognostic and Pharmacological Overview on Caesalpinia bonducella. Res J Pharm Biol Chem Sci. 2012;3(1):440-496.
- Mirzoyan Z, Sollazzo M, Allocca M, Valenza AM, Grifoni D, Bellosta P. Drosophila melanogaster: A Model Organism to Study Cancer. Front Genet. 2019;10:51.
Crossref - Ludington WB, Ja WW. Drosophila as a model for the gut microbiome. PLoS Pathog. 2020;16(4):e1008398.
Crossref - Pais IS, Valente RS, Sporniak M, Teixeira L. Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria. PLoS Biol. 2018;16(7):e2005710.
Crossref - Martino ME, Joncour P, Leenay R, et al. Bacterial Adaptation to the Host’s Diet Is a Key Evolutionary Force Shaping Drosophila-Lactobacillus Symbiosis. Cell Host Microbe. 2018;24(1):109-119.e6.
Crossref - Mazzucco R, Nolte V, Vijayan T, Schlotterer C. Long-Term Dynamics Among Wolbachia Strains During Thermal Adaptation of Their Drosophila melanogaster Hosts. Front Genet. 2020;11:482.
Crossref - Wong ACN, Chaston JM, Douglas AE. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 2013;7(10):1922-1932.
Crossref - Wang H, Zhao T, Liu Z, et al. The neuromodulatory effects of flavonoids and gut Microbiota through the gut-brain axis. Front Cell Infect Microbiol. 2023;13:1197646.
Crossref - Pei R, Liu X, Bolling B. Flavonoids and gut health. Curr Opin Biotechnol. 2020;61:153-159.
Crossref - Ajayi I, Ojelere O. Chemical Composition of Ten Medicinal Plant Seeds from South-west Nigeria. Adv Life Sci Technol. 2013;10:25-32.
- Kalidoss G, Velraja S, Narayanan P. Development and Formulation of Phytoestrogen-rich Supplement for Women with Polycystic Ovary Syndrome. Int J Infertil Fetal Med. 2021;12(2):31-36.
Crossref - Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients. 2019;11(5):1171.
Crossref - Mahmood N, Mahmood M, Yahya IK. Nutrient and Phytochemical of Fenugreek (Trigonella Foenum graecum) Seeds. International Journal of Sciences: Basic and Applied Research. 2017;36(3):203-213.
Crossref - Visuvanathan T, Than LTL, Stanslas J, Chew SY, Vellasamy S. Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants. 2022;11(11):1450.
Crossref - Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor a (ERa) and ERb in human cells. Toxicol Sci. 2004;80(1):14-25.
Crossref - Alyemeni M, Basahy AY, Sher H. Physico-chemical analysis and mineral composition of some sesame seeds (Sesamum indicum L.) grown in the Gizan area of Saudi Arabia. J Med Plants Res. 2011;5(2):270-274.
- Che L, Zhou Q, Liu Y, et al. Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct. 2019;10(12):8149-8160.
Crossref - Wei P, Zhao F, Wang Z, et al. Sesame (Sesamum indicum L.): A Comprehensive Review of Nutritional Value, Phytochemical Composition, Health Benefits, Development of Food, and Industrial Applications. Nutrients. 2022;14(19):4079.
Crossref - Arora T, Rudenko O, Egerod KL, et al. Microbial fermentation of flaxseed fibers modulates the transcriptome of GPR41-expressing enteroendocrine cells and protects mice against diet-induced obesity. Am J Physiol Endocrinol Metab. 2019;316(3):E453-E463.
Crossref - Bruce-Keller AJ, Richard AJ, Fernandez-Kim S-O, et al. Fenugreek Counters the Effects of High Fat Diet on Gut Microbiota in Mice: Links to Metabolic Benefit. Sci. Rep. 2020;10(1):1245.
Crossref - Jones KA, Richard AJ, Salbaum JM, et al. Cross-Omics Analysis of Fenugreek Supplementation Reveals Beneficial Effects Are Caused by Gut Microbiome Changes Not Mammalian Host Physiology. Int J Mol Sci. 2022;23(7):3654.
Crossref - Yu J, Sun H, Yang Y, Yan Y. Sesamolin Alleviates Nonalcoholic Fatty Liver Disease through Modulating Gut Microbiota and Metabolites in High-Fat and High-Fructose Diet-Fed Mice. Int J Mol Sci. 2022;23(22):13853.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.