ISSN: 0973-7510
E-ISSN: 2581-690X
Mangroves are one of the world’s most unique tropical coastal ecosystems. They are a rich repository of biological wealth, including specially adapted flora and fauna. The microbiome component of this ecosystem is a fascinating world that is yet to be fully explored for its functional and ecological inter-relationships with its hosts. The mangrove ecosystem is a hidden treasure of microbial diversity, without which mangrove biology is incomplete. In the present study, the isolation of a cellulase-producing, endophytic Bacillus sp. from the hypocotyl region of viviparous seedlings is described. This study urges us to look into the microbial diversity of mangrove propagules, by presenting a glimpse of a member of the endospheric microbiome of viviparous hypocotyls.
Mangrove, Rhizophora, Endophyte, Vivipary, Bacillus
Mangroves are a unique, tropical coastal ecosystem, mostly comprising salt-tolerant tree species.1-3 These plant species have unique morphological and physiological adaptations, which enable them to encounter harsh conditions of high salinity and pH range of 4-10.4-6 Mangrove biology has always remained an intriguing subject and currently, there is a resurgence in its studies. 7-14 Microbiome component, especially endophytes of ‘mangrove holobiont’,15,16 is one such area that needs further investigations to unveil the functional relationship between endophytes and their host.17-22 The term ‘holobiont’ is comprehended as a ‘single dynamic entity’, formed by the functional interaction between the macroorganism host and its associated microbes.23-26 In the case of mangroves, the interactions between the microbes and its mangrove host, forming a single dynamic entity can be apprehended as ‘mangrove holobiont’. Endophytes like Bacillus amyloliquefaciens, Bacillus subtilis, Gordonea terrae, Trichoderma harzianum, Piriformospora indica etc are non-pathogenic intra or intercellular, microbial residents of host plants.27-31 They are found to be ubiquitous, sharing an ecological niche similar to that of plant pathogens, and often, they enter into a mutualistic relationship with host plants, contributing to the overall host fitness.32-37
Rhizophora mucronata Lam. is a mangrove tree species, belonging to the family Rhizophoraceae. Members of this family, commonly called ‘red mangroves’, are one of the prominent mangrove taxa possessing several adaptive features common to mangroves including the viviparous type of seed germination.38-41 Vivipary is an important developmental phenomenon seen associated with the mangrove tree species.39,40 Here, the seeds germinate while still attached to the mother plant and later, seedlings detach and drop into the muddy environment where it grows further into an adult plant, overcoming extreme pH and salinity.40, 42 Exploration of the microbiome of these seedlings will help us to identify and understand the role of microorganisms in the establishment and growth of mangroves, and further, it will shed light on mangrove biology and its survival. In the present study, attached viviparous seedling hypocotyls of Rhizophora mucronata are explored for the presence of culturable endophytes.
Study site and collection of plant samples
Viviparous seedling hypocotyls of R. mucronata, in the attached condition, were cut from the plant and collected in polyethylene bags. They were collected from multiple mangrove sites at Koduvally (11°767’N, 75°482’W) and Ozhayilbhagam, Dharmadam (11°46’30.14″ N 75°28’25.5″ E) located near Thalassery, Kannur, Kerala, India.
Isolation of pure culture
The samples were cleaned thoroughly using 5% teepol solution, followed by surface sterilisation using 70% ethanol for 1 min and treatment with 5% sodium hypochlorite for 3 min.43,44 After surface sterilisation, the samples were washed three times using sterile distilled water and air-dried. Afterwards, the seedlings were cut into small pieces of 1-2 cm in length. The samples were split open longitudinally and the outer green-coloured epidermal portion was removed. Inner ‘core’ parts were placed on Luria-Bertani agar medium (Titan Biotech, Kerala, India) and incubated for 1 to 2 days at room temperature along with a control plate with sterile water.
Biochemical characterisation of endophytes
The following tests were carried out to characterise the biochemical properties of the bacterial isolates.
a) Test for Indole acetic acid (IAA) production
The bacterial isolates were cultured in Luria-Bertani broth containing L-tryptophan (1 µg/ml) for 24 h.45,46 Culture supernatant was collected by centrifugation (8000 rpm; 10 min) and 1 ml of it was taken and mixed with freshly prepared Salkowsky reagent (2 ml). The reaction mixture was incubated at 28°C for 25-30 min., and colour change noticed.
b) Cellulase activity
Fresh cultures of the bacterial isolates were spot inoculated on nutrient agar containing carboxy methyl cellulose (0.2%) and incubated at 30°C for 3-4 days.47 After the incubation, the culture plates were flooded with Congo-red stain solution (1 µg/ml) for 10-15 min., and washed with NaCl (1 M).
c) Starch hydrolysis
Nutrient agar medium containing soluble starch (0.3%) was inoculated with freshly grown bacterial cultures and incubated for 2-3 days at 30°C. The culture plates were stained with Gram’s iodine by flooding.44
d) Production of ammonia
For the detection of ammonia production, the bacterial cultures were grown in nutrient broth (30°C for 24-48 h) in a rotary shaker (Remi Instruments, India). After the incubation, Nessler’s reagent (0.5 ml) was added to each tube and colour development is noticed.46
e) Catalase activity
Fresh bacterial cultures were used to inoculate Yeast extract tryptone broth tubes and incubated for 3 days at 30°C. For testing catalase activity a few drops of 3% H2O2 were added to both cultures.48
f) Gelatin hydrolysis
Freshly grown bacteria was inoculated on nutrient gelatin medium and incubated for 3 days at 30°C along with control tubes. These tubes were then placed in ice to test the gelatinase activity.49,50
g) Phosphate solubilisation
To detect phosphate solubilisation activity, freshly grown bacterial cultures were spot inoculated on Pikovskaya agar medium, and incubated at 30°C for 1-8 days.51
Molecular analysis of 16S rRNA region of bacterial isolates
a) Amplification of 16S rRNA
Forward primer 5’-CCGAATTCG TCGACAACAGAGTTTGACCCTGGTTCAG-3′; and Reverse primer 5’-CCCGGGATCCAAGCTTAC GGCTACCTTGTTACGACTT-3’ was used to amplify the 16S rRNA gene from the microbe under the following PCR conditions: 98°C – 2 min; 30 cycles of 98°C – 30 sec; 55°C – 30 sec; 72°C – 1 min and Final extension, 72°C – 10 min.
The amplified products were analysed on 0.8% EtBr-Agarose gel.
b) Sequencing of PCR product
The PCR product were purified and subjected to direct sequencing in an automated sequencing machine (ABI prism, Applied biosystems, CA,USA). Forward primer given above was used for the sequencing purpose.
c) BLAST analysis of sequences
Good quality sequences were subjected to BLAST analysis (https://blast.ncbi.nlm.nih.gov). The sequences were searched against nucleotide database of NCBI to identify the bacterial strain.
d) Phylogenetic analysis
Phylogenetic analysis of the current bacterial isolate were carried out based on the 16SrRNA sequences using the software MEGA version 11.52 Neighbour- Joining Method was used to generate the phylogenetic tree (cladogram).
Isolation and characterization of endophytes from plant tissues
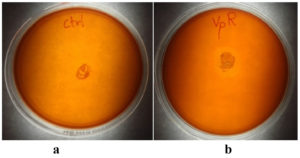
Out of the several bacterial colonies obtained, the bacterial colony which is found fast growing and emerging directly out of the tissues was selected and named as VpR. It was found to have rod-shaped morphology when observed under a microscope. The culture was positive for Gram’s stain reaction and also for biochemical tests like cellulase, catalase and ammonia production. The culture did not show any prominent hydrolytic activity for starch or gelatin. Tests for IAA production and Phosphate solubilisation were also negative. A summary of morphological and biochemical characterization is shown in Table. Figure 1 shows the results of the biochemical test for cellulase activity.
Table
Results of morphological and biochemical characterization of bacterial endophytes isolated from Rizhophora mucronata. VpR- Viviparous seedling hypocotyl inner core tissue
No. |
Reaction |
VpR |
|---|---|---|
1. |
Gram staining |
+ |
2. |
Cell morphology |
Rod |
3. |
Cellulase activity |
+ |
4. |
Starch hydrolysis |
– |
5. |
Catalase activity |
+ |
6. |
Gelatine hydrolysis |
– |
7. |
Test for IAA production |
– |
8. |
Production of ammonia |
+ |
9. |
Phosphate solubilisation |
– |
16S rRNA amplicon sequence analysis of bacterial isolates
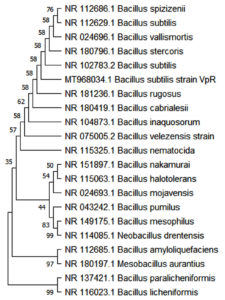
Molecular analysis of bacterial isolates was carried out based on the 16S rRNA region. 16S rRNA region of the bacterial culture samples was amplified and separated on 0.8% EtBr-Agarose gel. Sequencing of the purified PCR products was carried out in ABI prism automated sequencer. The 16S rRNA sequence showed maximum homology to that of Bacillus subtilis. The sequence obtained was deposited in GenBank: MT968034 (VpR). Result of the phylogenetic analysis is shown as a bootstrap consensus tree in Figure 2. The evolutionary relatedness of the present bacterial isolate with other bacterial groups can be inferred from this cladogram.
Figure 1. Cellulase activity by endophytic isolates from Rhizophora mucronata Lam.
a) Control plate (coli), b) VpR. The ‘clear zone’ seen around the colony is due to the cellulolytic activity of bacteria
The microbiome of mangroves is an underexplored ecological as well as functional component of ‘mangrove holobiont’.15 Several studies have highlighted the importance of microbial associates of mangrove plants which includes both plant protection and plant growth promotion.53,54 Isolation of salt-tolerant microbes from the mangroves is an example to apprehend the role microbes in mangrove ecosystem which is a unique ecosystem characterized by salinity.8 They can facilitate the uptake of nutrients like Phosphorus, Nitrogen, etc. which is very much crucial for the plant growth. Many of the resident microbes are capable of producing growth promoting phytohormones like IAA (indole acetic acid) besides the production of hydrolytic enzymes like cellulases. They can also ‘prime’ the plant defense mechanism.55 Many bioactive compounds are isolated from mangrove endophytes which could also find applications in agriculture as well as in medical fields besides their role in mangrove ecosystem.8,54 In general, they are partners in mangrove biology rendering many ecosystem services including nutrient cycling.13,56-60
In the present study, isolation and identification of Bacillus subtilis from the core tissues of viviparous seedling hypocotyls of Rhizophora mucronata is described. The occurrence of Bacillus subtilis in the endosphere of the undetached viviparous seedling hypocotyl of R. mucronata points to the various possibilities that this endophyte could have in the lifecycle of its host. This particular bacterial culture showed cellulase, catalase and ammonia-producing activities. Ammonia production by endophytic bacteria is a feature that is generally considered to be plant growth promotion in nature,61 but, contrary views and opinions are also presented by researchers.62,63 From an ecological point of view, the feature of ammonia production by bacteria and its relevance to mangrove biology will be an interesting study to follow. Microbial cellulase activity is considered to contribute significantly to the carbon cycle at the ecosystem level.64,65 Catalase activity is generally considered as a cellular mechanism to mitigate abiotic stress, but the endophytic catalase activity observed here could be an attribute of the microbe to adapt itself to the endophytic lifestyle.66
In most cases, endophytes are known to impart plant-beneficial attributes. They are found to reduce the severity of stress encountered by the host plant.30-32, 61,67,68 Bacillus subtilis and other members of the group Bacillus, occur in diverse habitats including endophytic conditions where they contribute to the benefit of host plants, especially in adaptive features like salinity and drought resistance.69-71 Endophyte-mediated plant resistance has emerged as a successful alternative agriculture strategy in many instances. Various species of Bacillus, (Bacillus cereus, Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus tequilensis, etc.), in several cases, have been shown to be successful as biocontrol agents in crops.29,36,37,54,68,70, 72-74
The term ‘holobiont’ is conceptualized as ‘a single dynamic entity’ formed of the functionally related partners – the macroorganism host and its associated or interacting microbes.23-26 Endophytes of these salt-tolerant trees are important partners of the ‘holobiont’ without which the mangrove biology will be incomplete. Besides, the microbiome component of the mangrove ecosystem is a promising resource for novel microbes with hitherto unknown attributes or features with biotechnological and agricultural application potential.7, 75-79
ACKNOWLEDGMENTS
The authors would like to thank the University Grants Commission, Government of India, New Delhi, for financial assistance for this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by the University Grants Commission, Government of India, New Delhi – (MRP(S)-0661/13-14/KLKA011/UGC-SWRO).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Kathiresan K and Bingham BL. Biology of mangroves and mangrove ecosystems. Adv Mar Biol. 2001;40:81- 251.
Crossref - Kathiresan K. Mangrove Forests of India. Curr Sci. 2018;114(05):976.
Crossref - Friess DA, Rogers K, Lovelock CE, et al. The State of the World’s Mangrove Forests: Past, Present, and Future. Ann Rev Environ Res. 2019;44(1):89-115.
Crossref - Kathiresan, K, Moorthy P, Ravikumar S. A Note on the influence of Salinity and pH on Rooting of Rhizophora mucronata Lamk. Seedlings. Indian Forester. 1996;122(8):763-764.
- Ball MC. Ecophysiology of mangroves. Trees. 1988;2:129-142.
Crossref - Parida AK, Jha B. Salt tolerance mechanisms In mangroves: a review. Trees. 2010; 24(2):199-217.
Crossref - Zhou J, Diao X, Wang T, et al. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South China Sea. PloS One. 2018;13(6):e0197359.
Crossref - Kathiresan K. Salt-tolerant Microbes in Mangroves: Ecological Role and Bioprospecting Potential. Dagar J, Yadav R, Sharma P. (eds). Research Developments in Saline Agriculture. 2019:237-255.
Crossref - Venil,CK, Usha R, Devi PR. Biotechnological potential of microbial pigments from mangrove ecosystems: a review. Patra JK, Mishra RR, Thatoi H (Eds.). Biotechnological Utilization of Mangrove Resources. 2020;13:303-313.
Crossref - Liu X, Yang C, Yu X, et al. Revealing structure and assembly for rhizophyte-endophyte diazotrophic community in mangrove ecosystem after introduced Sonneratia apetala and Laguncularia racemosa. Sci Total Environ. 2020;721:137807-137807.
Crossref - Dhal PK, Kopprio GA, Gardes A. Insights on aquatic microbiome of the Indian Sundarbans mangrove areas. PLOS ONE. 2020;15(2):e0221543.
Crossref - Segaran TC, Azra MN, Lananan F, et al. Mapping the link between climate change and mangrove forest: A global overview of the literature. Forests. 2023;18;14(2):421.
Crossref - Scherer BP, Mason OU, Mast AR. Bacterial communities vary across populations and tissue type in red mangroves (Rhizophora mangle, Rhizophoraceae) along an expanding front. FEMS Microbiol Ecol. 2022;98(12):fiac139.
Crossref - Anu K, Henna PK, Sneha VK, Busheera P, Muhammed J, Augustine A. Mangroves in environmental engineering: Harnessing the multifunctional potential of Nature’s coastal architects for sustainable ecosystem management. Results in Engineering. 2024;21:101765.
Crossref - Sanchez-Canizares C, Jorrin B, Poole PS, Tkacz A. Understanding the holobiont: the interdependence of plants and their microbiome. Curr Opin Microbiol. 2017;38:188-196.
Crossref - Hassani MA, Duran P, Hacquard S. Microbial interactions within the plant holobiont. Microbiome. 2018;6(1):58.
Crossref - Purahong W, Sadubsarn D, Tanunchai B, et al. First Insights into the Microbiome ofa Mangrove Tree Reveal Significant Differences in Taxonomic andFunctional Composition among Plant and Soil Compartments. Microorganisms. 2019;7(12):585.
Crossref - Allard SM, Costa MT, Bulseco AN, et al. Introducing the Mangrove Microbiome Initiative: Identifying Microbial Research Priorities and Approaches To Better Understand, Protect, and Rehabilitate Mangrove Ecosystems. mSystems. 2020;5(5):e00658-20.
Crossref - Inoue T, Shimono A, Akaji Y, Baba S, Takenaka A, Chan HT. Mangrove-diazotroph relationships at the root, tree and forest scales: diazotrophic communities create high soil nitrogenase activities in Rhizophora stylosa rhizospheres. Ann Bot. 2020;125(1):131-144.
Crossref - Yao H, Sun X, He C, Li XC, Guo LD. Host identity is more important in structuring bacterial epiphytes than endophytes in a tropical mangrove forest. FEMS Microbiol Ecol. 2020;96(4):fiaa038.
Crossref - de Carvalho FM, Laux M, Ciapina LP, et al. Finding microbial composition and biological processes as predictive signature to access the ongoing status of mangrove preservation. Int Microbiol. 2024:1-16.
Crossref - Ghose M, Parab AS, Manohar CS, Mohanan D, Toraskar A. Unraveling the role of bacterial communities in mangrove habitats under the urban influence, using a next-generation sequencing approach. J Sea Res. 2024;198:102469.
Crossref - Vandenkoornhuyse P, Quaiser A, Duhamel M, Le Van A, Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 201;206(4):1196-1206.
Crossref - Mesny F, Hacquard S, Thomma BP. Co-evolution within the plant holobiont drives host performance. EMBO Reports. 2023;6;24(9):e57455.
Crossref - Li JH, Aslam MM, Gao YY, et al. Microbiome-mediated signal transduction within the plant holobiont. Trends Microbiol. 2023;31(6):616-628.
Crossref - Berg G, Dorador C, Egamberdieva D, Kostka JE, Ryu CM, Wassermann B. Shared governance in the plant holobiont and implications for one health. FEMS Microbiol Ecol. 2024;100(3):fiae004.
Crossref - Stone JK, Bacon CW and White JF. An overview of endophytic microbes: endophytism defined. Bacon CW, White JF (Eds.). Microbial endophytes. Marceldekker Inc., New York. 2000;3:29-33.
- Bacon CW, White JF. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis. 2016;68(1-3):87-98.
Crossref - Omomowo OI, Babalola OO. Bacterial and fungal endophytes: tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms. 2019;7(11):481.
Crossref - Guha T, Biswas SM. Recent progress in the role of seed endophytic bacteria as plant growth-promoting microorganisms and biocontrol agents. World J Microbiol Biotechnol. 2024;40(7):218.
Crossref - Sena L, Mica E, Vale G, Vaccino P, Pecchioni N. Exploring the potential of endophyte-plant interactions for improving crop sustainable yields in a changing climate. Front Plant Sci. 2024;20;15:1349401.
Crossref - Lata R, Chowdhury S, Gond SK, White JF. Induction of abiotic stress tolerance inplants by endophytic microbes. Lett Appl Microbiol. 2018;66(4):268-276.
Crossref - Fan D, Subramanian S, Smith DL. Plant endophytes promote growth and alleviate salt stress in Arabidopsis thaliana. Sci Rep. 2020;10(1):12740.
Crossref - Abdelshafy Mohamad OA, Ma JB, Liu YH, et al. Beneficial Endophytic Bacterial Populations Associated With Medicinal Plant Thymus vulgaris Alleviate Salt Stress and Confer Resistance to Fusarium oxysporum. Front Plant Sci. 2020;11:47.
Crossref - Singh DP, Singh V, Gupta VK, et al. Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci Rep. 2020;10(1):4818.
Crossref - Kumar N, Dubey RC. Plant Growth Promoting Endophytic Bacteria Bacillus australimaris BLR41 and Enterobacter kobei BLR45 Enhance the Growth of Medicinal Plant Barleria lupulina Lindl. J Pure Appl Microbiol. 2022;16(4):2647-2658.
Crossref - Philip I, Sarojini S, Biswas S, Jayaram S. Exploring the Potential of Bacillus velezensis, an Endophytic Bacteria Isolated from Alternanthera philoxeroides for Plant Growth Promotion and Bioremediation Properties. J Pure Appl Microbiol. 2023;17(3):1748-1763.
Crossref - Hogarth PJ. The biology of mangroves. Oxford University Press (OUP). 1999. Oxford. ISBN 0-19-850222-2.
- Tomlinson PB, Cox PA. Systematic and functional anatomy of seedlings in mangrove Rhizophoraceae: vivipary explained? Bot J Linn Soc. 2000;134(1-2):215- 231.
Crossref - Robert EMR, Oste J, Van der Stocken T, et al. Viviparous mangrove propagules of Ceriops tagal and Rhizophora mucronata, where both Rhizophoraceae show different dispersal and establishment strategies. J Exp Mar Biol Ecol. 2015;468:45-54.
Crossref - Ankure S, Tah M, Mondal S, Murmu AK, Naskar S. Adaptive evolution of leaf anatomical features in mangrove Rhizophoraceae cues differential strategies of salt tolerance. Flora. 2023;1;300:152225.
Crossref - Das S, Ghose M. Seed structure and germination pattern of some Indian mangroves with taxonomic relevance. Taiwania 2003;48(4):287-298.
- Sun L, Lu Z, Bie X, et al. Isolation and characterization of a co-producer of fengycins and surfactins, endophytic Bacillus amyloliquefaciens ES-2, from Scutellaria baicalensis Georgi. World J Microbiol Biotechnol. 2006;22 :1259-1266.
Crossref - Gayathri S, Saravanan D, Radhakrishnan M, Balagurunathan R, Kathiresan K. Bioprospecting potential of fast growing endophytic bacteria from leaves of mangrove and salt-marsh plant species. Ind J Biotech. 2010;9(4):397-402.
- Gordon SA, Weber RP. Colorimetric estimation of Indole acetic acid. Plant Physiol, 1951;26(1):192-195.
Crossref - Gang S, Sharma S, Saraf M, Buck M, Schumacher J. Analysis of Indole-3-acetic Acid (IAA) Production in Klebsiella by LC-MS/MS and the Salkowski Method. Bio Protoc. 2019;9(9):e3230.
Crossref - Cho KM, Hong SY, Lee S M, et al. Endophytic bacterial communities in Ginseng and their antifungal activity against pathogens. Microb Ecol. 2007;54(2):341-351.
Crossref - Reiner K. Catalase test protocol. Am Soc Microbiol. 2010. https://asm.org/getattachment/72a871fc-ba92- 4128-a194-6f1bab5c3ab7/Catalase-Test-Protocol.pdf)
- De Clerck E, Vanhoutte T, Hebb T, Geerinck J, Devos J, DeVos P. Isolation, characterization, and identification of bacterial contaminants in semifinal gelatin extracts. Appl Environ Microbiol. 2004;70(6):3664-3672.
Crossref - Harley JP. Laboratory exercises in microbiology, 6th ed. McGraw-Hill Companies, Inc., New York, USA. 2005
- Pandey A, Das N, Kumar B, Rinu K ,Trivedi P. Phosphate solubilization by Penicillium spp. isolated from soil sample of Indian Himalayan region. World J Microbiol Biotechnol.2008;24(1):97-102.
Crossref - Tamura K, Stecher G, Kumar S, MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. 2021;38(7):3022-3027.
Crossref - Palit K, Rath S, Chatterjee S, Das S. Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: Threats, vulnerability, and adaptations. Environ Sci Poll Res Int. 2022;29(22):32467- 32512.
Crossref - Wainwright BJ, Millar T, Bowen L, et al. The core mangrove microbiome reveals shared taxa potentially involved in nutrient cycling and promoting host survival. Environ Microbiome. 2023;1;18(1):47.
Crossref - Conrath U, Beckers GJM, Flors V, et al. Priming: getting ready for battle. Mol Plant Microbe Interact. 2006;19(10):1062-1071.
Crossref - Basak P, Majumder NS, Nag S, et al. Spatiotemporal Analysis of Bacterial Diversity in Sediments of Sundarbans Using Parallel 16S rRNA Gene Tag Sequencing. Microb Ecol. 2014;69(3):500-511.
Crossref - Pylro VS, Roesch LFW, Ortega JM, et al. Brazilian Microbiome Project: Revealing the Unexplored Microbial Diversity-Challenges and Prospects. Microbial Ecol. 2013;67(2):237-241.
Crossref - Singh G, Chauhan R, Ranjan RK, Prasad MB, Ramanathan Al. Phosphorus dynamics in mangroves of India. Curr Sci. 2015;108(10):1874-1881.
- Shiau YJ, Chiu CY. Biogeochemical Processes of C and N in the Soil of Mangrove Forest Ecosystems. Forests. 2020;11(5):492.
Crossref - Abdellatif MM, Arafat HH. Endophytic Microbial Diversity, Heavy Metal Accumulation, and Antimicrobial Properties of Avicennia marina from Saudi Arabia. J Pure Appl Microbiol. 2024;18(2):995-1003.
Crossref - Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda Ma, Glick BR. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92-99.
Crossref - Weise T, Kai M, Piechulla B. Bacterial Ammonia Causes Significant Plant Growth Inhibition. Bornke F, ed. PLoS ONE. 2013;8(5):e63538.
Crossref - Avalos M, Garbeva P, Raaijmakers JM, van Wezel GP. Production of ammonia as a low-cost and long-distance antibiotic strategy by Streptomyces species. ISME J. 2020;14(2):569-583.
Crossref - Leschine SB. Cellulose Degradation in Anaerobic Environments. Ann Rev Microbiol. 1995;49(1):399 426.
Crossref - Behera P, Mohapatra M, Kim JY, Adhya TK, Pattnaik AK, Rastogi G. Spatial and temporal heterogeneity in the structure and function of sediment bacterial communities of a tropical mangrove forest. Environ Sci Pollut Res. 2018;26(4):3893-3908.
Crossref - Walitang DI, Kim K, Madhaiyan M, Kim YK, Kang Y, Sa T. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of Rice. BMC Microbiol. 2017;17(1):209.
Crossref - Ullah A, Nisar M, Ali H, et al. Drought tolerance improvement in plants: anendophytic bacterial approach. Appl Microbiol Biotechnol. 2019;103(18):7385-7397.
Crossref - White JF, Kingsley KL, Zhang Q, et al. Review: Endophytic microbes and their potential applications in crop management. Pest Manag Sci. 2019;75(10):2558- 2565.
Crossref - Radhakrishnan R, Hashem A, Abd_Allah EF. Bacillus: A Biological Tool for Crop Improvement through Bio- Molecular Changes in Adverse Environments. Front Physiol. 2017;8:667.
Crossref - Fira D, Dimkic I, Beric T, Lozo J, Stankovic S. Biological control of plant pathogens by Bacillus species. J Biotechnol. 2018;285:44-55.
Crossref - Lopes R, Tsui S, Goncalves PJRO, de Queiroz MV. A look into a multifunctional toolbox: endophytic Bacillus species provide broad and underexploited benefits for plants. World J Microbiol Biotechnol. 2018;34(7):94.
Crossref - Ahmed A, He P, He Y, et al. Biocontrol of plant pathogens in omics era-With special focus on endophytic bacilli. Crit Rev Biotechnol. 2024;18;44(4):562-580.
Crossref - Hazarika DJ, Goswami G, Gautom T, et al. Lipopeptide mediated biocontrol activityof endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol. 2019;19(1):71.
Crossref - Melnick RL, Zidack NK, Bailey BA, Maximova SN, Guiltinan M, Backman PA. Bacterial endophytes: Bacillus spp. from annual crops as potential biological control agents of black pod rot of cacao. Biological Control. 2008;46(1):46-56.
Crossref - Thatoi H, Behera BC, Mishra RR, Dutta SK. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Ann Microbiol. 2013;63(1):1-19.
Crossref - Xu J. Bioactive natural products derived from mangrove -associated microbes. RSC Adv. 2015;5(2):841-892.
- Hao L, Wang Y, Chen X, et al. Exploring the potential of natural products from mangrove Rhizosphere bacteria as biopesticides against plant diseases. Plant Dis. 2019;103(11):2925-2932.
Crossref - Das S, Sinha N, Sen M, Ghosh D. Isolation and Screening of Dye Degrading Lignocellulolytic Bacteria from Sundarban Mangrove Ecosystem, West Bengal, India. J Pure Appl Microbiol. 2023;17(1):609-626.
Crossref - Dey G, Maity JP, Banerjee P, et al. Characterization of halotolerant phosphate-solubilizing rhizospheric bacteria from mangrove (Avicennia sp.) with biotechnological potential in agriculture and pollution mitigation. Biocatal Agric Biotechnol. 2024;55:102960.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.