ISSN: 0973-7510
E-ISSN: 2581-690X
Dengue is a highly prevalent mosquito-borne disease that is endemic in over 100 countries. It has a wider impact in terms of severity of illness and mortality risk in the absence of an effective vaccine as yet. The purpose of this study was to use meta-analysis to find out how common the dengue virus (DENV) is in India based on public data and to figure out how much of a problem. We searched, read, and reviewed about DENV in India that were available online. Forty-four cross-sectional studies were selected from the 178 records. There were reports of dengue cases in 14 out of the 28 states. Out of the patients presenting with symptoms of Dengue 27% of people were confirmed to have DENV infection with 82.29% (95% CI. 81-84%), having dengue IgM, 41.67% (95% CI: 40.16-43.43), having dengue IgG, and 23.97% (95% C.I. 14-43%), having both IgG and IgM from positive sample n=27156. Hospital-based cross-sectional studies on suspected Dengue-like illness (DLI) found that 99.48% of people had confirmed dengue out of the patients with features of DLI, and community-based studies found that 0.52% of DLI cases had dengue. The seroprevalence rates for East, South, North, and Western regions were 35.38% (95% C.I.14-31%), 11.57% (95% C.I. 2-69%), 38.10% (95% C.I. 9-61%), and 14.87% (95% C.I. 6-38%) correspondingly. DENV is interestingly spreading across the whole country, and the disease’s frequency varies a lot from place to place and from 2010-2023. However this review does not find appropriate published literature from 50% of the Indian states. The identification of IgG-class antibodies to dengue virus is indicative of prior exposure to this pathogen. Almost all immunocompetent individuals should have developed IgG antibodies against the dengue virus within three weeks of exposure. The presence of dengue virus IgM-class antibodies is indicative of an acute phase of infection. National Vector borne Disease Control Programme (NVBDCP) has some surveillance information, appropriate designed research into prevalence and risk factors for DENV infection would be required to provide adequate information for public health intervention.
Seroprevalence, Dengue Virus, Systematic Review, Meta-Analysis, Dengue-like Illness
Dengue fever transmitted by mosquitoes is a global health crisis and (DENV, serotypes 1-4) is a highly significant arbovirus found in the tropics and subtropics. India is host to numerous viral diseases, including Crimean-Congo hemorrhagic fever, Kyasanur forest illness, chikungunya fever, West Nile virus, and Japanese encephalitis. There has been an increase in dengue epidemics in Indian cities since the mid-1990s. The disease quickly spread to previously uncharted regions, including Orissa, Arunachal Pradesh, and Mizoram. In 1780, the city of Madras (now known as Chennai) experienced the first recorded incidence of dengue fever in India. India has had several epidemics since the initial outbreak in Kolkata in 1963.1 There have been four different dengue serotypes in the country since 1956. There has been an explosion in dengue cases in India since 2001. During the early 2000s, dengue also severely affected the Indian states of Maharashtra, Karnataka, Pondicherry, Tamil Nadu, Delhi, Rajasthan, Haryana, Punjab, and Chandigarh. It has expanded to numerous states and regions of the union in recent years. The disease is increasing in both the quantity and severity of cases, and it has spread to numerous new regions. The spread of dengue fever from urban to rural areas has advanced.2 Based on the historical records of the spread and transmission of DENV, In the last 50 years, the number of dengue cases worldwide has increased.3 Approximately 50 million people are infected with dengue each year in over 100 countries and an additional 3.97 billion people from 128 nations are at risk of getting sick.4-6 Dengue transmission in India has been associated with unplanned urbanization, environmental changes, host-pathogen interactions, and community immune system factors. Insufficient measures to regulate vectors have additionally facilitated the transmission of the dengue virus and its mosquito vectors. In India, the Dengue virus is transmitted primarily by Aedes aegypti and Aedes albopictus mosquitoes.7 Symptoms of dengue can vary from non-existent to dengue shock syndrome. The Western Pacific and Southeast Asia (SEA) account for 75% of all dengue occurrences worldwide.8 Numerous nations have approved Dengvaxia(R), a dengue vaccine. Live attenuated tetravalent vaccination Dengvaxia(R) is undergoing phase,9 clinical trials in Asia and Latin America (Brazil, Honduras, Mexico, and Puerto Rico).10 Dengvaxia(R) was protected against virologically confirmed dengue by 50.2% to 76.6% across age groups and serotypes in clinical trials.11 Furthermore, Dengvaxia (R) has not received approval from the Indian Ministry of Health and Family Welfare. As evidenced by the increasing number of clinical trials, India requires them.12 Though the vaccines against DENV has not got approval in India it is quite demanding for an early introduction. Since the first virologically proven evidence of Dengue, it is spreading all over the country. 13,14 Also there is an apparent increase in case fatality rate globally, including India.15 A new serotype DENV-5 has also surfaced in the recent years (2013).16
Different studies on the prevalence of DENV in India have come up with different outcomes, likely because of changes in geography, time, and research methods.17 Even places where DENV is common are seeing changes in the types of viruses circulating.18 The goal of this study is to conduct a thorough review and meta-analysis of the high seroprevalence of Dengue virus infection in India. The purpose of this research is to understand the prevalence of Dengue in the country and also its clinical severity.
Searching strategy
To conduct the literature review and extract records, several databases, such as PubMed Web of Science, Scopus, Sciences, Google Scholar, EMBASE and Medline, ScienceDirect were searched with the following combination of search terms: (“India”) and “dengue” or “dengue fever” or “dengue prevalence” or “dengue incidence” or “Seroprevalence of Dengue” or “dengue virus” or “severe dengue” or “DENG” or “DENV” and the last search was conducted up to December 2023.
Criteria for inclusion
Studies were gathered irrespective of the type of research, geographical location, or year of survey implementation. Only Indian reports were incorporated. Case series, community and hospital-based studies, and case-control research that supplied information on dengue exposure were incorporated into our analysis. For identifying DENV infection, enzyme-linked immunosorbent assays (ELISAs) for IgG and/or IgM were the primary laboratory tests utilized. However, certain IgM-based investigations incorporated additional PCR testing to validate the infection and ascertain the serotype.
Articles lacking clarity, study design, study setting, laboratory investigation (Dengue IgG/IgM) and there interpretation were excluded from the study.
Data extraction and validity assessment
The meta-analysis utilised the New Castle-Ottawa Scale (NOS) procedure to evaluate the absence of randomization in participant enrolment and any other possible bias in the studies.18 The following information was extracted from each eligible study: demographics of the research participants, author, location, survey year, methodology, type of diagnostic testing, study design, sample size, and dengue testing results, prevalence and severity.
Statistical analysis
The study was done with STATA-13 from the School of Biotechnology, KSBT, KIIT, Odisha, India. The data was evaluated using random effects modelling to investigate the status of Dengue virus (DENV) infection in different regions of India. DENV seroprevalence was the main outcome measure. The binomial probability distribution was employed to calculate the standard error of the prevalence estimate from the cross-sectional studies. A random effects model was used to determine the overall and subgroup pooled effect size by finding the confidence interval and pooled proportion.19,20 The chi-square (Q) value at the 10% significant level was used to measure how different the studies were from each other. It’s not likely that the studies are similar because they were done by different experts in different places. Our research was conducted utilizing the random effect model, which postulated that the actual effect magnitude would vary across studies. A fixed-effect model, in contrast, calculates the mean of a collection of effects. In our analysis model, we incorporated both within-study and between-study variance to ensure that no methodological bias existed. This model is predicated on the relative importance of each study. The objective is to obtain an approximation of the mean effect from multiple studies, irrespective of the sample sizes.21
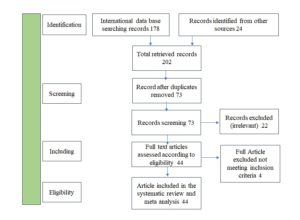
Out of the 202 studies found, 10 duplicates were removed. Among the 73 remaining records, 29 were excluded for reporting diseases other than dengue yet referencing dengue reports. Forty-four studies were reviewed, and 4 more records were removed because they did not provide sufficient data on research participants and infection rates (Figure 1).
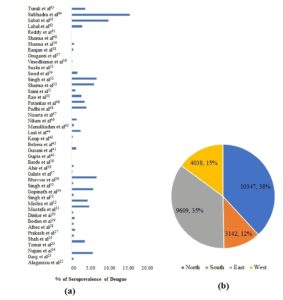
Forty-four papers met all the inclusion criteria and were included in the analysis. The studies selected encompassed various regions of India: five from the Eastern region, 11 from the West, 10 from south India, and 17 from North India (Table and Figure 2a).
Table:
List of studies that met the criteria to be included in the analysis
| No. | Author/Year of Publication | Region | State | Study type | Diagnostic test | Sample size | No. of case | Reported Prevalence (%) | Detected Serotype | Age Group | Sex | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kalichamy Alagarasu et al. | West | Pune, Maharastra | Population-based | ELISA-IgM & IgG | 230 | 141 | 61.30 | DENV1 to4 | >60 | M+F | 22 | ||
| 2 | Suneela Garg et al. | North | Delhi | Cross-sectional hospital-based | ELISA-IgG | 2609 | 1511 | 57.91 | DENV1 to 3 | 5 to 10 | M+F | 23 | ||
| 3 | Zinia T. Nujum et al. | South | Thiruvananthapuram | Cross-sectional hospital-based | ELISA-IgG | 126 | 16 | 12.70 | DENV | 24 | F | 24 | ||
| 4 | Shilpa Jagatram Tomar et al;2022 | West | Pune, Maharashtra | Cross-sectional hospital-based | ELISA-IgM & IgG | 2520 | 948 | 37.62 | CHIKV | 25 | M+F | 25 | ||
| 5 | Paresh S. Shah et al. | West | Pune, Maharastra | Cross-sectional hospital-based | ELISA-IgG | 819 | 168 | 20.51 | DENV1 to5 | 10 | M+F | 26 | ||
| 6 | Prabhu Prakash et al. | North | Rajasthan | Cross-sectional hospital-based | ELISA-IgM&IgG | 1664 | 250 | 15.02 | DENV | 10 to 40 | M+F | 27 | ||
| 7 | Pragya Ranjan et al. | North | Delhi | Cross-sectional hospital-based | ELISA-IgM&IgG | 200 | 144 | 72.00 | DENV | 19 to 51 | M+F | 28 | ||
| 8 | Gaurav Badon et al. | North | Uttarakhand | Cross-sectional hospital-based | ELISA-IgM&IgG | 279 | 222 | 79.57 | DENV & CHIKV | >80 | M+F | 29 | ||
| 9 | Anju Dinkar et al. | North | Banaras | Cross-sectional hospital-based | ELISA-IgM | 186 | 108 | 58.06 | DENV | 20 to 50 | M+F | 30 | ||
| 10 | Zeeshan Mustafa et al. | North | Uttar Pradesh | Cross-sectional hospital-based | ELISA-IgM | 7256 | 1277 | 17.60 | DENV1 to4 | >40 | M+F | 31 | ||
| 11 | Akhilesh C. Mishra et al. | West | Pune, Maharastra | Cross-sectional hospital-based | ELISA-IgG | 1434 | 1163 | 81.10 | DENV | >70 | M+F | 32 | ||
| 12 | Sweta Singh et al. | North | UP India | Prospective Cross-Sectional study | ELISA-IgM | 1,067 | 138 | 12.93 | DENV | 18 to 62 | M+F | 33 | ||
| 13 | Kanwardeep Singh et al. | North | Amritsar | Cross-sectional hospital-based | ELISA-IgM | 2709 | 1538 | 56.77 | DENV | 21 to 40 | M+F | 34 | ||
| 14 | Jitendra Singh et al. | West | Banaras | Cross-sectional hospital-based | ELISA-IgM | 161 | 110 | 68.32 | DENV | >18 | M+F | 35 | ||
| 15 | Amit Bhavsar et al. | South | Hyderabad | Cross-sectional hospital-based | ELISA-IgG | 2556 | 1789 | 69.99 | DENV | 5 to 10 | M+F | 36 | ||
| 16 | Lata Baswanna Galate et al. | West | Mumbai | Cross-sectional hospital-based | ELISA-IgM | 200 | 62 | 31.00 | DENV & CHIKV | 13 to 60 | M+F | 37 | ||
| 17 | Hitesh R et al. | West | Gujrat | Cross-sectional hospital-based | ELISA-IgM | 1146 | 148 | 12.91 | DENV | M+F | 38 | |||
| 18 | Pradip V Barde et al. | Centre | Madhya Pradesh | Cross-sectional hospital-based | ELISA-IgM | 119 | 21 | 17.65 | DENV | M+F | 39 | |||
| 19 | Ekta Gupta et al. | West | Delhi | Cross-sectional hospital-based | ELISA-IgM | 75 | 19 | 25.33 | DENV | 10 to 86 | M+F | 40 | ||
| 20 | Jigar Kiritkumar Gusani et al. | West | Gujrat | Prospective & observational study | ELISA-IgM | 765 | 331 | 43.27 | DENV | 21 to 30 | M+F | 41 | ||
| 21 | Arshi Islam et al. | North | Delhi | Cross-sectional hospital-based | ELISA-IgM | 62 | 18 | 29.03 | DENV1 to4 | 21 to 30 | M+F | 42 | ||
| 22 | Soumya Kaup et al. | South | Tumkur | Cross-sectional hospital-based | ELISA-IgM&IgG | 278 | 91 | 32.73 | DENV | >75 | M+F | 43 | ||
| 23 | H. Lall et al. | North | Delhi | Retrospective study | ELISA-IgM | 3163 | 646 | 20.42 | DENV | 11 to 30 | M+F | 44 | ||
| 24 | Anoop Manakkadan et al. | South | Kerala | Cross-sectional hospital-based | ELISA-IgG | 709 | 162 | 22.85 | DENV | >55 | M+F | 45 | ||
| 25 | Nikam AP et al. | North | Delhi | Observational study | ELISA-IgM&IgG | 1090 | 354 | 32.48 | DENV | 0 to 15 | M+F | 46 | ||
| 26 | Ankita Nisarta et al. | West | Gujrat | Cross-sectional hospital-based | ELISA-IgM&IgG | 90 | 21 | 23.33 | DENV | 16 to 35 | M+F | 47 | ||
| 27 | Sanghamitra Padhi et al. | east | Odisha | Retrospective study | ELISA-IgM&IgG | 5102 | 1074 | 21.05 | DENV | >60 | M+F | 48 | ||
| 28 | Manisha Patankar et al. | West | Gujrat | Cross-sectional hospital-based | ELISA-IgM | 4401 | 927 | 21.06 | DENV | 18 to 35 | M+F | 49 | ||
| 29 | Srinivas Rao M.S et al. | South | Andhra Pradesh | Cross-sectional hospital-based | ELISA-IgM&IgG | 1327 | 706 | 53.20 | DENV | >50 | M+F | 50 | ||
| 30 | S. Saini et al. | South | Maharashtra | Cross-sectional hospital-based | ELISA-IgM&IgG | 917 | 281 | 30.64 | DENV | >60 | M+F | 51 | ||
| 31 | Yukti Sharma et al. | North | Delhi | Cross-sectional hospital-based | ELISA-IgM&IgG | 8138 | 1600 | 19.66 | DENV | >50 | M+F | 52 | ||
| 32 | Kanwardeep Singh et al. | North | Amritsar | Cross-sectional study | ELISA-IgM | 5781 | 1790 | 30.96 | DENV | >60 | M+F | 53 | ||
| 33 | Smita Sood et al. | North | Rajasthan | Cross-sectional hospital-based | ELISA-IgM&IgG | 2169 | 412 | 18.99 | DENV | >90 | M+F | 54 | ||
| 34 | K. Mary Sushi et al. | South | Tamilnadu | Prospective study | ELISA-IgM | 100 | 8 | 8.00 | DENV | 20 to 45 | M+F | 55 | ||
| 35 | C.S. VinodKumar et al. | South | Karnataka | Cross-sectional hospital-based | ELISA-IgM&IgG | 72 | 42 | 58.33 | DENV | 5 to 10 | M+F | 56 | ||
| 36 | Ganesh Oruganti et al. | South | Andhra Pradesh | Cross-sectional hospital-based | ELISA-IgM | 200 | 21 | 10.50 | DENV | 25 to 45 | M+F | 57 | ||
| 37 | Pragya Ranjan et al. | North | Delhi | Cross-sectional hospital-based | ELISA-IgM | 200 | 116 | 58.00 | DENV | >51 | M+F | 58 | ||
| 38 | Kritika Sharma et al. | North | Rajasthan | Cross-sectional hospital-based | ELISA-IgM&IgG | 200 | 196 | 98.00 | DENV | 15 to 24 | M+F | 59 | ||
| 39 | Sharma G.K et al. | North | Rajasthan | Prospective cross sectional study | ELISA-IgM&IgG | 107 | 26 | 24.30 | DENV | 3 to 11 | M+F | 60 | ||
| 40 | M.N Reddy et al. | South | Kerala | Cross-sectional hospital-based | ELISA-IgM | 100 | 26 | 26.00 | DENV | 30 to 40 | M+F | 61 | ||
| 41 | Saloni Labala et al. | East | Odisha | Cross-sectional hospital-based | ELISA-IgM | 2892 | 763 | 26.38 | DENV | 31-40 | M+F | 62 | ||
| 42 | J. Sabat | East | Odisha | Cross-sectional hospital-based | ELISA-IgM | 5320 | 2644 | 49.69 | DENV | >60 | M+F | 63 | ||
| 43 | Subhra Subhadra | East | Odisha | Cross-sectional hospital-based | ELISA-IgM | 5198 | 4154 | 79.91 | DENV | >60 | M+F | 64 | ||
| 44 | Jyotirmayee Turuk | East | Odisha | Cross-sectional hospital-based | ELISA-IgM | 2902 | 974 | 33.56 | DENV | >40 | M+F | 65 | ||
There were 43 cross-sectional studies conducted in hospitals, and one cross-sectional research conducted in the community. Most of these investigations were conducted during or after well-known disease epidemics.
A total of 27136 cases registered in these studies, highest number of cases reported from North India (38%) followed by East (35%) ,West (15%) and South (12%) respectively (Figure 2b).
Figure 2. The approximated case study for seroprevalence percent in various Indian regions (n=27156)
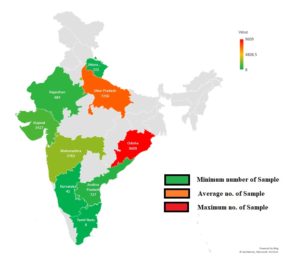
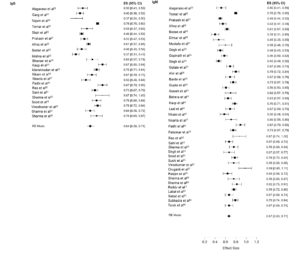
As per the meta-analysis, the aggregate seroprevalence of cases presenting with DLI in India is 35.43% (95% C.I. 13-32%). The differences in DENV infection estimates (p = 0.11 and 99.46%) may be attributed to differences in disease transmission/infection rate-related factors such as the diagnostic methodologies used to assess DENV infection, the study design and spatiotemporal variance (Figure 3 and Table).
Figure 3. Displays a map of India highlighting states where dengue virus infection has been documented. States are color-coded to reflect the number of DENV found: red for extremely high, yellow for medium, and green for low
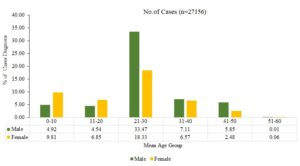
The meta-analysis indicated that different mean age groups had varying impacts on the total seroprevalence of DENV in India: 0-10 age group (14%)(95% CI: 11.1-16.6), 11-20 age group (11.39%)(95% CI: 10.1-13.6), 21-30 age group (51.81%)(95% CI: 50.13-54.43), 31-40 age group (13.68%)(95% CI: 11.32-15.16), 41-50 age group (8.33%)(95% CI: 7.45-9.32), and 51-60 age group (0.07%)(95% CI: 0.01-0.6), (p=0.239) (Figure 4). The study revealed a spectrum of DENV seroprevalence, ranging from 21 to 30 in all age groups. The highest prevalence was detected in both male and female individuals compared to others. Additionally, the percentage of males in the mean age group of 21 to 30 was higher than that of females (Figure 3).
Figure 4. Displays the estimated percentage of DENV seroprevalence according to the mean age group between male and female from the analysis of all studies included
By conducting sub-group analysis according to the geographic location of the study site in North, East, South, and Western India, the regional malady prevalence was ascertained. It was estimated that the seroprevalence of DENV infections in East India was 35.38% (95% C.I. 14-31%), in South India it was 11.57% (95% C.I. 2-69%), in North India it was 38.10% (95% C.I. 9-61%), and in Western India it was 14.87% (95% C.I. 6-38%).
Additional statistical analyses were performed to investigate the significance and variation of the DENV seroprevalence in general, as well as the seroprevalence pertaining to the types of studies, geographic regions, and diagnostic approaches utilised for DENV detection. The analysis reveals substantial heterogeneity, which explains 99.46% (95% CI 15-30%) of the estimated seroprevalence (p = 0.17) (Figure 5). As a result, the influence of confounding variables on the results is significantly reduced.
Figure 6 displays the estimated dengue burden based on subgroup analysis categorized by the geographical location of the research regions. Region: North India South India, West India East India.
Figure 6. Shows the estimated dengue burden based on sub-group analysis categorized by the geographical location
Subsequent examination was conducted by subdividing and scrutinizing the studies by the laboratory test employed to distinguish between recent acute infection (IgM) and prior exposure to DENV (IgG) (Figure 7). As determined by IgM ELISA, the estimated seroprevalence of DENV was 82.29% (95% CI. 81-84%), and the I2 was 94.02%. IgG ELISA estimated the seroprevalence of DENV to be 41.67% (95% C.I. 14-43%), and the I2 was 96.04%, which is consistent with the IgG antibody response being more durable.
This research endeavoured to conduct an exhaustive review and meta-analysis of the serotype prevalence of DENV in India from 2010 to 2023. Finally, the meta-analysis showed that 35.43% of people in India have DENV and that the virus is common in many parts of the country. Still, this study showed that India’s people are at high risk for DENV and other arboviral infections because of the abundance of vector and conducive environment. In some cases, surveys were done up to 21 years apart. Arbovirus surveillance needs to be more thorough and consistent right away to find epidemics and move valuable public health resources in the right direction.66 This monitoring system needs to have a good way for people to talk to each other so that information about disease outbreaks and prevalence can be shared quickly.67
Subgroup analysis was performed in order to improve the accuracy of the disease prevalence estimates and reduce the potential for bias that may have been introduced by combining multiple studies. This involves conducting a subgroup analysis of the studies according to their location, study type, and the DENV infection detection test utilized. Temporal variation was examined in subgrouping studies based on the diagnostic test conducted (IgG for prior exposure to the virus and IgM for the most recent acute infection).68 DENV-IgM was much more common than DENV-IgG n=22347; 82.29% (95% CI 10-28%) vs n=11317; 41.67% (95% C.I. 14-43%) which suggests that DENV transmission was widespread.69 The seroprevalence of DENV is higher in hospital-based studies compared to community-based research. This means that DENV is common all over the country. To look at how DENV seroprevalence changes with space, studies were divided into groups based on the parts of the country where they were performed.
The present investigation reported a pooled seroprevalence of dengue infection, as determined by indicators such as IgM and IgG antibodies, which were found to be 41.67% and 23.97% respectively. After doing a study, it was found that the highest rates of DENV were found in northern (38%) and eastern (35%) part of India. The seroprevalence was 15% and 14% for Southern and Western part of India, respectively. The values align with those documented by Li et al., who observed a global seroprevalence of dengue infection at 38%. The South East Asia region had the highest seroprevalence of dengue, namely 56%, while the European arena had the lowest, specifically 4%.70 Nevertheless, the combined prevalence of IgM, IgG, and DENV-RNA in febrile individuals from Africa was documented as 8.4%, 10.8%, and 24.8%, respectively.71 Humphrey et al. reported a seroprevalence of 25% (ranging from 0% to 62%) in the general population in the Middle East and North Africa from 1941 to 2015.72
Our investigation unveiled that a mean of 51% of dengue-positive patients were between the ages of 21 and 30. The elevated incidence of dengue-positive infections among individuals aged 21-30 could potentially be attributed to daytime Aedes mosquito bite susceptibility caused by local activities, such as attending school or college and engaging in outdoor pursuits.73 According to additional research conducted in Delhi, West Bengal, Odisha, and Central India, dengue fever is most prevalent among those aged 11 to 20, with those aged 21 to 30 following suit.74-77 To prevent dengue, there should be increased public awareness. It is advisable to use insect repellents, particularly on small children, and to dispose of trash and obstruct rainwater collection in waste containers, tyres, bags, and other suitable locations.78-81 The majority of dengue patients in this study presented with retro-orbital pain, arthralgia, myalgia, fever, headache, and shivers. Prior research conducted in Delhi, Assam, Uttar Pradesh, and West Bengal has documented that dengue patients exhibit similar symptoms.
Several types of the dengue virus were found in different parts of the country, as our study showed. The DENV-2 strain is thought to have more severe manifestation of the four serotypes.82 Different DENV strains may be more common in some places than others. This could be because of selection pressures during DENV evolution or because some lines are more fit to live in humans or mosquitoes than others.83 As a result, serotype variation kinetics are complex processes. Additionally, during the 2016 season, a new subgroup of DENV-4 (genotype I) emerged in Pune, India despite the fact that all four varieties were prevalent in India.84
DENV serotypes all four are reported in India.85 All four DENV serotypes are present in dengue outbreaks in Uttar Pradesh. According to a study conducted in Uttar Pradesh between 2009 and 2012, serotype-2 of DENV was the most prevalent, followed by serotype-3 and serotype-1. There was no detection of serotype-4.86 Separate studies conducted in Delhi, North India, between 2013 and 2015 identified serotype-2 as the prevailing DENV serotype, with serotype-1 following suit.87-89 Conversely, an alternative investigation conducted in the midst of the 2014 dengue outbreak in New Delhi revealed serotype-1 to be the most prevalent, with serotype-2 following suit.90 DENV serotype-3 was the most prevalent during the 2016-2017 dengue outbreak in New Delhi, according to one study.91,92 Serotype-2 was the most prevalent. While these studies do not provide a definitive trend in the evolution of serotypes across time and space, this aspect can be further investigated in the future to gain a clearer understanding of the epidemiologic risks associated with the dissemination to uninformed regions.
India urgently needs to strengthen the existing arboviral disease surveillance programme to assess DENV prevalence, vector distribution, and virus serotypes and genotypes. It should aim to depict DENV risk and dynamics with precision and assist in disease prevention and control. It would oversee vector management and identify risk factors for disease transmission in India in light of the country’s current dynamic environmental conditions.
To investigate, prevent, and control endemic diseases, we, therefore, urge international donors, research and disease control funding agencies, health partners, and research institutes to establish coordinated funding mechanisms, develop capacity, and collaborate with institutions in endemic countries.
In conclusion, dengue is still an ongoing health challenge for the country despite efforts taken by the national health program. It affects all zones of India with the potential to spread over time and geographical locations that need comprehensive and integrated attention.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Newredmars Education for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not Applicable.
- Ramakrishnan SP, Geljand HM, Bose PN, Sehgal PN, Mukherjee RN. The epidemic of acute haemorrhagic fever, Calcutta, 1963; epidemiological inquiry. Indian J Med Res. 1964;52:633-650.
- Arunachalam N, Murty US, Kabilan L et al. Studies on dengue in rural areas of Kurnool District, Andhra Pradesh, India. J Am Mosq Control Assoc. 2004;20(1):87-90.
- World Health OrganizationDengue:Guidelines for Diagnosis. Treatment, Prevention and Control:New Edition. World Health Organization:Geneva. 2009.
- Lam SK, Burke D, Gubler D, Mendez-Galvan J, Thomas L. Call for a World Dengue Day. Lancet. 2012;379(9814):411-412.
Crossref - Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504-507.
Crossref - Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidencebased consensus. PLoS Negl Trop Dis. 2012;6(8):e1760.
Crossref - Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480-496.
Crossref - Aguiar M, Rocha F, Pessanha JEM, Mateus L, Stollenwerk N. Carnival or football, is there a real risk for acquiring dengue fever in Brazil during holidays seasons? Sci Rep. 2015;5:8462.
Crossref - Chakravarti A, Arora R, Luxemburger C. Fifty years of dengue in India. Trans R Soc Trop Med Hyg. 2012;106(5):273-282.
Crossref - World Health Organization. Dengue vaccine:WHO position. Wkly Epidemiol Rec. 2016;30:349-364.
- Villar L, Dayan GH, Arredondo-Garcia JL, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372(2):113-123.
Crossref - Halstead SB. Dengue. Lancet. 2007;370(9599):1644- 1652.
Crossref - Pandya G. Prevalence of dengue infections in India. Def Sci J. 1982;4:359-370.
- Chaturvedi UC, Nagar R. Dengue and dengue haemorrhagic fever: Indian perspective. J Biosci. 2008;33(4):429-441.
Crossref - Low GK, Ogston SA, Yong MH, Gan SC, Chee HY. Global dengue death before and after the new World Health Organization 2009 case classification: A systematic review and meta-regression analysis. Acta Trop. 2018;182:237-245.
Crossref - Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India. 2015;71(1):67-70.
Crossref - Torres-Flores JM, Reyes-Sandoval A, Salazar MI. Dengue Vaccines: An Update. BioDrugs. 2022;36(3):325-336.
Crossref - Wells G. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non Randomised Studies in Meta-Analyses. 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed on 20 May 2019.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177-188.
Crossref - Higgins JP, Thompson SG, Deeks JJ, Altman DDG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
Crossref - Moher D, Liberati A, Tetzlaff J, Altman DG, PRIZMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Crossref - Alagarasu K, Tomar S, Patil J, et al. Seroprevalence of dengue virus infection in Pune City in India, 2019:A decadal change. J Infect Public Health. 2023;16(11):1830-1836.
Crossref - Garg S, Chakravarti A, Singh R, et al., DNG10 study group. Dengue serotype-specific seroprevalence among 5- to 10-year-old children in India:a community-based cross-sectional study. Int J Infect Dis. 2017;54:25-30.
Crossref - Nujum ZT, Saritha N, Prathibha Raj MR, et al. Seroprevalence of dengue infection in pregnant women and placental antibody transfer. Med J Armed Forces India. 2019;75(1):90-95.
Crossref - Tomar SJ, Alagarasu K, More A, et al. Decadal Change in Seroprevalence of Chikungunya Virus Infection in Pune City, India. Viruses. 2022;14(5):998.
Crossref - Shah PS, Alagarasu K, Karad S, et al. Seroprevalence and incidence of primary dengue infections among children in a rural region of Maharashtra, Western India. BMC Infect Dis. 2019;19(1):296.
Crossref - Prakash P, Gupta E, Nareda P, et al. Seroprevalence and incidence of primary dengue infection and its correlation with fetomaternal prognosis in Western Rajasthan. J Family Med Prim Care. 2023;12(8):1525-1530.
Crossref - Afroz S, Giddaluru J, Abbas MM, Khan N. Transcriptome meta-analysis reveals a dysregulation in extra cellular matrix and cell junction associated gene signatures during Dengue virus infection. Sci Rep. 2016;6:33752.
Crossref - Badoni G, Gupta PK, Gupta P, et al. Dengue-chikungunya infection in the tertiary care hospital of northern India:Cross-sectional latent class cluster analysis in viral infection. Heliyon. 2023;9(3):e14019.
Crossref - Dinkar A, Singh J, Prakash P, Das A, Nath G. Hidden burden of chikungunya in North India;A prospective study in a tertiary care centre. J Infect Public Health. 2018;11(4):586-591.
Crossref - Mustafa Z, Khan HM, Azam M, et al. Insight into the seroepidemiology and dynamics of circulating serotypes of dengue virus over a 4 year period in western Uttar Pradesh, India. Access Microbiol. 2023;5(6):acmi000567.v4.
Crossref - Mishra AC, Arankalle VA, Gadhave SA, et al. Stratified sero-prevalence revealed overall high disease burden of dengue but suboptimal immunity in younger age groups in Pune, India. PLoS Negl Trop Dis. 2018;12(8):e0006657.
Crossref - Singh S, Patel SS, Sahu C, Ghoshal U. Seroprevalence trends of Scrub typhus among the febrile patients of Northern India:A prospective cross-sectional study. J Family Med Prim Care. 2021;10(7):2552-2557.
Crossref - Gopinath R, Sundaram ALM, Dhanasezhian A, Arundadhi M, Thangam GS. Seroprevalence of Various Viral Diseases in Tamil Nadu, India. J Glob Infect Dis. 2023;15(4):144-148.
Crossref - Singh J, Dinkar A, Singh RG, Siddiqui MS, Sinha N, Singh SK. Clinical profile of dengue fever and coinfection with chikungunya. Ci Ji Yi Xue Za Zhi. 2018;30(3):158-164.
Crossref - Bhavsar A, Tam CC, Garg S, et al. Estimated dengue force of infection and burden of primary infections among Indian children. BMC Public Health. 2019;19(1):1116.
Crossref - Galate LB, Agrawal SR, Shastri JS, Londhey V. Chikungunya Fever Among Patients with Acute Febrile Illness Attending a Tertiary Care Hospital in Mumbai. J Lab Physicians. 2016;8(2):85-89.
Crossref - Ahir HR, Vaghela HG. Seroprevalence of Dengue Viral Infection in Patients attending Tertiary Care Hospital, South Gujarat, India. Int J Curr Microbiol Appl Sci. 2016;5(11):92-96.
Crossref - Barde PV, Shukla MK, Bharti PK, Kori BK, Jatav JK, Singh N. Co-circulation of dengue virus serotypes with chikungunya virus in Madhya Pradesh, central India. WHO South-East Asia J Public Health. 2014;3(1):36-40.
Crossref - Gupta E, Mohan S, Bajpai M, Choudhary A, Singh G. Circulation of Dengue virus-1 (DENV-1) serotype in Delhi, during 2010-11 after Dengue virus-3 (DENV-3) predominance:A single centre hospitalbased study. Vector Borne Zoonotic Dis. 2012;49(2):82-85.
- Gusani JK, Assudani HJ, Kothari K, Ghosh AN. Serological and Epidemiological Picture of Dengue during the Year 2014:An Exclusive Study of Kutch District, Gujarat, India. Int J Curr Microbiol App Sci. 2017;6(5):2100-2106.
Crossref - Behera SP, Bhardwaj P, Deval H, Srivastava N, Singh R, Misra BR, Agrawal A, Kavathekar A, Kant R. Co-circulation of all the four Dengue virus serotypes during 2018-2019: first report from Eastern Uttar Pradesh, India. PeerJ. 2023;11:e14504.
Crossref - Kaup S, Sankarankutty J. Seroprevalence and seasonal trend of dengue virus infection at a teaching hospital in Tumkur, India. Sch J Appl Med Sci. 2014;2(3A):922-926.
- Lall H, Gupta P, Debbarma M, et al. Sero-Prevalance of Dengue in Tertiary Care Hospital in Delhi. Int J Curr Microbiol Appl Sci. 2016;5(6):439-445.
Crossref - Manakkadan A, Joseph I, Prasanna RR, Kanju RI, Kailas L, Sreekumar E. Lineage shift in Indian strains of Dengue virus serotype-3 (Genotype III), evidenced by detection of lineage IV strains in clinical cases from Kerala. Virol J. 2013;10(1):37.
Crossref - Nikam AP, Bhise PR, Deshmukh MM. Seroprevalence of Dengue infection in clinically suspected cases of dengue at tertiary care hospital in central India. Int J Med Res Rev. 2015;3(6):593-596.
Crossref - Nisarta A, Ahir H. Study of Seroprevalence of Dengue Virus Infection in a Tertiary Care Hospital in Patan, Gujarat, India. Int J Curr Microbiol App Sci. 2016;5(10):819-824.
Crossref - Padhi S, Dash M, Panda P, et al. A three year retrospective study on the increasing trend in seroprevalence of dengue infection from southern Odisha, India. Indian J Med Res. 2014;140(5):660-664. PMID:25579149
- Patankar M, Patel B, Gandhi V, Shah P, Vegad M. Seroprevalence of Dengue in Gujarat, Western India:A study at a tertiary care hospital. Int J Med Sci Public Health. 2014;3(1):16-18.
Crossref - Rao MS, Pavani K, Dass M, Kareem MA, Vinayaraj EV. Seroprevalence of dengue virus in a tertiary care hospital, Andhra Pradesh, South India. Int J Res Med Sci. 2013;1(4):448-450.
Crossref - Saini S, Kinikar A, Deorukhkar S, Bhalerao D, Roushani SB. Epidemiology and seropositivity of dengue fever cases in a rural tertiary care hospital of western Maharashtra, India. Int J Bio Med Res. 2013;4(7):473-477.
Crossref - Sharma Y, Kaur M, Singh S, Pant L, Kudesia M, Jain S. Seroprevalence and trend of dengue cases admitted to a government hospital, delhi-5-year study (2006-2010):a look into the age shift. Int J Prev Med. 2012;3(8):537-543. PMID:22973483
- Singh K, Sidhu SK, Devi P, Kaur M, Kaur M, Singh N. Seroprevalence of Common Viral Diseases:A Hospital Based Study from Amritsar, India. J Clin Diagn Res. 2016;10(12):DC15-DC19.
Crossref - Sood S. A hospital based serosurveillance study of dengue infection in jaipur (rajasthan), India. J Clin Diagn Res. 2013;7(9):1917-1920.
Crossref - Sushi KM, Sivasangeetha K, Kumar AS, et al. Seroprevalence of Leptospirosis, Enteric fever and Dengue in patients with acute febrile illness in Tamil Nadu, India. Indian J Basic Applied Med Res. 2014;3(2):615-623.
- Vinodkumar CS, Kalapannavar NK, Basavarajappa KG, et al. Episode of coexisting infections with multiple dengue virus serotypes in central Karnataka, India. J Infect Public Health. 2013;6(4):302-306.
Crossref - Oruganti G, Dinaker M, Tez KSRS, Rajesh JG, Vijay YV, Reddy PS. High sero-prevalence of dengue IgG antibodies among healthy individuals in Andhra Pradesh, India. Indian J Public Health Res Dev. 2014;5(1):131-135.
Crossref - Ranjan P, Natarajan V, Bajpai M, Gupta E. High Seroprevalence of Dengue Virus Infection in Blood Donors From Delhi:A Single Centre Study. J Clin Diagn Res. 2016;10(10):DC08.
Crossref - Sharma K, Yadav A. Association of mean platelet volume with severity, serology & treatment outcome in dengue fever:prognostic utility. J Clin Diagn Res. 2015;9(11):EC01-EC03.
Crossref - Sharma G, Bhatt D, Garg GK, Sharma D, Gulati RK. A prospective seroepidemiologic study on dengue in children in Southeastern Rajasthan, India. Pediatric Review: Int J Pediatr. 2016;3(10).
Crossref - Reddy MN, Dungdung R, Valliyott L, Pilankatta R. Occurrence of concurrent infections with multiple serotypes of dengue viruses during 2013-2015 in northern Kerala, India. Peer J. 2017;5:e2970.
Crossref - Labala S, Sinha A, Panda S, Turuk J, Pati S, Sahoo PK. Mapping the distribution and trends of co-circulating dengue virus serotypes in Odisha, India:A retrospective facility-based analysis. Natl Med J India. 2022;35(6):344-347.
Crossref - Sabat J, Subhadra S, Thakur B, et al. Molecular and phylogenetic analysis of the dengue strains circulating in Odisha, India. Virusdisease. 2019;30(3):380-386.
Crossref - Subhadra S, Sabat J, Dwibedi B, et al. Prevalence and trend of emerging and re-emerging arboviral infections in the state of Odisha. Virusdisease. 2021;32(3):504-510.
Crossref - Turuk J, Palo SK, Rath S, et al. Viral characteristics and clinical presentation in dengue co-infection- Findings from a facility based observational study in Odisha, India. J Family Med Prim Care. 2021;10(8):2958-2963.
Crossref - Ganeshkumar P, Murhekar MV, Poornima V, et al. Dengue infection in India:A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(7):e0006618.
Crossref - Rather IA, Parray HA, Lone JB, Paek WK, Lim J, Bajpai VK, Park YH. Prevention and Control Strategies to Counter Dengue Virus Infection. Front Cell Infect Microbiol. 2017;7:336.
Crossref - Luo R, Fongwen N, Kelly-Cirino C, Harris E, Wilder-Smith A, Peeling RW. Rapid diagnostic tests for determining dengue serostatus:a systematic review and key informant interviews. Clin Microbiol Infect. 2019;25(6):659-666.
Crossref - Moorthy M, Chandy S, Selvaraj K, Abraham AM. Evaluation of a rapid immunochromatographic device for the detection of IgM & IgG antibodies to dengue viruses (DENV) in a tertiary care hospital in south India. Indian J Med Microbiol. 2009;27(3):254-256.
Crossref - Li Z, Wang J, Cheng X, et al. The worldwide seroprevalence of DENV, CHIKV and ZIKV infection:a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(4):e0009337.
Crossref - Simo FBN, Bigna JJ, Kenmoe S, et al. Dengue virus infection in people residing in Africa:a systematic review and meta-analysis of prevalence studies. Sci Rep. 2019;9(1):13626.
Crossref - Humphrey JM, Cleton NB, Reusken CB, Glesby MJ, Koopmans MP, Abu-Raddad LJ. Dengue in the Middle East and North Africa:a systematic review. PLoS Negl Trop Dis. 2016;10(12):e0005194.
Crossref - Dutta P, Khan SA, Borah J, Mahanta J. Demographic and clinical features of patients with dengue in Northeastern region of India:a retrospective crosssectional study during 2009-2011. J Virol Microbiol. 2012;2012:786298.
Crossref - Deshwal R, Qureshi MI, Singh R. Clinical and laboratory profile of dengue fever. J Assoc Physicians India. 2015;63(12):30-32.
- Mandal SK, Ganuly J, Sil K, et al. Clinical profiles of dengue fever in a teaching hospital of Eastern India. Natl J Med Res. 2013;2:173-176.
Crossref - Gupta B, Reddy BPN. Fight against dengue in India:progresses and challenges. Parasitol Res. 2013;112(4):1367-1378.
Crossref - Hang VTT, Holmes EC, Veasna D, et al. Emergence of the Asian 1 genotype of dengue virus serotype 2 in Vietnam:in vivo fitness advantage and lineage replacement in South-East Asia. PLoS Negl Trop Dis. 2010;4(7):e757.
Crossref - Shrivastava S, Tiraki D, Diwan A, et al. Co-circulation of all the four dengue virus serotypes and detection of a novel clade of DENV-4 (genotype I) virus in Pune, India during 2016 season. PLoS ONE. 2018;13(2):e0192672.
Crossref - Malhotra G, Yadav A, Dudeja P. Knowledge, awareness and practices regarding dengue among rural and slum communities in north Indian city, India. Int J Med Sci Public Health. 2014;3(2):295.
Crossref - Sharma Y, Kaur M, Singh S, Pant L, Kudesia M, Jain S. Seroprevalence and trend of dengue cases admitted to a government hospital, delhi – 5-year study (2006-2010):a look into the age shift. Int J Prev Med. 2012;3:537-543.
- Hati AK. Dengue serosurveillance in Kolkata, facing an epidemic in West Bengal, India. J Vector Borne Dis. 2009;46:197-204.
- Poddar et al.Seroprevalence and changing trend of dengue in a tertiary care hospital. Indian Journal of Microbiology Research. 2022;9(1):50–54
- Deshkar ST, Raut SS, Khadse RK. Dengue infection in central India:a 5 years study at a tertiary care hospital. Int J Res Med Sci. 2017;5(6):2483.
Crossref - Laul A, Laul P, Merugumala V, Pathak R, Miglani U, Saxena P. Clinical profiles of dengue infection during an outbreak in Northern India. J Trop Med. 2016;2016:5917934.
Crossref - Bharaj P, Chahar HS, Pandey A, et al. Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J. 2008;5:1.
Crossref - Mishra G, Jain A, Prakash O, et al. Molecular characterization of dengue viruses circulating during 2009-2012 in Uttar Pradesh, India. J Med Virol. 2015;87(1):68-75.
Crossref - Afreen N, Deeba F, Naqvi I, et al. Molecular investigation of 2013 dengue fever outbreak from Delhi, India. PLoS Curr. 2014;6:ecurrents.
Crossref - Afreen N, Naqvi IH, Broor S, Ahmed A, Parveen S. Phylogenetic and molecular clock analysis of dengue serotype 1 and 3 from New Delhi, India. PLoS One. 2015;10(11):e0141628.
Crossref - Islam A, Abdullah M, Tazeen A, et al. Detection of all the four serotypes of dengue virus in New Delhi, India during post monsoon season of 2015. Ind Jour Healt Scie. 2016;3(1):24.
Crossref - Tazeen A, Afreen N, Abdullah M, et al. Occurrence of co-infection with dengue viruses during 2014 in New Delhi, India. Epidemiol Infect. 2017;145(1):67-77.
Crossref - Parveen N, Islam A, Tazeen A, et al. Circulation of single serotype of dengue virus (DENV-3) in New Delhi, India during 2016:a change in the epidemiological trend. J Infect Public Health. 2019;12(1):49-56.
Crossref - Islam A, Abdullah M, Tazeen A, et al. Circulation of dengue virus serotypes in hyperendemic region of New Delhi, India during 2011-2017. J Infect Public Health. 2020;13(12):1912-1919.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.