ISSN: 0973-7510
E-ISSN: 2581-690X
Methicillin resistant Staphylococcus aureus (MRSA) is a known human pathogen capable of causing community and hospital acquired infections worldwide. Treatment options available for MRSA infections are limited, with vancomycin being one of the most common drugs used. It is described in the literature that vancomycin can be ineffective against MRSA isolates with MIC values between 1-2 mg/litre. This slow and steady shift of vancomycin MIC values towards higher side over a period of time is known as “MIC creep”. The present retrospective study was carried out over five year period from January 2019 to June 2023. Staphylococcus aureus isolates from all clinical samples isolated during study period were included in the study. MIC50, MIC90, geometric mean MIC values were determined and analysed using Microsoft Excel. In the present study, the prevalence of MRSA was high (79.6%) in pus and tissue samples followed by blood sample (9.7%). Most of the MRSA isolates (55.80%) in present study exhibited vancomycin MIC of 1 µg/ml, there is no increasing trend of MIC values over a five year period. MIC creep is a slow and steady process which is multifactorial in origin. Regular monitoring of vancomycin MIC trend is advisable as vancomycin is the first-line treatment for culture proven severe infection with MRSA.
Treatment Failure, MIC Creep, Clonal Dissemination, Antimicrobial Stewardship, Hospital Infection Control
Methicillin resistant Staphylococcus aureus (MRSA) is a known human pathogen capable of causing community and hospital acquired infections worldwide.1 MRSA infections are associated with extended antibiotic therapy, increased duration of hospitalization, increased morbidity and mortality.2,3 Treatment options available for MRSA infections are very few vancomycin being one of the commonest drug used.3,4-10
Various studies are conducted globally showing therapeutic failure in patients with MRSA infections with higher vancomycin MIC for the isolate though within susceptible range.8,11 It is described in the literature that vancomycin can be ineffective against MRSA isolates with MIC values between 1-2 mg/litre.12-14 This slow and steady shift of vancomycin MIC values towards higher side over a period of time is known as “MIC creep”.15 MIC creep results in slow clinical response, increased morbidity, higher relapse rates and therapeutic failure.16
American Thoracic Society and Infectious Diseases Society of America addressed the issue of clinical failure in MRSA infections due to higher MIC values for vancomycin.17,18 In some local institutions MIC creep can be attributed to the clonal dissemination of these MRSA strains with higher MIC values.1 For the clinical management of MRSA infections in particular geographic area it is important to study the susceptibility profile and MIC distribution pattern for the local MRSA isolates.19 The present study was conducted to assess the vancomycin MIC distribution for MRSA isolates in a tertiary care hospital in western Maharashtra, India.
The present retrospective study was carried out covering a five year period from January 2019 to June 2023 in the Microbiology diagnostic laboratory in a tertiary care centre. Ethical committee approval was obtained for this study.
Staphylococcus aureus isolates from all clinical samples isolated during study period were included in the study. Identification and antimicrobial susceptibility by microbroth dilution method of Staphylococcus aureus had been done by Vitek 2 automated system by Biomerieux, France. Methicillin resistance in Staphylococcus aureus was also determined by the automated system (Vitek 2) system by microbroth dilution method.1,15
MIC50, MIC90, geometric mean MIC values were determined and analysed using Microsoft Excel. Statistical significance was calculated using related samples Friedman’s two-way analysis of variance by rank test.
In the present study, total 900 MRSA isolates were obtained from all clinical samples over a period of five years. Sample wise distribution of MRSA isolates is shown in Table 1.
Table (1):
Showing sample wise distribution of MRSA isolates
Type of samples |
Number of MRSA isolates (Total MRSA isolates=900) |
Percentage MRSA |
|---|---|---|
Pus and tissue |
716 |
79.6% |
Blood |
87 |
9.7% |
Respiratory samples (Sputum, ETT, BAL) |
35 |
3.9% |
Urine |
45 |
5% |
Body fluids (Ascitic fluid, CSF, Pleural fluid) |
17 |
1.9% |
MIC parameters for Vancomycin in MRSA isolates
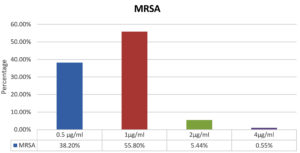
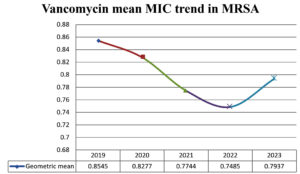
MIC distribution of all MRSA isolates is shown in Figure 1. MIC range for vancomycin in MRSA was from 0.5 to 4 µg/ml. Out of total 900 isolates of MRSA, 99.4% were vancomycin susceptible (VSMRSA) and 0.55% isolates were vancomycin intermediate (VISA). Vancomycin resistant MRSA (VRSA) strains were not observed in present study. Most of the MRSA isolates (55.8%) exhibited MIC value of 1 µg/ml. Year wise vancomycin MIC distribution is shown in Table 2. The percentage of VISA in 2019 were 1.03%, there after there was decreasing trend in 2020-2021 and VISA isolates were absent in 2022-2023. The vancomycin MIC related parameter analysis is shown in Table 3. The Vancomycin MIC50 value was 1 µg/ml in all five years. MIC90 value for vancomycin for 2019-2020 was 2 µg/ml after that it decreased to 1 µg/ml in 2021 to 2023. Geometric mean (GM) MIC value trend over a period of five years is shown in Figure 2. Highest mean MIC values were observed in 2019 and lowest MIC values were seen in 2022. The declining trend of mean MIC values was observed from 2019-2022. This difference in the geometric mean MIC values over a period of 5 years was found to be statistically insignificant (p > 0.05).
Table (2):
Year wise Vancomycin MIC distribution in MRSA isolates
| Vancomycin MIC distribution in MRSA isolates | ||||
|---|---|---|---|---|
| Year | 0.5 µg/ml | 1 µg/ml | 2 µg/ml | 4 µg/ml |
| 2019 | 32.9 | 58.4 | 7.56 | 1.03 |
| 2020 | 35.06 | 57.79 | 6.49 | 0.64 |
| 2021 | 40.78 | 55.86 | 2.79 | 0.55 |
| 2022 | 46.26 | 49.25 | 9.47 | 0 |
| 2023 | 37.33 | 58.66 | 4 | 0 |
Table (3):
Vancomycin MIC related parameters in MRSA
Year |
MIC 50 µg/ml |
MIC 90 µg/ml |
Geometric mean µg/ml |
|---|---|---|---|
2019 |
1 |
2 |
0.8545 |
2020 |
1 |
2 |
0.8277 |
2021 |
1 |
1 |
0.7744 |
2022 |
1 |
1 |
0.7485 |
2023 |
1 |
1 |
0.7937 |
MRSA is a very important pathogen in both community and hospital set up.20 In the present study prevalence of MRSA was high (79.6%) in pus and tissue samples. (Table 1) Lohan et al.(61.7%) and Mallick and Basak (61.4%) also showed similar finding in their studies.21,22
The present study is the first Indian study showing vancomycin MIC trend over a period of 5 years. In this study, MIC50 values for vancomycin were found to be constant over five year period. Studies conducted by Alos et al.23 and Arshad et al.24 have also not shown change in MIC 50 values over a period of four years. However, Steinkraus et al. have shown increase in MIC50 value from 0.75 µg/ml to 1 µg/ml over a period of five years. 25 The geometric MIC values showed decreasing trend from 2019-2022. Though most of the MRSA isolates (55.80%) in present study exhibited vancomycin MIC of 1 µg/ml, there is no increasing trend of MIC values over a five year period. Studies have reported that geometric MIC value is a sensitive marker to reflect the changes in MIC values as compared to other markers like MIC50, MIC90, percentage susceptible and percentage resistant.24-29
Vancomycin remains the mainstay of treatment for infections caused by MRSA. There is large numbers of work done describing vancomycin MIC creep, which means sustained increase in the MICs of vancomycin within susceptible range against Staphylococcus aureus.6,7,27 But the results of these studies are conflicting. Various studies conducted globally have demonstrated MIC creep phenomenon.1,24,28-31 Few studies have shown no change in vancomycin MICs over period of years.25,32,33 On the contrary, studies conducted by Joana et al., Haas et al. and Lu et al. have shown decreasing trend of vancomycin MICs in MRSA isolate.34-36 Some authors have reported that pooling date from multiple centers can obscure the MIC trend that exists in individual set up. Also there can be variation in MIC values in two different institutes in the same geographic area.19
The present study did not show any vancomycin MIC creep phenomenon. The decreasing trend of vancomycin MICs in the present study was mainly associated with decrease in percentage of MRSA isolates with MIC >1µg/ml over a period of 5 years. The development of vancomycin MIC creep is found to be multifactorial.1 It is affected by drug over use, clonal spread, geographical area, methodologies used to detect vancomycin MIC, guidelines used for interpretation1,26 and MIC parameters analysed.24 Other important factors affecting the MIC creep are antimicrobial stewardship and hospital infection control practices in the institute.1,26,24 Recognition of this creep phenomenon is important as it can be precursor of hVISA and VISA and can lead to therapeutic failure with poor outcome.4,5,7,8,37,38 Joana et al. reported that MIC creep phenomenon is not generalised. So each institution should independently monitor vancomycin MICs in their set up.34
The present study had certain limitations. The MIC values were taken from the automated susceptibility testing system and were not confirmed by any other method. The performance of the automated system for detection of glycopeptide resistance are said to underestimate the MICs,37-39 but still these results were useful for observing the trend of vancomycin MICs over a long period in present study.
The “MIC creep” for vancomycin was not observed in present study. MIC creep is a slow and steady process which is multifactorial in origin. Regular monitoring of vancomycin MIC trend is advisable as vancomycin is the first-line treatment for culture proven severe infection with MRSA. An analysis of regional variation is essential as they may differ from global trends.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Bharati Vidyapeeth (Deemed to be University) Medical College, Pune, India, with reference number BVDUMC/IEC/127.
- Aljohani S, Layqah L, Masuadi E et al. Occurrence of vancomycin MIC creep in methicillin resistant isolates in Saudi Arabia. J Infect Public Health. 2020;13(10):1576-1579.
Crossref - Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40(1):135-136.
Crossref - Charles PGP, Ward PB, Johnson PDR, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38(3):448-451.
Crossref - Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51(7):2582-2586.
Crossref - Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138-2144.
Crossref - Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering Jr. RC. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis. 2004;38(12):1700-1705.
Crossref - Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering Jr RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42(6):2398-402.
Crossref - Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(2):193-200.
Crossref - Clinical and Laboratory Standard Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically ; Approved Standard — Seventh Edition. M07-A7. 2006;26(2).
- Anitha TK, Rao MR, Shankaregowda R, Mahale RP, Sowmya GS, Chitharagi VB. Evaluation of Vancomycin Minimum Inhibitory Concentration in the clinical isolates of Methicillin Resistant Staphylococcus aureus (MRSA). J Pure Appl Microbiol. 2019;13(3):1797-1801.
Crossref - Wilcox M, Al-Obeid S, Gales A, et al. Reporting elevated vancomycin minimum inhibitory concentration in methicillin-resistant Staphylococcus aureus: Consensus by an International Working Group. Future Microbiol. 2019;14(4):345-352.
Crossref - Gould IM. Is vancomycin redundant for serious staphylococcal infection? Int J Antimicrob Agents. 2010;36(Suppl 2):S55-S57.
Crossref - Moise PA, North D, Steenbergen JN, Sakoulas G. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect Dis. 2009;9(10):617-624.
Crossref - Sader HS, Fey PD, Fish DN, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother. 2009;53(10):4127-32. doi: 10.1128/AAC.00616-09. Erratum in: Antimicrob Agents Chemother. 2010;54(3):1383. Fish, Douglas N [corrected to Madinger, Nancy].
Crossref - Edwards B, Milne K, Lawes T, Cook I, Robb A, Gould M. Is vancomycin MIC “creep” method dependent? Analysis of methicillin-resistant Staphylococcus aureus susceptibility trends in blood isolates from North East Scotland from 2006 to 2010. J Clin Microbiol. 2012;50(2):318-325.
Crossref - Sharma R, Hammerschlag MR. Treatment of MethicillinResistant Staphylococcus aureus (MRSA) Infections in Children: a Reappraisal of Vancomycin. Curr Infect Dis Rep. 2019;21(10):37.
Crossref - Liu C, Bayer A, Cosgrove SE, et al. Clinical Practice Guidlines by the Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. doi: 10.1093/cid/ciq146. Erratum in: Clin Infect Dis. 2011;53(3):319.
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111. doi: 10.1093/cid/ciw353. Erratum in: Clin Infect Dis. 2017;64(9):1298. Erratum in: Clin Infect Dis. 2017;65(8):1435. Erratum in: Clin Infect Dis. 2017;65(12):2161.
- Kehrmann J, Kaase M, Szabados F et al. Vancomycin MIC creep in MRSA blood culture isolates from Germany: A regional problem? Eur J Clin Microbiol Infect Dis. 2011;30(5):677-683.
Crossref - Kumara J, Shenoy S, Mahaprabha C, Katara V, Bhat KG. In vitro activity of vancomycin and daptomycin against healthcare-associated methicillin-resistant staphylococcus aureus isolated from clinical specimens. Asian J Pharm Clin Res. 2016;9(3):44-46.
- Lohan K, Sangwan J, Mane P, Lathwal S. Prevalence pattern of MRSA from a rural medical college of North India: A cause of concern. J Family Med Prim Care. 2021;10(2):752-757.
Crossref - Mallick SK, Basak S. MRSA-too many hurdles to overcome: a study from Central India. Trop Doct. 2010;40(2):108-110.
Crossref - Alos JI, Garcia-Canas A, Garcia-Hierro P, Rodriguez-Salvanes F. Vancomycin MICs did not creep in Staphylococcus aureus isolates from 2002 to 2006 in a setting with low vancomycin usage. J Antimicrob Chemother. 2008;62(4):773-775.
Crossref - Arshad F, Saleem S, Jahan S, Tahil R. Assessment of Vancomycin MIC Creep Phenomenon in Methicillin-Resistant Staphylococcus aureus isolates in a Tertiary Care Hospital of Lahore. Pak J Med Sci. 2020;36(7):1505-1510.
Crossref - Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J Antimicrob Chemother. 2007;60(4):788-94.
Crossref - Husain A, Rawat V, Umesh, Kumar M, Kumar M, Verma PK. Vancomycin, linezolid and daptomycin susceptibility pattern among clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) from Sub- Himalyan Center. J Lab Physicians. 2018;10(2):145-148.
Crossref - Maclayton DO, Suda KJ, Coval KA, York CB, Garey KW. Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 microg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin Ther. 2006;28(8):1208-1216.
Crossref - Niveditha N, Sujatha S. Worrisome trends in rising minimum inhibitory concentration values of antibiotics against methicillin resistant Staphylococcus aureus – Insights from a tertiary care center, South India. Braz J Infect Dis. 2015;19(6):585-589.
Crossref - Dhand A, Sakoulas G. Reduced vancomycin susceptibility among clinical Staphylococcus aureus isolates (‘the MIC Creep’): implications for therapy. F1000 Med Rep. 2012;4:4.
Crossref - Chang W, Ma X, Gao P, Lv X, Lu H, Chen F. Vancomycin MIC creep in methicillin-resistant Staphylococcus aureus (MRSA) isolates from 2006 to 2010 in a hospital in China. Indian J Med Microbiol. 2015;33(2):262-266.
Crossref - Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44(11):3883-3886.
Crossref - Diaz R, Afreixo V, Ramalheira E, Rodrigues C, Gago B. Evaluation of vancomycin MIC creep in methicillin-resistant Staphylococcus aureus infections-a systematic review and meta-analysis. Clin Microbiol Infect. 2018;24(2):97-104.
Crossref - Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY Antimicrobial Surveillance Program. Open Forum Infect Dis. 2019;6(Suppl 1):S47-S53.
Crossref - Joana S, Pedro P, Elsa G, Filomena M. Is vancomycin MIC creep a worldwide phenomenon? Assessment of S. aureus vancomycin MIC in a tertiary university hospital. BMC Res Notes. 2013;6:65.
Crossref - Haas K, Meyer-Buehn M, von Both U, Hubner J, Schober T. Decrease in vancomycin mics and prevalence of hgisa in MRSA and MSSA isolates from a German pediatric tertiary care center. Infection. 2023;51(3):583-588.
Crossref - Lu C, Guo Y, Wang S, et al. Decreased Vancomycin mics among Methicillin-Resistant Staphylococcus aureus Clinical Isolates at a Chinese Tertiary Hospital over a 12-year Period. Front Microbiol. 2016;7:1714.
Crossref - Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52(9):3315-3320.
Crossref - Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23(1):99-139.
Crossref - Choi EY, Huh JW, Lim CM, et al. Relationship between the MIC of vancomycin and clinical outcome in patients with MRSA nosocomial pneumonia. Intensive Care Med. 2010;37(4):639-647.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.