Severe Acute Respiratory Syndrome (SARS-CoV-2) causes the coronavirus disease (COVID-19), which is characterised by severe respiratory syndrome and other complications. It is a serious threat to global public health if proper vaccination is not followed. The efficient COVID-19 management requires vaccination along with other precautionary measures. Public transmission of COVID-19 seems to have decreased and immune response to viral infections has improved by vaccination. The present review discusses in detail about the current situation of the COVID-19, based on the latest reports about approved vaccine types and their efficacy, vaccination status, and various SARS-CoV-2 variants. This review also includes insights into the post-COVID complications in recovered patients. Besides, some of the ill-effects of drugs in inducing other diseases in COVID-19 recovered patients, are also discussed in this review article. This study will help the researchers to prepare strategies for further research on vaccine production and prevent the occurrence of the disease in future.
COVID-19, SARS-CoV-2, Vaccines, Adverse Effects, Efficacy
A novel coronavirus disease brought on by the SARS-CoV-2 virus had first emerged in China in the month of December, 2019. The World Health Organisation (WHO) declared the coronavirus pneumonia outbreak as a “public health emergency of international concern” on January 30, 2020. On 11th of February, 2020, the disease was termed as COVID-19 (the abbreviation of “Coronavirus Disease 2019”). The disease had a devastating effect on the entire world due to the quick spread and difficulty in the detection of silent infections and mild cases. Even if the virus can be totally eradicated from the community, the transfer from the host to the human remains unclear due to the population’s general susceptibility.1,2 Several COVID-19 complicacies include respiratory failure, myocardial injury, stroke, thromboembolic complications, neurological complications (movement disorders and ataxia), inflammatory complications (Guillain-Barre syndrome and elevated inflammatory markers), and secondary infections.3 By December 31, 2021, there had been 5.94 million documented fatalities attributed to COVID-19, with a mortality rate of 9.22 per 100,000 people worldwide.4 Around 242 vaccine candidates and a total of 821 clinical trials are underway in which around 80 countries are participating in vaccine trials. About 50 vaccines have been approved so far and 66 vaccine candidates undergoing phase I trials, 72 in phase II trials, and 92 in phase III trials, as per the information provided in a publicly accessible COVID-19 vaccine tracker website (Data was last updated on 2nd December 2022).5 Some prominent vaccines like Pfizer-BioNTech BNT162b2, Moderna’s mRNA-1273, Oxford-AstraZeneca, Johnson & Johnson, Sinopharm-Beijing, Sinovac, Sputnik V, Novavax, and Covaxin vaccines are some of the vaccines that were approved for use.6
Mechanism of action
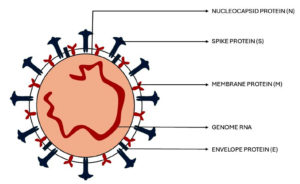
SARS-CoV-2 virus consists of several structural and non-structural proteins. The structural proteins are named as Spike (S) protein, envelope (E) protein, membrane/matrix (M) protein, and nucleocapsid (N) protein (Figure).7 Infection results from the binding of S proteins to certain receptors. The S protein is the most crucial target antigen for the development of vaccines against this virus since it can activate the T-cell immune response. Besides, the M and N proteins stimulate the body to create a powerful cellular immunological response. Upon its entry, the virus binds to a receptor called angiotensin-converting enzyme 2 (ACE2). Every organ expresses ACE2, but the lungs, brain, and gut express more of it than other organs.8 The virus spreads widely after this binding, and it can even harm tissues beyond the respiratory system. In various circumstances, the distribution of ACE2 causes alterations in the symptoms.1,2,9
The S protein has two subunits such as S1 and S2. The S1 being the receptor-binding domain of the virus, attaches to the host making it a target for pharmaceutical applications. To initiate the infection inside the body, the S2 component encourages viral fusion with cells. The main goal of the vaccine’s design is to produce antibodies that would attach to the S protein’s epitope and potentially neutralise and stop viral intercellular propagation.10
Types of vaccines
The following Table 1 includes different categories of vaccines currently in development. Each type of vaccine has its own characteristic features, mechanism of action, advantages and disadvantages which have been discussed here.
Table (1):
Various types of vaccines with their mechanism of action, advantages, and disadvantages
Types of Vaccines |
Mechanisms |
Advantages |
Disadvantages |
Manufacturers and Products |
|---|---|---|---|---|
DNA VACCINES [11,12] |
It leads to the formation of MHC class II-peptide complex. This complex is the result of the fragments formed upon the degradation of the virus by the vaccine. This complex will trigger the cellular immune response in the body. |
It triggers both the cellular and antibody mediated immunity. The virus will be neutralized before it enters the host cell. |
may cause cell death at the site of injection, pain to patient when the vaccine is administered. |
Inovio Pharmaceuticals, Inc.: INO-4800 |
mRNA vaccines [13,14] |
mRNA instructs our own cell to produce proteins, resulting in an immune response. |
It allows fast modifications to the transgene to target specific variants. The mRNA is safer as it does not contain any mutagenic or infectious part. |
mRNA is unstable and easily degraded. Can trigger unnecessary immune response and has low immunization potential. |
BioNTech and Pfizer: BNT16b2 (Comirnaty®) |
Non-replicating viral vector vaccines [15,16] |
it is incompletely understood. But the hypotheses says that the adenoviral vector vaccine mechanism is dependent on the adenoviral vector used in the vaccine. |
With the help of genetic engineering the vaccines can be optimized to the new variants. It is stable in nature making it safer to use. |
It is very complex to manufacture these vaccines. It has a limited capacity for the transgene. |
Panacea Biotec: Sputnik V |
Inactivated vaccines [17,18] |
As the inactivated virus contains all the structural and non-structural proteins without the virulence part it will activate the B cells to produce antibodies against these proteins. |
Can be used in the regions where there are limited ultra-cold storage. |
the mechanism of this vaccines is limited by the original antigenic sin(OAS) |
Bharat Biotech: COVAXIN® |
Live attenuated vaccines [19,20] |
This vaccine will activate the immune system in the nasal issues by which it provides protection against the virus by clearing the infections and preventing them. |
immunogenic due to the lack of non-structural protein 1 which inhibits the immune response. Safe for all age groups. |
It can lead to secondary mutations which may reverse the virulence and cause the disease. Shows some adverse effects after the vaccinations |
Beijing Wantai Biological Pharmacy Enterprise: dNS1-RBD |
Subunit vaccines [21-23] |
The vaccines targeting the RBD of the spike protein will generate a neutralizing antibody response. This response will provide protection against the original strain along with its variants |
safe, easy manufacturing and precise immune targeting. Induce both humoral and cell mediated immunological responses. |
Reduced immunogenicity. It’s difficult to isolate the specific antigens to invoke the necessary immune response. |
The Vektor State Research Center of Virology and Biotechnology in Russia: EpiVacCorona |
Vaccination status
A total of 5,33,574 (1.18%) deaths were reported out of the total 4,50,35,573
COVID patients, and 4,45,01,431 patients were discharged, with a recovery rate of 98.81% (Statistical data obtained on April 14, 2024, from the website of Government of India). A total of 93,58,79,465 people have been tested positive for COVID-19 till date. About 2,20,68,93,569 vaccine doses were reported to have been administered as of April 14, 2024.24 Nearly 67% of total population worldwide, have been administered with the primary dose of COVID-19 vaccine and 32% of them with at least one booster dose given. Globally, 13.59 billion doses have been administered.6 According to The New York Times, as of March 13, 2023, 72.3% of the world’s population is immunised, with 67% of those vaccinations being complete. Out of 100 people, 35 had taken an additional dose.25
On 22 July 2022, the first COVID-19 vaccine product was introduced.6 According to statistics recorded on November 30, 2022, the vaccines being utilised in various nations include as given in Table 2.25
Table (2):
Different COVID-19 vaccines used in various countries (as of November 30, 2022)
Vaccine |
No. of Countries used |
|---|---|
Oxford-AstraZeneca |
185 |
Pfizer-BioNTech |
165 |
Moderna |
114 |
Johnson & Johnson |
103 |
Sinopharm-Beijing |
72 |
Sinovac |
41 |
Gamaleya (Sputnik V) |
35 |
Novavax |
33 |
Bharat Biotech (Covaxin) |
31 |
CanSino |
27 |
Sputnik Light |
06 |
Abdala |
04 |
Sinopharm-Wuhan |
03 |
Soberana |
02 |
Sanofi/ GSK |
02 |
QazVac |
02 |
Vector Institute (EpiVacCorona) |
02 |
KoviVac/Chumakov |
01 |
Medicago |
01 |
IMBCAMS |
01 |
KCONVAC |
01 |
Soberana Plus |
01 |
Valneva |
01 |
Corbevax |
01 |
COVIran Barekat |
01 |
Medigen |
01 |
Turkovac |
01 |
ZF2001 |
01 |
COVID-19 variants
Since its discovery, the SARS-CoV-2 virus transformed into several forms. As this S protein is bound to the angiotensin-converting enzyme 2 receptor, antigenic impact, cell entry transmissibility, virulence, evasion of host cell immunity, and mutations in the spike S protein (critical S mutation) have occurred. The humoral immune response against the virus produces neutralising antibodies that bind to the spike protein. The SARS-CoV-2 variations were categorised by the Centre for Disease Control and Prevention (CDC) into four categories which are mentioned below.26,27
- Variants of concern (VOC)
- Variants being monitored (VBM)
- Variants of interest (VOI)
- De-escalated variants
Variants of Interest (VOI) and Variants of Concern (VOC)
Research findings showed that these variants have potential effects on causing the disease, based on genetic characteristics, epidemiological evidence, in-vitro evidence with major impact on immunity, transmissibility, and severity. Some of the VOIs and VOCs reported on the website of European Centre for Disease Prevention and Control are mentioned in Table 3.
Table (3):
Different variants of VOI and VOC
Virus variants |
Descendants |
|---|---|
B.1.351 |
B.1.351.2, B.1.135.1, B.1.351, B.1.351.3, B.1.351.5 |
P.1 |
P.1.15, P.1.12, P.1.4, P.1.16, P.1.10.2 |
B.1.1.7 |
Q.3, Q.2, Q.7, B.1.1.7, Q.8 |
B.1.617.2 |
AY.39.3, AY.49, AY.57, AY.14, AY.4.9 |
XBB.1.5 |
FL.4.5, FL.30, EG.5.2.3, GK.1.11.1, XBB.1.5.43 |
Deescalated variants
These variants (Table 4) are listed as de-escalated variants as they follow at least one of the criteria26,27:
- Not a single case has been reported of the infection of this variant.
- The variant still being active for a long time without showing any noticeable effects.
- The analysis indicates that these strains do not exhibit any alarming characteristics.
Table (4):
Different types of de-escalated variants
Type |
Lineage and mutations |
Detection details |
|---|---|---|
Alpha |
B.1.1.7 |
United Kingdom, September 2020 |
Epsilon |
B.1.427, B.1.429 |
USA, September 2020 |
Eta |
B.1.525 |
Nigeria, December 2020 |
Theta |
P.3 |
The Philippines, January 2021 |
Kappa |
B.1.617.1 |
India, December 2020 |
Variants being monitored (VBM)
There are no variants currently meeting the criteria to be listed under the variants being monitored.
Currently available and approved COVID-19 vaccines
Since the outbreak of SARS-CoV-2, efforts have been made globally to undergo antiviral treatments and vaccine development. The therapeutic objectives were concentrated on actions that could reduce the length of hospitalisation and improve the survival of infected patients. It was essential to create effective vaccinations against SARS-CoV-2 due to its pandemic spread and its related effects, such as constrained ventilators and hospital capacity. Vaccines against COVID-19 have mostly been produced in five different forms such as inactivated, DNA-based, RNA-based, replicating and non-replicating vaccines, protein subunit, and virus-like particles (VLPs).28 About 185 nations used the AstraZeneca vaccine, which according to the data analysed, is the most widely used vaccine. Pfizer-BioNTech, Moderna, and Johnson & Johnson administered the vaccines in 165, 114, and 103 countries, respectively.25 The other vaccines being used are Sinopharm-Beijing, Sinovac, Gamaleya, Novavax, etc. Table 5 includes the details of the available COVID-19 vaccines, their efficacy, dosage and immunogenicity.
Table (5):
Currently available and approved COVID-19 vaccines with their properties
Vaccine and Developer |
Efficacy |
Type |
Injection and dosage interval |
Antigenicity/Immunogenicity |
|---|---|---|---|---|
BNT16b2 Pfizer/BioNTech |
95%
[29] |
RNA-based |
2 doses administered intramuscularly 4 weeks apart |
High neutralizing and antibody titres and CD4+ and CD8+ T cells responses [30] |
mRNA-1273 Moderna |
94.1% [29] |
RNA-based |
2 doses administered intramuscularly 4 weeks apart |
Two doses resulted in neutralizing antibodies similar to the antibodies present in the sera of recovered patients was observed [32] |
CVnCoV CureVac |
48.2% [31] |
RNA-based |
2 doses administered intramuscularly 4 weeks apart |
High neutralizing antibodies and CD4+ T cell responses [30] |
AZD1222 AstraZeneca and Oxford University |
66.7% [33] |
Recombinant vaccine |
2 doses Administered intramuscularly 4-12 weeks apart |
High production of neutralizing antibodies in a single dose [34] |
Ad26.COV2. S Johnson & Johnson |
66.9% [35] |
Recombinant vaccine |
A single dose is given intramuscularly |
Production of neutralizing antibodies against the virus due to the rapid binding inducing the T cell mediated immunity [36] |
Sputnik V Gamaleya National Center of Epidemiology and Microbiology |
91.6% [37] |
Recombinant vaccine |
2 doses are given intramuscularly with a time difference of 21 days |
High titres were observed in both frozen and lyophilized forms of the vaccine [38] |
NVX-CoV2373 Novavax |
89.7% [39] |
Protein-based |
Intramuscularly 2 doses 21 days apart |
High number of antibodies against the D614G strain was observed [40] |
EpiVacCorona VECTOR |
82.5% [41] |
Protein-based |
2 doses administered intramuscularly with 21 days gap |
The vaccine was responsible for inducing neutralizing and antigen-specific antibodies against the virus. [42] |
ZF2001 “Institute of Microbiology, Chinese Academy of Sciences, and Anhui Zhifei Longcom Biopharmaceutical” (36, 28) |
86.5% [43] |
Protein-based |
Intramuscular |
The vaccine produced neutralizing antibodies against the omicron BA strain of coronavirus [44] |
Convidecia™ Ad5-nCoV CanSino |
85% [45] |
Recombinant vaccine |
Intramuscularly A single dose |
Safe and production of significant levels of neutralizing antibodies [46] |
CoronaVac SinoVac Biotech |
83.5% [47] |
Killed vaccine |
2 doses given intramuscularly with 21-28 days gap |
Induced strong humoral response against the SARS-CoV-2 virus [48] |
BBIBP-COrV Beijing Bio-Institute of Biological Products |
78.1% [49] |
Killed vaccine |
2 doses given intramuscularly with 21-28 days gap |
Safe and high antibody titres [50] |
Wuhan Sinopharm/Chinese Academy of Science |
79% [51] |
Killed vaccine |
2 doses with a gap of 28 days are given intramuscularly |
Found to be immunogenic against Coronavirus, influenza virus, and S. pnuemoniae [52] |
Covaxin Bharat Biotech |
81% [53] |
Killed vaccine |
2 doses with a gap of 28 days are given intramuscularly |
The clinical studies found that the vaccine was able to induce the humoral response with 80% of IgG antibodies produced inside the body against the Spike protein [54] |
CIGB-66 Cuban Genetic Engineering and Biotechnology Centre |
61.8% [55] |
Protein-based |
3 doses are given intramuscularly at 0,14 and 28 days |
the clinical studies in the subjects ranging from 19 to 80 years resulted in the inducing of humoral response when immunised with the vaccine [56] |
QazCovid-In Kazakhstan Research Institute for Biological Safety Problems |
77% [57] |
Killed vaccine |
2 doses with a gap of 21 days are given intramuscularly |
Induced a fourfold increased production of neutralizing antibodies [58] |
Coviran Barkat Shifa Pharmed Industrial Group |
86.4% [59] |
Killed vaccine |
Intramuscularly 2 doses 28 days apart |
High production of IgG antibodies was produced specifically against the S and N protein [60] |
COVISHIELD™ Serum Institute of India Pvt Ltd |
90% [45] |
Recombinant vaccine |
intramuscularly 2 doses 12-16 weeks apart |
Binds to B cells making them produce a greater number of antibodies leading to immunoreactivity against the spike protein [61] |
LNP-nCoVsaRNA Imperial College London |
N/A |
RNA-based |
intramuscular |
Triggers humoral response to produce neutralizing antibodies and cell mediated immunity [62] |
INO-4800, Inovio Pharmaceuticals |
100% in phase 1 [63] |
DNA-based |
intradermal 2 doses 28 days apart |
Induce immune response and safe [63] |
ZyCoV-D Zydus Cadila |
66.6% [64] |
DNA-based |
intradermal doses at an interval of 28 and 56 days |
Induced immune response in preclinical trials [64] |
With the help of emerging techniques of biotechnology, the current vaccines are being developed to tackle the new variants. As mentioned above in the table some of the currently available vaccines are efficient against the new variants of SARS-CoV-2 such as omicron, and delta strains. The new FDA announcements made on June 14, 2024; the vaccines developed should consider the new variants which are responsible for most cases in a particular region. As mentioned in an announcement made on June 7, 2024, by FDA that the COVID-19 vaccines for the use in United states should be monovalent meaning that it should be closely related to the current circulating strains. With the help of new biotechnological advancements, the COVID-19 vaccines efficacy can be increased to tackle the new variants.65,66
Adverse effects in recovered patients
Reports are there of many ill-effects of SARS-CoV-2 virus in recovered COVID-19 patients. The effects varied from mental illness to various other health issues. Many studies have been conducted to assess the effects of the virus in different regions of the world.
Study conducted by Ahuja et al. on fifty Indian patients recovered from COVID-19, observed that SARS-CoV-2 has the potential for neurotropism, affecting central and peripheral nervous system. The feet electrochemical skin conductance (FESC) sue to COVID-19 infection is more linked to the sensory and motor symptoms than to autonomic symptoms. This study also identified FESC as a biomarker for the prediction of peripheral neuropathy in patients infected with SARS-CoV-2 virus. The COVID-19 treatment groups were found to have several neurological manifestations including mild symptoms like headache, hypogeusia to more severe symptoms including peripheral neuropathy, and cerebrovascular stroke. It was also observed that the patients suffered from sweat dysfunction. The patients with sweat dysfunction were mostly belonged to older age and were treated at home with antiviral drugs. Reports are there of a patient suffering from acute dysautonomia during SARS-CoV-2 infection.67,68
Many case studies related to the adverse effects of coronavirus in recovered patients revealed the mental illnesses like insomnia, depression, anxiety, and post-traumatic stress. This might be due to the lifestyle during the COVID-19 pandemic being inside the house without any physical activities and interaction with other people. A study in Milan, on the recovered patients at one month follow up after hospital treatment, observed various neurological disorders as mentioned here. About 28% of the people suffered from post-traumatic stress disorder (PTSD), 31% with depression, 42% with anxiety, 20% with obsessive compulsive symptoms, and 40% with insomnia. The results were collected from the patients through a questionnaire designed to access the mental state of a person. It was clear from these case reports that SARS-CoV-2 induced significant immunological responses and affected the central nervous system, which may lead to the development of neuropsychiatric symptoms. It was concluded that sociodemographic and environmental factors play vital role in the development of COVID-19 related neuropsychiatric syndromes. Some symptoms of this condition were observed to be delusion, depression, emotional disorder, and loss of memory.69,70
The data taken by a healthcare team in all population and age groups in Australia, revealed the insights into the effects of SARS-CoV-2 infection. Cardiac manifestations were observed and children with the infection recovered well, but a small population developed Kawasaki-like illness, and multisystem inflammatory syndrome temporarily associated with the SARS-CoV-2 infection. It was also seen that the pandemic has affected paediatric population with physical deconditioning and psychological harm.71
A study conducted to assess the post COVID-19 fitness, had shown a change in the lung computed tomography (CT). The CT observed were of glass ground opacities and fibrotic changes. This case study comprised of 43 occupational and recreational divers. It was observed that thirteen divers were restrained from further diving due to the changes in lung CT results. The results from fitness to dive (FTD) assessment showed that there were chances of air trapping regardless the severity of the disease. The CT results have shown hypo- and hyper-inflated zones, bronchiectasis and reticulations which were associated with pulmonary fibrosis and attributed to small airway disease and air trapping. The case study included 35 males and 8 females which were assessed post COVID-19 recovery. It was seen that a total of 5 subjects (4 male, 1 female) have shown cardiovascular disease, 3 subjects (3 males) have shown the symptoms of lung disease and 3 subjects (3 male) have shown the symptoms of diabetes. The study population consisted of divers with asymptomatic SARS-CoV-2 infected cases along with symptomatic infected cases. The common findings from this study were that post COVID-19 divers showed the symptoms of ground glass opacities (GGO), fibrotic changes, alveolar consolidation, and emphysematous changes.72,73
A patient got admitted in a hospital of Portugal with a condition of rhabdomyolysis 3 days after being administered the first dose of Pfizer coronavirus vaccine Comirnaty® and it was found that the disease onset might be related to the vaccine administration. Following the immunization of the vaccine a total of 69 reports of rhabdomyolysis were reported in Europe, as stated by European Medicines Agency in 2021. The other causes of rhabdomyolysis were excluded as there were no abnormal levels of myoglobin and creatinine kinase (CK) before. The patient had a history of ischemic heart disease with electrocardiographic abnormality, cerebrovascular disease, and interstitial lung diseases medicated with nintedanib. The medication of nintedanib was started recently and showed no effects of the drug during the hospital stay which was same with the drug statin. The patient after being treated with more than one medication of rhabdomyolysis, it was seen that the disease onset has links with the vaccination by Comirnaty® vaccine. This case provided the insights into the effects of Pfizer coronavirus vaccine Comirnaty® with the onset of the disease rhabdomyolysis.74,75
A study conducted in China to assess the effects of SARS-CoV-2 virus in recovered pregnant women and their foetus growth, comprised a total of 12 pregnant women recovered from COVID-19 prior to pregnancy termination. The study was conducted to provide clinical references for other countries with regard to the effects of COVID-19 in pregnant women. In this study group, two women chose labour induction (due to worries of SARS-CoV-2 infection), eight of them gave birth by caesarean and two by vaginal delivery. This study concluded that there were no effects of COVID-19 in pregnant women and their foetal growth. There were some cases of women undergoing abortion due to the anxiety of COVID-19 treatment. The conclusions were supported with placental pathological examination results, which was observed to be consistent in all the subjects studied. The results were supported by the finding that the foetus was protected from the infection of SARS-CoV-2 by the placenta after the mothers were cured with COVID-19 treatment.76,77
A study conducted to assess the clinical course and outcomes of liver transplant recipients with hepatic cirrhosis, who have recovered from the infection of coronavirus and underwent liver transplant from dead donors. This study focused to determine the management, timing, and safety of liver transplant in the COVID-19 recovered patients. The study was conducted at Shiraz Transplant Center in Iran during the COVID-19 pandemic. It was concluded that the liver from the deceased can be transplanted into patients recovered from COVID-19, especially in those with increasing Model for End-Stage Liver Disease (MELD) scores and deteriorating clinical status. It was also discussed that the liver transplant can be performed in cases where patients have worsening clinical conditions. It was found that the reverse transcriptase polymerase chain reaction (RT-PCR) results were positive for SARS-CoV-2 after the liver transplantation in patients who had been previously infected with COVID-19. Further studies are required to find the implications of positive RT-PCR results post-transplantation and the long-term outcomes of such patients.78,79
A study in which the data from the European Renal Association COVID-19 database (ERACODA) was analysed to compare the outcomes between peritoneal dialysis (PD) and haemodialysis (HD) patients with COVID-19. The study consisted of adult patients with kidney failure who were treated with dialysis and developed COVID-19. The study was mainly focused on finding the mortality rates, functional and mental health status among the survivors, and to compare the outcomes with HD and PD patients with COVID-19. The study was conducted to determine the effect of COVID-19 on kidney failure patients treated with dialysis, so that potential strategies can be designed for improving care and clinical outcomes of the patients during the COVID-19 pandemic. It was observed that the mortality rate of PD patients was higher as compared to HD patients. It was concluded that special attention and specialized care should be provided to the PD patients who were at higher mortality risk due to COVID-19 infection. The main insights of this study were the importance of specialised care for the vulnerable group and the need for proactive strategies to enhance the clinical outcomes of the population at risk by lowering the mortality rates of the vulnerable patients.80,81
A study comprising the patients with both coronavirus infected and non-infected groups who have undergone total hip arthroplasty (THA) or total knee arthroplasty (TKA), was conducted to evaluate the complications associated with COVID-19. The results of this study revealed that the patients diagnosed with COVID-19 within 90 days of surgery had higher rates of readmission, pneumonia, deep vein thrombosis (DVT), kidney failure and acute respiratory distress syndrome as compared to the COVID-19 negative group. The potential reasons for the complications were combined risk of thromboembolism from COVID-19 and total joint arthroplasty (TJA), residual symptoms like fatigue, dyspnea, joint pain and chest pain, pulmonary complications such as acute respiratory distress syndrome (ARDS) which showed inflammation-mediated necrosis of the alveolar-capillary endothelium and epithelium along with interstitial and intra-alveolar edema.82,83
A study was performed to determine the safety of surgery on patients recovered from COVID-19 and the challenges in the preoperative evaluation of the COVID-19 recovered patients. It was discussed that the surgeries are recommended to be postponed whenever possible and the surgeries should be performed based on the clinical status of the patients. It was observed that there is lung damage due to COVID-19 infection caused by the SARS-CoV-2 virus which increases the risks of pulmonary complications in COVID-19 recovered patients. It was suggested to use protective mechanical ventilation during and after the surgery to prevent the pulmonary complications. It also discussed the parameters to operate the protective mechanical ventilation for use during and after the surgery of recovered COVID-19 patients.84,85
Since the outbreak of COVID-19 there has been a concern that the infection may adversely affect the patients due to immune thrombocytopenia (ITP). A study conducted on 52 ITP patients after administered with COVID vaccine, was able to provide insights into the effects of COVID-19 in the patients. The study revealed that 73% of the subjects had no new symptoms and no decline in the platelet count. However, 12% of the patients had an average platelet drop of 96% within 2-5 days from the day of vaccine administration. The results were related to the vaccines which were produced by dead bacteria or viruses which are the simulators of the immune system of the body. Some of the subjects have shown the same symptoms before when vaccinated for Neisseria meningitis and Streptococcus pneumoniae.86,87
Remdesivir was one of the drugs used to treat COVID-19 infection at earlier stages before the vaccines were prepared. This drug was seen to decrease the time for recovery from the infection in adults. The effects of this drug were not known in the children as very few studies were conducted till then. The study included 77 SARS-CoV-2 infection cases who were administered with remdesivir drug. It was concluded that among the 77 cases most had recovered, and few adverse effects were observed. The side effects reported with the use of this drug include serious allergic reactions including infusion-related reaction and anaphylaxis. This study also evaluated the safety, quality, pharmacokinetics, and efficacy of the drug with the help of a control arm.88,89
Globally, the COVID-19 pandemic had serious social, economic, and health effects. According to the reports, COVID-19 infection caused the deaths of at least 6.9 million people. To date, none of the licenced medications have been found to be potent enough to heal the afflicted patients. Numerous effective vaccinations have been released throughout the pandemic by the enormous efforts of scientists in order to defend the virus. The goal of universal immunisation was accomplished, and the COVID-19 pandemic is now mostly under control, even though the vaccines that were developed by various pharmaceutical companies and research institutes have demonstrated varying efficacies. Although, there was a wide coverage of vaccination worldwide, the rise of new variants of the virus poses challenge in eliminating the disease. Additionally, in most of these instances, vaccine effectiveness was decreased in altered forms, particularly in Delta and Omicron variants, and antibody titres against COVID-19 were noticeably decreased. Although the newly circulating COVID-19 variants showed signs of vaccine efficacy decline, the mortality rate of COVID-19 was significantly reduced in 2022 as a result of the widespread immunisation in 2021.
The newly developed COVID-19 vaccines have been very much updated to tackle the new strains, which play a main role in the spreading of the infections again. However, accurate observation of the recently emerging SARS-CoV-2 variants is crucial for the early detection of strains, which will help in stopping the future spread of the virus throughout the world. It is essential to closely monitor the recently emerging sub-lineages and take preventive measures to lessen the likelihood of disease burden.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Conceptualization – RBN and MKJ; Writing original draft – RBN; Writing: Review and Editing – MKJ, SK, PK, and GM; Guidance – MKJ.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Han X, Xu P, Ye Q. Analysis of COVID-19 vaccines: Types, thoughts, and application. J Clin Lab Anal. 2021;35(9):e23937.
Crossref - Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-98.
Crossref - Motamedi H, Ari MM, Dashtbin S, et al. An update review of globally reported SARS-CoV-2 vaccines in preclinical and clinical stages. Int Immunopharmacol. 2021;96:107763.
Crossref - COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet. 2022 Apr 16;399(10334):1513-1536. doi: 10.1016/S0140-6736(21)02796-3. Epub 2022 Mar 10. Erratum in: Lancet. 2022 Apr 16;399(10334):1468.
Crossref - World Health Organization. Draft landscape of COVID-19 candidate vaccines. www.who.int. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed date, 30 March 2023
- World Health Organization 2023 data.who.int, WHO Coronavirus (COVID-19) dashboard > Vaccines. https:// data.who.int/dashboards/covid19/vaccines. Accessed on June 22, 2023.
- Islam MJ, Islam nN, Alom MS, Kabir M, Halim MA. A review on structural, non-structural, and accessory proteins of SARS-CoV-2: Highlighting drug target sites. Immunobiology. 2023;228(1):152302.
Crossref - Shirbhate E, Pandey J, Patel VK, et al. Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: a potential approach for therapeutic intervention. Pharmacol Rep. 2021;73(6):1539-1550.
Crossref - McIntosh K, Chao RK, Krause HE, Wasil R, Mocega HE, Mufson MA. Coronavirus infection in acute lower respiratory tract disease of infants. The Journal of infectious diseases, 1974;130(5):502-507.
Crossref - Mahmoudi S, Balmeh N, Mohammadi N, Sadeghian-Rizi T. The Novel Drug Discovery to Combat COVID-19 by Repressing Important Virus Proteins Involved in Pathogenesis Using Medicinal Herbal Compounds. Avicenna J Med Biotechnol. 2021;13(3):107-115.
Crossref - Khalid K, Poh CL. The development of DNA vaccines against SARS-CoV-2. Adv Med Sci. 2023;68(2):213-226.
Crossref - Bian L, Gao F, Zhang J, et al. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20(4):365-373.
Crossref - Mirtaleb MS, Falak R, Heshmatnia J, et al. An insight overview on COVID-19 mRNA vaccines: Advantageous, pharmacology, mechanism of action, and prospective considerations. Int Immunopharmacol. 2023;117:109934.
Crossref - Lappeman J, Munyai K, Kagina BM. Negative sentiment towards COVID-19 vaccines: A comparative study of USA and UK social media posts before vaccination rollout. F1000Research. 2021;10(472):472.
Crossref - Deng S, Liang H, Chen P, et al. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms. 2022;10(7):1450.
Crossref - WHO. COVID-19 Vaccine Tracker and Landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- Kan AKC, Li PH. Inactivated COVID-19 vaccines: potential concerns of antibody-dependent enhancement and original antigenic sin. Immunol Lett. 2023;259:21-23.
Crossref - Khoshnood S, Arshadi M, Akrami S, et al. An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. J Clin Lab Anal. 2022;36(5):e24418.
Crossref - Chen J, Wang P, Yuan L, et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci Bull. 2022;67(13):1372-1387.
Crossref - Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947-953.
Crossref - Lidder P, Sonnino A. Biotechnologies for the management of genetic resources for food and agriculture. Adv Genet. 2012;78:1-167.
Crossref - Hou Y, Chen M, Bian Y, Zheng X, Tong R, Sun X. Advanced subunit vaccine delivery technologies: From vaccine cascade obstacles to design strategies. Acta Pharm Sin B. 2023;13(8):3321-3338.
Crossref - Thuluva S, Paradkar V, Gunneri S, et al. Immunogenicity and safety of Biological E’s CORBEVAXTM vaccine compared to COVISHIELDTM (ChAdOx1 nCoV-19) vaccine studied in a phase-3, single blind, multicentre, randomized clinical trial. Hum Vaccin Immunother. 2023;19(1):2203632.
Crossref - India Fights Corona COVID-19 in India, vaccination, Dashboard, Corona Virus Tracker | Mygov.in. https://www.mygov.in/covid-19/.
- Holder J. Covid World Vaccination Tracker. The New York Times. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html. Published March 13, 2023
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases; SARS-CoV-2 variant classifications and definitions, 2021.
- SARS-CoV-2 variants of concern as of 26 July 2024. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/covid-19/variants-concern. Published July 1, 2024.
- Savina K, Sreekumar R, Soonu VK, Variyar EJ. Various vaccine platforms in the field of COVID-19. Beni Suef Univ J Basic Appl Sci. 2022;11(1):35.
Crossref - Rotshild V, Hirsh-Raccah B, Miskin I, et al. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11:22777.
Crossref - Phetsouphanh C, Khoo WH, Jackson K, et al. High titre neutralizing antibodies in response to SARS-CoV-2 infection require RBD-specific CD4 T cells that include proliferative memory cells. Front Immunol. 2022;13:1032911.
Crossref - Kremsner PG, Ahuad Guerrero RA, Arana-Arri E, et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2022;22(3):329-340.
Crossref - Corbett KS, Werner AP, Connell SO, et al. mRNA-1273 protects against SARS-CoV-2 beta infection in nonhuman primates. Nat Immunol. 2021;22(10):1306-1315.
Crossref - Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671-681.
Crossref - Ekstrom N, Haveri A, Solastie A, et al. Strong Neutralizing Antibody Responses to SARS-CoV-2 Variants Following a Single Vaccine Dose in Subjects With Previous SARS-CoV-2 Infection. Open Forum Infect Dis. 2022;9(12):ofac625.
Crossref - Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187-2201.
Crossref - Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA. 2021;325(15):1535-1544.
Crossref - Voysey M, Clemens SAC, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881-891.
Crossref - Rossi AH, Ojeda DS, Varese A, et al. Sputnik V vaccine elicits seroconversion and neutralizing capacity to SARS-CoV-2 after a single dose. Cell Rep Med. 2021;2(8):100359.
Crossref - Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172-1183.
Crossref - Lyke KE, Atmar RL, Dominguez Islas C, et al. Immunogenicity of NVX-CoV2373 heterologous boost against SARS-CoV-2 variants. NPJ Vaccines. 2023;8(1):98.
Crossref - Ryzhikov AB, Ryzhikov EA, Bogryantseva MP, et al. Assessment of Safety and Prophylactic Efficacy of the EpiVacCorona Peptide Vaccine for COVID-19 Prevention (Phase III). Vaccines. 2023;11(5):998.
Crossref - Saleem A, Akhtar MF, Haris M, Abdel-Dami MM. Recent updates on immunological, pharmacological, and alternative approaches to combat COVID-19 . Inflammopharmacol. 2021;29(5):1331-1346.
Crossref - Dai L, Gao L, Tao L, et al. Efficacy and Safety of the RBD-Dimer-Based Covid-19 Vaccine ZF2001 in Adults. N Engl J Med. 2022;386(22):2097-2111.
Crossref - Gao L, Li Y, He P, et al. Safety and immunogenicity of a protein subunit COVID-19 vaccine (ZF2001) in healthy children and adolescents aged 3-17 years in China: a randomised, double-blind, placebo-controlled, phase 1 trial and an open-label, non-randomised, non-inferiority, phase 2 trial. Lancet Child Adolesc Health. 2023;7(4):269-279.
Crossref - Halperin SA, Ye L, MacKinnon-Cameron D, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022.
Crossref - Li JX, Wu SP, Guo XL, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir Med. 2022;10(8):739-748.
Crossref - Tanriover MD, Doganaf HL, Akova M. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213-222.
Crossref - Muena NA, Garcia-Salum T, Pardo-Roa C, et al. Induction of SARS-CoV-2 neutralizing antibodies by CoronaVac and BNT162b2 vaccines in naןve and previously infected individuals. EBioMedicine. 2022;78:103972.
Crossref - Zhang Y, Belayachi J, Yang Y, et al. Real-world study of the effectiveness of BBIBP-CorV (Sinopharm) COVID-19 vaccine in the Kingdom of Morocco. BMC Public Health. 2022;22(1):1584.
Crossref - Zamani B, Hasan-Abad AM, Piroozmand A, Dehghani M, Arfaatabar M, Motedayyen H. Immunogenicity and safety of the BBIBP-CorV vaccine in patients with autoimmune inflammatory rheumatic diseases undergoing immunosuppressive therapy in a monocentric cohort. Immun Inflamm Dis. 2023;11(5):e858.
Crossref - World Health Organization: WHO. The Sinopharm COVID-19 vaccine: What you need to know. https://www.who.int/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know#:~:text=The%20vaccine%20is%20safe%20and,COVID%2D19%20in%20the%20past.
- Chen H, Huang Z, Chang S, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine (Sinopharm BBIBP-CorV) coadministered with quadrivalent split-virion inactivated influenza vaccine and 23-valent pneumococcal polysaccharide vaccine in China: A multicentre, non-inferiority, open-label, randomised, controlled, phase 4 trial. Vaccine. 2022;40(36):5322-5332.
Crossref - Behera P, Singh AK, Subba SH, et al. Effectiveness of COVID-19 vaccine (Covaxin) against breakthrough SARS-CoV-2 infection in India. Hum Vaccin Immunother. 2022;18(1):2034456.
Crossref - Das S, Kar SS, Samanta S, Banerjee J, Giri B, Dash SK. Immunogenic and reactogenic efficacy of Covaxin and Covishield: a comparative review. Immunol Res. 2022;70(3):289-315.
Crossref - Mahase E. COVID-19: Russian vaccine efficacy is 91.6%, show phase III trial results. BMJ. 2021;372:n309.
Crossref - Hernandez-Bernal F, Ricardo-Cobas MC, Martin-Bauta Y, et al. Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: A randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study). EClinicalMedicine. 2022;46:101383.
Crossref - Nabirova D, Horth R, Smagul M, et al. Effectiveness of four vaccines in preventing SARS-CoV-2 infection in Almaty, Kazakhstan in 2021: retrospective population-based cohort study. Front Public Health. 2023;11:1205159.
Crossref - Zakarya K, Kutumbetov L, Orynbayev M, et al. Safety and immunogenicity of a QazCovid-in® inactivated whole-virion vaccine against COVID-19 in healthy adults: A single-centre, randomised, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. EClinicalMedicine. 2021;39:101078.
Crossref - Mohraz M, Vahdat K, Ghamari SH, et al. Efficacy and safety of an inactivated virus-particle vaccine for SARS-CoV-2, BIV1-CovIran: randomised, placebo controlled, double blind, multicentre, phase 3 clinical trial. BMJ. 2023;382:e070464.
Crossref - Abdoli A, Aalizadeh R, Aminianfar H, et al. Safety and potency of BIV1-CovIran inactivated vaccine candidate for SARS-CoV-2: A preclinical study. Rev Med Virol. 2022;32(3):e2305.
Crossref - Bhatnagar T, Chaudhuri S, Ponnaiah M, et al. Effectiveness of BBV152/Covaxin and AZD1222/Covishield vaccines against severe COVID-19 and B.1.617.2/Delta variant in India, 2021: a multi-centric hospital-based case-control study. Int J Infect Dis. 2022;122:693-702.
Crossref - Huang Q, Zeng J, Yan J. COVID-19 mRNA vaccines. J Genet Genomics. 2021;48(2):107-114.
Crossref - Inovio, Powering DNA medicines: News details, INOVIO receives authorization to conduct phase 3 efficacy trial of its COVID-19 Vaccine candidate, INO-4800, August 26, 2021. https://ir.inovio.com/news-releases/news-releases-details/2021/INOVIO-Receives-Authorization-to-Conduct-Phase-3-Efficacy-Trial-of-its-COVID-19-DNA-Vaccine-Candidate-INO-4800/default.aspx
- Tebas P, Yang S, Boyer JD, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31:100689.
Crossref - FDA. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines.
- COVID-19 Vaccines Advice. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice. Accessed date: 18 August, 2022
- Hinduja A, Moutairou A, Calvet JH. Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiol Clin. 2021;51(2):193-196.
Crossref - Eshak N, Abdelnabi M, Ball S, et al. Dysautonomia: an overlooked neurological manifestation in a critically ill COVID-19 patient. Am J Med Sci 2020;360(4):427-429.
Crossref - Smith CJ, Renshaw P, Yurgelun-Todd D, Sheth C. Acute and chronic neuropsychiatric symptoms in novel coronavirus disease 2019 (COVID-19) patients: A qualitative review. Front Public Health. 2022;10:772335.
Crossref - World Health Organization. 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-$-$21-september-2021 (accessed May 1, 2022).
- Turner CJ. COVID-19: The disease, the vaccine and the heart. J Paediatr Child Health. 2023;59(6):786-793.
Crossref - Mirasoglu B, Yetis G, Erelel M, Toklu AS. Post COVID-19 fitness to dive assessment findings in occupational and recreational divers. Diving Hyperb Med. 2022;52(1):35-43.
- Shaw B, Daskareh M, Gholamrezanezhad A. The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Radiol Med. 2021;126(1):40-46.
Crossref - Elias C, Cardoso P, Goncalves D, Vaz I, Cardoso L. Rhabdomyolysis following administraton of Cominrnaty®? EJCRIM 2021;8.
Crossref - Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
- Fan C, Guo Y, Qu P, et al. No obviously adverse pregnancy complications and outcomes of the recovered pregnant women from COVID-19. Reprod Toxicol. 2021;100:163-166.
Crossref - Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM. 2020;2(3):100134.
Crossref - Eshraghian A, Nikoupour H, Dehghani M, et al. Early Liver Transplant In Patients With Liver Cirrhosis Recovered From COVID-19 Infection. 2022;20(10):925-929.
Crossref - Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430-436.
Crossref - Abrahams AC, Noordzij M, Goffin E, et al. Outcomes of COVID-19 in peritoneal dialysis patients: A report by the European Renal Association COVID-19 Database. Perit Dial Int. 2023;43(1):23-36.
Crossref - Couchoud C, Bayer F, Ayav C, et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 2020;98(6):1519-1529.
Crossref - Lee A, Durst CR, Rezzadeh KT, Rajaee SS, Penenberg BL, Than JP. Higher Complication Rate in COVID-19 Recovered Patients Undergoing Primary Total Joint Arthroplasty. J Arthroplasty. 2023;38(Suppl 2):S111-S115.
Crossref - COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107(11):1440-1449.
Crossref - Cariello C, Corradi F, Isirdi A, Marino F, Forfori F. Is surgery on patients recently recovered from COVID-19 safe? Minerva Anestesiol. 2021;87(5):615-617.
Crossref - Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623.
Crossref - Kuter DJ. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br J Haematol. 2021 Nov;195(3):365-370.
Crossref - David P, Shoenfeld Y. ITP following vaccination. Int J Infect Dis. 2020;99:243-244.
Crossref - Goldman DL, Aldrich ML, Hagmann SHF, et al. Compassionate Use of Remdesivir in Children With Severe COVID-19. Pediatrics. 2021;147(5):e2020047803.
Crossref - Kim L, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of children aged ,18 years hospitalized with laboratoryconfirmed COVID-19 – COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1081-1088.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.