Nipah virus (NiV) continues to remain a significant threat to health of the public, characterized by its ability to cause respiratory and neurological complications. The World Health Organization (WHO) has identified NiV as a priority disease for its R and D Blueprint. However, despite extensive research efforts, specific antiviral therapies for NiV infection are currently unavailable. This highlights the crucial need to focus on supportive care for patients affected by the NiV. Therapies Prompt medical attention, including mechanical ventilation and intensive care, is crucial in case of NiV infection. Preventive measures such as avoiding consumption of raw date palm sap and implementing control of infection practices, gives a major role in halting the spread of NiV. This review provides a comprehensive overview of NiV, including its unique characteristics, clinical manifestations, diagnostic methods, treatment strategies, and preventive measures. The article also provides details on vaccines currently undergoing clinical trials, including ChAdOx1, PHV02, mRNA-1215, HeV-sG-V, and CD40.NiV. In addition, it highlights that the m102.4 monoclonal antibody and nucleotide analogue remdesivir has shown effective in the Non-Human Primate (NHP) model was also reviewed.
Nipah Virus, Neurological, Respiratory, Preventive Measure, Treatment
Nipah Virus (NiV) stands as a formidable threat, characterized by its high pathogenicity, zoonotic nature and for its severe outbreaks. It is originated from Paramyxoviridae family, specifically the henipavirus genus, NiV represents the biosafety level-4 (BSL-4) pathogen.1,2
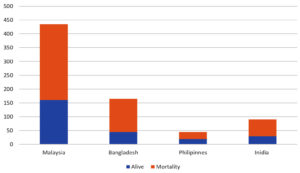
The name Nipah, given after its initial outbreak in a Malaysian town in 1998-1999, has caused over 250 instances of febrile encephalitis among agriculture and slaughterhouse workers, resulting in significant economic disruption and public concern.3 Although no new outbreaks have surfaced in Malaysia, the virus remains a danger, especially in Bangladesh and India. Graphically elaborated in Figure 1. The current outbreak in Kerala in May 2018, which included over 2000 recorded cases, highlights the ongoing threat posed by this new virus.4,5 The Nipah virus’s proclivity for person-to-person transmission, wide species tropism, many viable mechanisms of transmission, high mortality rate, and confirmed incidences of healthcare professional infection during outbreaks have perplexed the medical world. Despite continued efforts to solve its secrets, much is still unknown about the virus and its clinical implications.6-8
The purpose of this article is to enlighten these challenges, the article aims to elucidate the virology, molecular characteristics, pathogenesis, clinical manifestations, epidemiology, and diagnostic methods of NiV. By synthesizing existing literature and analyzing recent advancements in NiV research, this review seeks to address knowledge gaps and inform future strategies for NiV surveillance, prevention, and control. Furthermore, by highlighting the ongoing threat of NiV and the need for collaborative efforts in combating this viral pathogen, this review aims to underscore the urgency of sustained investment in NiV research and preparedness initiatives.
Virology and molecular characteristics of Nipah virus
NiV member of the Paramyxoviridae family, specifically with in the Henipavirus genus, and is classed as a Biosafety Level-4 (BSL-4) pathogen due to its high pathogenicity and the absence of viable therapy or vaccine.9,10 Distinct from typical paramyxoviruses, NiV presents subtle yet notable differences in its composition, including reticular cytoplasmic inclusions near the endoplasmic reticulum and a larger size.11 The strong cross-reactivity observed in serological testing between NiV and the Hendra virus (HeV) underscores their classification as two of the three Henipaviruses, despite their relatively minor ultrastructural disparities.12,13 The NiV is characterized by having a (-ve)-sense, non-segmented, single-stranded, enveloped RNA virus with helical symmetry, containing approximately 18.2 kb of genetic material. Its RNA genome comprises six genes arranged in a 3’-5’ orientation, encoding the phosphoprotein, matrix, glycoprotein fusion, nucleocapsid, and long polymerase. The nucleocapsid, phosphoprotein, and long polymerase collectively form the virus ribonucleoprotein, while the fusion glycoprotein and attachment glycoprotein play major role in virus entry into the host cells. Interaction between viral receptor on the host cell and NiV glycoprotein triggers the activation of the fusion glycoprotein, leading to membrane fusion. Upon activation, the fusion glycoprotein is cleaved into two subunits, F1 fusion glycoprotein and F2 fusion glycoprotein, with the F1 fusion glycoprotein subunit driving the fusion of viral and host cell membrane for virus entry. The attachment glycoprotein facilitates binding to host receptor present in the surface of the cell, while the fusion glycoprotein mediates membrane fusion, allowing viral entry into host cells. Studies have shown that binding of monomeric viral receptor ephrin B2 induce allosteric changes in NiV glycoprotein, promoting its activation and facilitating receptor-activated virus entry into cells of the host.14
Pathogenesis and clinical manifestations of Nipah virus infection
Pathogenesis
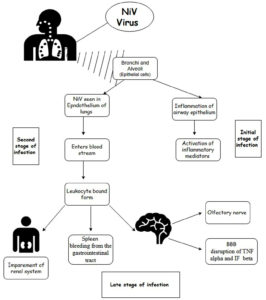
During the early stages of viral infection, NiV was diagnosed in the epidermal cells, particularly in the bronchioles and alveoli, as detected in animal models. Cytokines are released from swollen epithelial cells of the respiratory tract due to infection, leading to the development of acute respiratory distress syndrome (ARDS). Other inflammatory mediators, such as interleukins and granulocyte-colony stimulating factors, are also secreted in the later stages of infection. NiV enters the epithelial cells of the lungs from the respiratory tract epithelium. As the infection progresses, NiV enters the bloodstream and affects organs such as the respiratory tract, digestive system, excretory, and brain. The two major routes responsible for viral entry are the hematological route and olfactory nerve. In the hematological route, the virus entry disrupts the blood- brain barrier (BBB), leading to the activation of IL-1b and tumor necrosis factors, ultimately cribriform plate to the olfactory bulb.14,20-22
Clinical manifestation
Patients afflicted with Nipah virus encounter a spectrum of clinical manifestations, encompassing acute encephalitis, respiratory distress, and atypical pneumonia, often culminating in fatal outcomes. With an incubation period spanning 4 to 45 days, symptoms typically manifest 4 to 2 weeks after a clinically quiescent period. The onset of Nipah virus symptoms can range from overt to subtle, with varying rates of subclinical infections documented during epidemics in Malaysia, Singapore, Bangladesh, and India. Notably, asymptomatic infections were rare during the Bangladesh pandemic and infrequent in India. Research indicates that individuals with physical contact with Nipah-positive individuals but no exposure to bodily fluids have a diminished risk of subclinical infections, while latent infections may recur months or years after acute illness.23
Nipah virus infection affects multiple major organs, such as the heart, brain, lungs, kidneys, and spleen. Early symptoms mimic flu-like infections but can swiftly progress to an encephalitic syndrome characterized by severe neurological symptoms, along with fever and headache. Patients often exhibit reduced consciousness, convulsions, tachycardia, and prominent cerebellar symptoms, with severe brain stem dysfunction observed in over 50% of cases in Malaysia and a higher frequency of comparable symptoms during the Bangladesh outbreak. Respiratory complications, including colds, coughs, dyspnea, and acute respiratory distress syndrome, are prevalent, particularly in Bangladesh and India, attributed to differences in NiV strains and heightened human-to-human transmission. These insights illuminate the complex clinical profile and epidemiological dynamics of Nipah virus infection across affected regions.24
Nipah virus majorly infects the respiratory system or brain (central nervous system) and also show impact on spleen kidney and on heart.16
In respiratory system
- Symptoms include coughing, pneumonia, difficulty breathing, and leading to acute respiratory distress syndrome (ARDS).
- Initial signs resemble influenza: fever, headache, sore throat and muscle aches.
In nervous system
- Nipah virus induces brain swelling (encephalitis), seizures, and coma with 24 to 48 hours.
- Neurological symptoms such fever, headaches, drowsiness, confusion, seizures, and coma, often following initial flu-like symptoms.
- Brain inflammation occurs as the virus multiplies in brain cells, potentially causing nerve damage.
- Serious complications include meningitis and meningoencephalitis, involving inflammation of the brain and spinal cord.
- Survivors may face long-term neurological issues like cognitive impairment, motor deficits, and behavior disorders.
History and origin of Nipah virus
The discovery of NiV can be traced back to a widespread epidemic of encephalitis in Malaysia during 1998-1999, which resulting in approximately 265 instance of acute encephalitis and 105 deaths, nearly causing the demise of the billion-dollar pigs-farming industry.25,26
The outbreak in India over various years and the number of reported cases are discussed in Table 1. The virus emerged as a novel paramyxovirus and was named after the Sungai Nipah Village, where the initial outbreak occurred. In the latter parts of September 1998, cases of acute febrile encephalitis with a high death rate were observed in a pig farm in the suburb of Ipoh, Perak, in Peninsular Malaysia. Initially, the outbreak was mistaken for Japanese encephalitis, as four serum sample from 28 patients tested positive for this disease. The second epicenter of the outbreak was in Negri Sembilan, located 300 km south of Ipoh, which reported 89 deaths and 180 patients due to the important of pigs from Ipoh. In March 1999, Singapore reported 11 cases and 1 death among individuals who had imported pigs from Malaysia. Despite early management methods, such as anti-mosquito fogging and pig immunization against Japanese encephalitis, proving ineffective. Healthcare workers attending to the infected patients were initially convinced that the outbreak was not caused by Japanese encephalitis. However, in early March 1999, a medical virologist at the University of Malaya isolated the NiV from the cerebrospinal fluid of an epidemic victim in Sungai Nipah Village, revealing that the infection was caused by an agent previously unknown to science. The novel virus, like the Hendra virus (HeV), is now classified as a new genus within the Paramyxoviridae family.27,28
Table (1):
In India outbreaks cases of Nipah virus
Place of Outbreak |
No of Cases |
Year |
|---|---|---|
Siliguri15 |
66 |
First outbreak was reported in 2001, since then, 17 outbreaks has been documented until 2015. |
Nadia16 |
05 |
2007 |
Kerala17, 19 (Kozhikode) |
19 (89% mortality) |
2018 |
Since then, spontaneous occurrences of the viruses have been reported throughout Southeast Asia, notably in Bangladesh, India, Malaysia, and Singapore, posing a significant threat to public health due to its high mortality rate, person-to-person transmission capability, and lack of specific treatments or vaccines.29
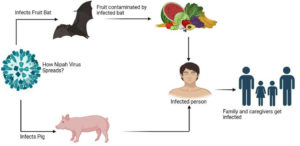
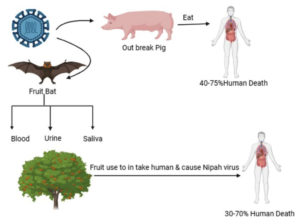
Research has revealed that fruit bats, specifically members of the Pteropus genus (genus of megabats), serve as the natural reservoir hosts for the NiV. Humans can contact the disease through direct contact with infected bats, consumptions of fruits contaminated by bat excretions, or contact with infected persons or animals. Pigs and other domestic animals act as intermediate hosts, facilitating the spread of the virus to humans. The virus can also be transmitted directly from bats to humans through saliva or excrements, particularly in areas where human activities encroach upon bat habitats.30 The detailed Pathophysiology was discussed in Figure 2. Once the NiV infects humans, it can lead to encephalitis, fever, headaches, respiratory ailments, and other severe symptoms, often resulting in high mortality rates. Limited instances of person-to-person transmission have been reported, typically within hospital environments. Epidemics of the NiV have occurred across Asia, with outbreaks frequently linked to contact with contaminated fruits or infected bats.31,32
Transmission route
The virus can be spread to humans via direct contact with infected animals, ingestion of contaminated food, or close association with Nipah virus-infected individuals.33 Diagrammatically elaborated in the Figure 3 and 4.
Figure 3. Defines the Nipah Virus as zoonotic, which refers to transmission from animals to humans32
Epidemiological variation
The epidemiology of NiV is characterized by its sporadic yet severe outbreaks primarily in Southeast Asia, with occasional cases reported in other regions. Understanding the epidemiology of NiV is essential for devising effective prevention and control strategies against NiV.34 The detailed epidemiology of NiV is discussed in Table 2 and Figure 1.
Table (2):
Epidemiology of NiV36,37
No. |
Country’s |
Reservoir Hosts |
Transmission Dynamics |
Cases Reported in each year |
No. of Deaths |
Seasonal patterns |
|---|---|---|---|---|---|---|
1 |
Malaysia |
Bat-bitten fruit |
Pig-to human |
265 |
105 |
Winter and spring months |
2 |
Singapore |
Bat-bitten fruit |
Pig- to human |
11 |
1 |
Winter and spring months |
3 |
Bangladesh |
Bat saliva contamination and excreta-contamination palm sap |
Bat-to human Human to human |
13 |
9 |
Winter and spring months |
4 |
India |
Bat-bitten fruit |
Bat-to human
Human to human |
66 |
45 |
Winter and spring months |
The initial incidence took place in Malaysia, where 265 cases and 105 deaths were reported. The virus then spread to Singapore in 1999 through transported pigs, resulting in 11 reported cases and fatalities.35
Since 2001, Bangladesh has experienced annual outbreaks, as detailed in Table 3. In India, outbreaks were reported in 2001 with 66 cases and 45 deaths, 2007 with 5 cases and fatalities, 2018 with 18 cases and 17 deaths, 2019 with 1 case and no death, and 2021 with 1 case and one death. According to a recent WHO report, six laboratory-confirmed and cases were confirmed in India between September 12 and 15, 2023.36,37
Table (3):
Number of cases reported in each year in Bangladesh38
No. |
Year |
Cases |
Deaths |
|---|---|---|---|
1 |
2001 |
13 |
9 |
2 |
2003 |
12 |
8 |
3 |
2004 |
67 |
50 |
4 |
2005 |
12 |
11 |
5 |
2007 |
18 |
9 |
6 |
2008 |
11 |
7 |
7 |
2009 |
4 |
1 |
8 |
2010 |
18 |
16 |
9 |
2011 |
43 |
37 |
10 |
2012 |
17 |
12 |
11 |
2013 |
31 |
25 |
12 |
2014 |
37 |
16 |
13 |
2015 |
15 |
11 |
14 |
2017 |
3 |
2 |
15 |
2018 |
4 |
3 |
16 |
2019 |
8 |
7 |
17 |
2020 |
7 |
5 |
18 |
2021 |
2 |
0 |
19 |
2022 |
3 |
2 |
20 |
2023 |
11 |
8 |
21 |
2024 |
2 |
0 |
Diagnostic method
Laboratory diagnosis involves the detection of Nipah virus RNA in clinical specimens, along with serological tests and immunohistochemistry for the confirmation of infection. A combination of clinical, laboratory, and epidemiological investigation is usually used in the diagnosis of Nipah virus. The following are some of the majorly used diagnostic techniques for identifying Nipah virus infections.
Clinical evaluation
While detecting the Nipah virus infection doctors determine through the clinical signs and symptoms. The symptoms include encephalitis, or inflammation of the brain resulting in altered mental status, fever, headache, respiratory symptoms (cough, difficulty breathing), seizures, and coma.39
Testing in a laboratory
Nucleic acid amplification testing
NAAT: the process requires 2-3 hours and target human and animal population. It is used for the detection of active viral infection and is highly sensitive.40
Polymerase Chain Reaction (PCR): PCR is assay which is used to carry out to identify the RNA (viral genetic material) in a clinical specimen, such as tissue, blood, CSF, and respiratory secretion. Nipah virus RNA can be detected quickly and accurately using PCR techniques.41 The most sensitive technique was considered was RT-PCR having the sensitivity ranging from 500- 1000 copies of RNA templates and the lowest range is 0.37 pg/µL of RNA.40
Serology
Blood samples can be examined to determine whether the body has produced antibodies in response to an infection with the Nipah virus. It directly detects the presence of NiV antigen, as well as IgM and IgG antibodies produced against NiV. Antibodies specific to the Nipah virus can be found via serological tests such as the enzyme-linked immunosorbent assay (ELISA). ELISA is carried out for about 3-4 hours and test for both human and animal.40
Virus isolation
Virus isolation includes the isolation of the virus from the clinical specimen like blood, tissue and respiratory secretion. This is carried out in the specialized lab settings with strict containment measures. The time required the processing is 5 days for cell culturing.42
Immunohistochemistry
Viral antigens in affected tissues can be found by employing immunohistochemical staining techniques to examine postmortem tissue samples taken during autopsies.39,41
Advanced methods
In this Nipah virus is isolated by using advanced molecular and sequencing method. These methods include molecular epidemiology to comprehend transmission dynamics, phylogenetic analysis to track the virus’s origin and spread, and genome sequencing to examine genetic diversity. In order to prevent the virus from spreading further, early diagnosis of Nipah virus infection is essential for timely medical management, infection control strategies, and public health initiatives. It’s crucial to remember that Nipah virus testing should only be done in specialized biosafety level 3 (BSL-3) laboratories that are outfitted with the security needed to handle extremely contagious pathogens.43
Challenges
The early PCR tests were primarily development for specific strains (NiV-B from Bangladesh-India or NiV from Malaysia-Singapore), indicating the potential value of a consensus-driven pan-NiV probe set. Further research on viral and immune kinetics is necessary to elucidate NiV pathogenesis in both blood and non-blood samples. This understanding would aid in identifying the optimal timing for intervention and improving monitoring of transmission and recovery.
In rural and remote NiV outbreak settings, deploying sensitive and accurate diagnostics is crucial, especially at the point of care (POC). POC and “near-POC” NAAT platforms require lower infrastructure and training, making them suitable for decentralized labs or fields settings. With existing assays for commercial NAAT platforms, NiV PCR assays could be easily adapted to POC formats.
Treatment strategies
Currently, there exists no specific antiviral therapy for Nipah virus infection, rendering supportive care, including mechanical ventilation and intensive care, the primary approach to management. Adherence to fundamental clinical procedures, such as maintaining fluid and electrolyte balance, prophylaxis against venous thrombosis, and ensuring airway patency, is crucial upon confirmation of NiV infection.44 In cases of severe respiratory symptoms, mechanical ventilation is employed, alongside the administration of broad-spectrum antibiotics. Despite extensive efforts, the efficacy of traditional antiviral agents like acyclovir and ribavirin remains questionable.45
However, promising studies have been made in exploring alternative treatment modalities. Chloroquine, an antimalarial medication, exhibited potency in suppressing NiV in cell cultures, though its efficacy in animal models remains uncertain.46 Encouraging outcomes were observed following the administration of Favipiravir (T705) and the monoclonal antibody m102.4 in animal trials, with Phase I human trials underway for the latter. Notably, treatment-related adverse events were manageable, with no reported fatalities.47
Additionally, research into novel compounds like Griffithsin (GRFT) and its synthetic derivative, oxidation-resistant GRFT (Q-GRFT), has shown potential in inhibiting NiV replication. In vivo evaluations in golden Syrian hamsters demonstrated significant protection against lethal NiV challenge, highlighting GRFT’s broad-spectrum antiviral activity. Detailed in Table 4 with their effect on human and animals. These advancements underscore a promising frontier in Nipah virus treatment, offering hope for improved clinical outcomes and enhanced patient care.48-50
Table (4):
Drugs under clinical and preclinical studies51
No. |
Drug |
Effect in human |
Effect in animals |
In vitro tests |
|---|---|---|---|---|
1 |
Ribavirin |
In Malaysia outbreak during 1998/1999, a 36% reduction in mortality. In 2018 outbreak in Kerala, out of 23 cases showed 2 survivors. (drug given orally) |
No effect in animals |
– |
2 |
Remdesivir (GS-5734; Veklury®) |
Not tested in humans |
Shows 100% survival rate in AGM model against NiV-B |
– |
3 |
Favipiravir (T-705; Avigan®) |
Not tested in humans |
Full protection against NiV- M |
– |
4 |
Griffithsin (GRFT) A homodimeric high-mannose oligosaccharide-binding lectin |
Not tested in humans |
In syrian hamster model against NiV-B shows 15% and 35% survival rate. |
– |
5 |
4’ azidocytidine (R1479) |
Not tested in human |
Not tested in animals |
Shows activity against NiV, but its prodrug is inactive; therefore withdrawn from clinical trials |
6 |
4′-chloromethyl-2′-deoxy-2′-fluorocytidine (ALS-8112) |
Phase I and II of clinical trials against respiratory syncytial virus infection |
Not tested in animals |
Strong effect against NiV in vitro at low micromolar range. |
7 |
Lipopeptide fusion inhibitors |
50% survival rate in Syrian golden hamster’s model. In AGMs model shows 33% survival rate. |
– |
– |
Vaccine against NiV
Vaccine development
Efforts are underway to develop safe and effective vaccines to protect against Nipah virus, with promising candidates showing potential in preclinical studies. There are few clinical trials for vaccines that protect against NiV because there aren’t many new viral outbreaks. Animal models are used to test potential preparations’ efficacy. Beyond ten vaccinations based on viral vector, messenger RNA, recombinant protein subunits, or virus like particles have been studied to date. Thus far, the most researched subunit vaccine has been developed using soluble recombinant HeV-sG, which also triggers a cross-immune response against NiV. In horses, cats, ferrets, and non-human primates, it has been confirmed 100% efficient in preventing the spread of NiV MY, NiV BD, and HeV; however, in pigs, it was ineffective. Equivac, made by Zoetis, Inc., is the only vaccination that has been formally authorized and licensed by the Australian Pesticides and Veterinary Medicines Authority (APVMA). It is applied to horses as a preventive measure.52-58 The details of the vaccine and the phases they are in are mentioned in detail in Table 5.
Table (5):
Vaccine under clinical trial which acts against Nipah virus
Vaccine Name |
Country |
Phase trial |
|---|---|---|
ChAdOx152, 53 |
United States (Oxford) |
Phase 2 |
PHV02 54, 55 |
United States |
Phase 1 |
mRNA-1215 56 |
United States |
Phase 1 |
HeV-sG-V 57, 58 |
USA |
Phase 1 |
CD40.NiV 59 |
Vaccine Research Institute of the ANRS MIE/Inserm |
– |
Preventive measures
Preventive strategies include avoiding consuming of raw date palm sap, implementing infection control practices in healthcare settings, and promoting awareness of bat-related risks. It appears reasonable to concentrate the efforts of researchers and organizations in charge of keeping an eye on epidemiological threats on halting the spread of NiV and providing them with efficient supervision, given the scarcity of effective therapeutic options and the absence of a vaccine.59
Preventive measures to stop the spread of epidemic outbreaks that have already started mainly involve avoiding contaminated food and direct contact with the virus’s hosts, fruit bats and pigs, as well as their secretions. On the one hand, it is advised to thoroughly inspect and clean the fruit of the trees that are home to bats. However, measures are put in place to restrict their access to locations and containers used for gathering date palm juice (the bamboo skirt method). Additionally, it is advised against planting fruit trees close to the piggery that draw bats. Wearing the proper protective gear is necessary for any job involving direct contact with farm animals, particularly during the procedures of slaughter and disposal.59
Preventive measure should include disseminating accurate and timely information through various channels, includes media, news channels, and educational materials. Furthermore, community engagement plays an important role in preventing strategies by involving local peoples. Community-based surveillance systems, participatory workshops and outreach programs can strengthen community resilience and empower individuals to take responsibility and health care.60
Nipah virus remains a threat to public health, despite extensive research, not much information is available about the behavior of the virus, transmission dynamics, and clinical implementation. The unique characteristics of the Nipah virus include its ability to cause respiratory issues and neurological complications.
In conclusion, we provide, a comprehensive overview compasses various aspects of NiV, including its virology, clinical manifestation, diagnostic methods, treatment strategies and preventive measures. Promising advancement in vaccine development, such as ChAdOx, PHV02, mRNA-1215, HeV-sG-V, and CD40.NiV, offers the hope to future prevention efforts.
The article also overviewed the potential therapeutics, including monoclonal antibodies like m102.4 and nucleotide analog like remdesivir holds a promising clinical outcome and enhance patients care. In light of these challenges, it is essential to continue prioritizing research and development efforts aimed at combating NiV, while also emphasizing the importance of proactive public health interventions, risk communication, and community engagement.
Future scope
The study is required to achieve a better understanding of the pathogenesis and epidemiology of the virus. There is a lot of research being conducted to develop anti-viral drugs and vaccines that show promising effects and better results in preclinical stages.
There should be more efforts should be put on the development of vaccine such as vectors of virus, messenger RNA, recombinant protein subunits and virus like particles offers opportunity for development of novel vaccine candidate. There should also be a need for improvement in the diagnostic technology such as point-of-care and near-patient testing platforms hold promise for enhancing early detection and rapid response capabilities in NiV outbreak. Research should be focused on improving the sensitivity and accessibility of the diagnostic methods.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Jitender Joshi, Chancellor, and Prof. (Dr.) Dharam Buddhi, Vice Chancellor of Uttaranchal University-Dehradun for the encouragement to publish this research work. Authors are grateful to the Division of Research and Innovation (DRI) and Central Instrumentation Facility (CIF), Uttaranchal University-Dehradun, India, for providing all facilities during the experimental work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Sharma N, Jamwal VL, Nagial S, Ranjan M, Rath D, Gandhi SG. Current status of diagnostic assays for emerging zoonotic viruses: Nipah and Hendra. Expert Review of Molecular Diagnostics. 2024:1-3.
Crossref - Singhai M, Jain R, Jain S, Bala M, Singh S, Goyal R. Nipah Virus Disease: Recent Perspective and One Health Approach. Ann Glob Health. 2021;87(1):102.
Crossref - Banerjee S, Gupta N, Kodan P, et al. Nipah virus disease: A rare and intractable disease. Intractable Rare Dis Res. 2019;8(1):1-8.
Crossref - Chua KB. Nipah virus outbreak in Malaysia. J Clin Virol. 2003;26(3):265-275.
Crossref - Kulkarni DD, Tosh C, Venkatesh G, Senthil Kumar D. Nipah virus infection: current scenario. Indian J Virol. 2013;24(3):398-408.
Crossref - Hassan D, Ravindran R, Hossain A. Nipah Virus Mystery: Insight into Transmission and Mechanism of Disease Progression. J Pure Appl Microbiol. 2022;16(1):26-34.
Crossref - Pallivalappil B, Ali A, Thulaseedharan NK, et al. Dissecting an outbreak: A clinico-epidemiological study of Nipah virus infection in Kerala, India, 2018. J Glob Infect Dis. 2020;12(1):21-27.
Crossref - WHO. Technical Brief: Enhancing readiness for a Nipah virus event in countries not reporting a Nipah virus event. 2024. https://www.who.int/publications-detail-redirect/9789290211273. Accessed March 23, 2024.
- Balaji AP, Bhuvaneswari S, Raj LS, Bupesh G, Meenakshisundaram KK, Saravanan KM. A review on the potential species of the zingiberaceae family with anti-viral efficacy towards enveloped viruses. J Pure Appl Microbiol. 2022;16(2):796-813.
Crossref - Raju S, Sahoo D, Bhari VK. In-silico Design of Multi-epitope Vaccine against Nipah Virus using Immunoinformatics Approach. J Pure Appl Microbiol. 2021;15(1):212-231.
Crossref - Ksiazek TG, Rota PA, Rollin PE. A review of Nipah and Hendra viruses with an historical aside. Virus Res. 2011;162(1-2):173-183.
Crossref - Daszak P, Plowright RK, Epstein JH, et al. The emergence of Nipah and Hendra virus: pathogen dynamics across a wildlife-livestock-human continuum. Disease Ecology: Community Structure and Pathogen Dynamics. 2006:186-201.
Crossref - Wacharapluesadee S, Lumlertdacha B, Boongird K, et al. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005;11(12):1949.
Crossref - Rana S, Negi P, Devi M, Butola M, Ansori ANM, Jakhmola V. Systematic Review On New Face of Monkeypox Virus. J Pure Appl Microbiol. 2022;16(suppl 1):3119-3129.
Crossref - Chadha M, Comer JA, Lowe L, et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12(2):235-240.
Crossref - Ching PG, de Los Reyes V, Sucaldito M, et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg Infect Dis. 2015;21(2):328-331.
Crossref - Ajith Kumar AK, Anoop Kumar AS. Deadly Nipah outbreak in Kerala: Lessons learned for the future. Indian J Crit Care Med 2018;22(7):475-476.
Crossref - Centers for Disease Control and Prevention (CDC. Update: outbreak of Nipah virus—Malaysia and Singapore, 1999. Morb Mortal Wkly Rep. 1999;48(16):335-337.
- Thomas B, Chandran P, Lilabi MP, et al. Nipah virus infection in Kozhikode, Kerala, South India, in 2018: epidemiology of an outbreak of an emerging disease. Indian J Community Med. 2019;44(4):383-387.
Crossref - Talukdar P, Dutta D, Ghosh E, Bose I, Bhattacharjee S. Molecular Pathogenesis of Nipah Virus. Appl Biochem Biotechnol. 2023;195(4):2451-2462.
Crossref - Escaffre O, Borisevich V, Vergara LA, Wen JW, Long D, Rockx B. Characterization of Nipah virus infection in a model of human airway epithelial cells cultured at an air-liquid interface. J Gen Virol. 2016;97(5):1077-1086.
Crossref - Escaffre O, Saito TB, Juelich TL, et al. Contribution of human lung parenchyma and leukocyte influx to oxidative stress and immune system-mediated pathology following nipah virus infection. J Virol. 2017;91(15):e00275-17.
Crossref - Sharma P, Kumar R, Sharma A, Hajam YA, Kumar N. Nipah Virus: An Active Causative Agent For Respiratory And Neuronal Ailments. Epidemiology and Transmission of Infectious Diseases. 2020:78-101.
- Skowron K, Bauza-Kaszewska J, Grudlewska-Buda K, et al. Nipah virus-another threat from the world of zoonotic viruses. Front Microbiol. 2022;12:811157.
Crossref - Bansal P, Gupta M, Sangwan S, et al. Computational purposing phytochemicals against cysteine protease of Monkeypox virus: an in-silico approach. J Pure Appl Microbiol. 2022;16(Suppl 1):3144-3154.
Crossref - Subedi D, Pantha S, Chandran D, Bhandari M, Acharya KP, Dhama K. FIFA World Cup 2022 and the Risk of emergence of Zoonotic Diseases. J Pure Appl Microbiol. 2022;16(4):2246-2258.
Crossref - Chua KB. Introduction: Nipah virus-discovery and origin. Curr Top Microbiol Immunol. 2012:359:1-9.
Crossref - Gurley ES, Spiropoulou CF, De Wit E. Twenty years of Nipah virus research: where do we go from here?. J Infect Dis. 2020;221(Suppl 4):S359-S362.
Crossref - Lo Presti A, Cella E, Giovanetti M, et al. Origin and evolution of Nipah virus. J Med Virol. 2016;88(3):380-388.
Crossref - Angeletti S, Presti AL, Cella E, Ciccozzi M. Molecular epidemiology and phylogeny of Nipah virus infection: A mini review. Asian Pac J Trop Med. 2016;9(7):630-634.
Crossref - Epstein JH, Field HE, Luby S, Pulliam JR, Daszak P. Nipah virus: impact, origins, and causes of emergence. Curr Infect Dis Rep. 2006;8(1):59-65.
Crossref - Looi LM, Chua KB. Lessons from the Nipah virus outbreak in Malaysia. Malays J Pathol. 2007;29(2):63-67.
- Clayton BA. Nipah virus: transmission of a zoonotic paramyxovirus. Curr Opin Virol. 2017;22:97-104.
Crossref - Weingartl H. Hendra and Nipah viruses: pathogenesis, animal models and recent breakthroughs in vaccination. Vaccine: Development and Therapy. 2015;5:59-74.
Crossref - Jwaziri AK, Esghaei M, Niya MHK, Mehrjoo M, Sayah HAZ, Keyvani H. Association of viral load and autophagy-related genes polymorphisms with hepatitis B virus pre-core/core mutations in chronic hepatitis B virus Iraqi patients. Immunopathol Persa. 2024;10(1):e40575.
Crossref - Mishra G, Prajapat V, Nayak D. Advancements in Nipah virus treatment: Analysis of current progress in vaccines, antivirals, and therapeutics. Immunology. 2024;171(2):155-169.
Crossref - WHO report. Nipah virus infection- India. 2023. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON490. Assessed on 02 June, 2024
- WHO report Nipah virus infection- Bangladesh. 2024. https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON508#:~:text=On%2030%20January%20and%207,unlinked%20cases%20of%20NiV%20infection.&text=The%20first%20 patient%20is%20a,from%20 Manikganj%20district%2C%20Dhaka%20division. Assessed on 02 June, 2024
- Singh RK, Dhama K, Chakraborty S, et al. Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies-a comprehensive review. Vet Q. 2019;39(1):26-55.
Crossref - Garbuglia AR, Lapa D, Pauciullo S, Raoul H, Pannetier D. Nipah virus: an overview of the current status of diagnostics and their role in preparedness in endemic countries. Viruses. 2023;15(10):2062.
Crossref - Jensen KS, Adams R, Bennett RS, Bernbaum J, Jahrling PB, Holbrook MR. Development of a novel real-time polymerase chain reaction assay for the quantitative detection of Nipah virus replicative viral RNA. PLoS One. 2018;13(6):e0199534.
Crossref - Pollak NM, Olsson M, Marsh GA, Macdonald J, McMillan D. Evaluation of three rapid low-resource molecular tests for Nipah virus. Frontiers in microbiology. 2023;13:1101914.
Crossref - Mazzola LT, Kelly-Cirino C. Diagnostics for Nipah virus: a zoonotic pathogen endemic to Southeast Asia. BMJ Global Health. 2019;4(Suppl 2):e001118.
Crossref - Urmi TJ, Dewan SMR, Rahman JM, Sharmin SN, Hassan MM. Development of preventive measures and treatment strategies against Nipah virus is a timely need: Bangladeshi perspective. Clin Pathol. 2023;16:2632010X231183314.
Crossref - Wong SSY, Yuen KY. Antiviral therapy for respiratory tract infections. Respirology. 2008;13(7):950-971.
Crossref - Chorawala M, Pandya A, Shah I, Prajapati BG, Kothari N, Shah A. Recent Advances in Combating Nipah Virus Disease. Rising Contagious Diseases: Basics, Management, and Treatments. 2024:145-63.
Crossref - Dawes BE, Kalveram B, Ikegami T, et al. Favipiravir (T-705) protects against Nipah virus infection in the hamster model. Sci Rep. 2018;8(1):7604.
Crossref - Lo MK, Spengler JR, Krumpe LRH, et al. Griffithsin inhibits Nipah virus entry and fusion and can protect Syrian golden hamsters from lethal Nipah virus challenge. J Infect Dis. 2020;221(Suppl 4):S480-92.
Crossref - Zamora JL, Ortega V, Johnston GP, et al. Third helical domain of the Nipah virus fusion glycoprotein modulates both early and late steps in the membrane fusion cascade. J Virol. 2020;94(19):10-128.
Crossref - Shrivastava-Ranjan P, Lo MK, Chatterjee P, et al. Hantavirus infection is inhibited by griffithsin in cell culture. Front Cell Infect Microbiol. 2020;10:561502
Crossref - Johnson K, Vu M, Freiberg AN. Recent advances in combating Nipah virus. Fac Rev. 2021;10.
Crossref - Oxford scientists launch first human vaccine trials for deadly Nipah virus. 2024. https://www.moneycontrol.com/news/trends/health/oxford-scientists-launch-first-human-vaccine-trials-for-deadly-nipah-virus-12055571.html. Accessed February 20, 2024.
- Van Doremalen N, Avanzato VA, Goldin K, et al. ChAdOx1 NiV vaccination protects against lethal Nipah Bangladesh virus infection in African green monkeys. npj Vaccines. 2022;7(1):171.
Crossref - Monath TP, Nichols R, Feldmann F, et al. Immunological correlates of protection afforded by PHV02 live, attenuated recombinant vesicular stomatitis virus vector vaccine against Nipah virus disease. Front Immunol. 2023;14:1216225
Crossref - Nipah virus vaccine enters Phase I trial. https://www.europeanpharmaceuticalreview.com/news/173100/nipah-virus-vaccine-enters-phase-i-trial/ Accessed February 20, 2024.
- National Institutes of Health. NIH launches clinical trial of mRNA Nipah virus vaccine.2022 https://www.nih.gov/news-events/news-releases/nih-launches-clinical-trial-mrna-nipah-virus-vaccine. Accessed on 18 July, 2024.
- Rodrigue V, Gravagna K, Yao J, Nafade V, Basta NE. Current progress towards prevention of Nipah and Hendra disease in humans: A scoping review of vaccine and monoclonal antibody candidates being evaluated in clinical trials. Trop Med Int Health. 202429(5):354-364.
Crossref - A promising vaccine against Nipah virus infection. 2024 https://presse.inserm.fr/en/a-promising-vaccine-against-nipah-virus-infection/68157/. Accessed March 27, 2024.
- Soman Pillai V, Krishna G, Valiya Veettil M. Nipah virus: past outbreaks and future containment. Viruses. 2020;12(4):465.
Crossref - Sayed A, Bottu A, Qaisar M, Mane MP, Acharya Y. Nipah virus: a narrative review of viral characteristics and epidemiological determinants. Public Health. 2019;173:97-104.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.