ISSN: 0973-7510
E-ISSN: 2581-690X
In plant roots, arbuscular mycorrhizal fungi (AMF) are the most prevalent microsymbionts, and thereby provide many key ecosystem services to natural and agricultural ecosystems. Despite AMF’s significance for the environment and the economy, little is known about the mycorrhizal inoculum potential and diversity of AMF associated with orphan African cereal crops, specially fonio millet (Digitaria exilis stapf.) under field conditions. We hypothesized that the type of fonio millet agroecosystem influences the AMF density and distribution in soils. We therefore, assessed the inoculum potential, density and diversity of AMF spores and soil enzyme activities in five fonio millet agroecosystems belonging to three climatic zones (Sudanian, Sudano-Sahelian and Sudano-Guinean). By combining AMF spore identification from field-collected soils and trap culture, 20 species belonging to 8 genera (Acaulospora, Ambispora, Dendiscutata, Gigaspora, Glomus, Racocetra, Sclerocystis and Scutellospora) were identified. Glomus was the most represented genus with 8 species, followed by Gigaspora (5 species) and Acaulospora (2 species); the remaining genera were each represented by one species. Except for Ambispora which was not found in the Sudanian area, all genera occurred in the three climatic zones. The abundance and diversity of AMF species and FDA-hydrolytic and phosphatase activities varied between fonio millet agroecosystems as well as between climatic zones. Soil pH and soil texture were the variables that best explained the density and distribution of AMF spores. Our results contribute to paving the way towards the development of microbial engineering approaches for agronomic improvement of fonio millet.
AMF Spore Diversity, Soil Properties, Digitaria exilis, Orphan Crop, Species, Trap Culture
Fonio millet (Digitaria exilis, stapf), also called “Acha”, is one of the oldest cereal crops originated in West Africa.1 It has very good prospects for semi-arid and upland areas as it tolerates poor soils and drought conditions, and matures very quickly (6-8 weeks).2 Moreover, Fonio grains contain higher amounts of amino acids (e.g., methionine and cystine),3 iron, potassium, calcium and phosphorus.4,5
However, fonio consumption is still low, particularly in urban areas where it has long been considered as an orphan crop.6,7 In recent years, this crop has attracted considerable research interest.8-10 So, it has been reported as a priority crop for West Africa due to its organoleptic qualities, nutritional and health benefits, and potential contribution to crop diversification and food security.11-13
In the other hand, arbuscular mycorrhizal fungi (AMF) form the most prevalent microbial symbiotic association with the majority of terrestrial plant species.14,15 These beneficial soil microorganisms have a great potential for contributing to crop production and thereby helping to achieve sustainable global food security.16,17 Indeed, AMF can provide to their host plants multiple benefits, including increase of nutrient uptake, stimulation of phytohormones production, tolerance to drought stress, as well as protection against pathogens.18-20 Furthermore, AMF play a crucial role in enhancing the physicochemical and biological characteristics of soil.21-23 Hence, harnessing the potential of AMF is considered as a potential less costly solution to increase crop yields.16,24,25 Meanwhile, abiotic and biotic factors influence the effects of AMF taxa on plant development and production.24,26,27 In addition, it has been reported that AMF abundance and diversity varied depending on the ecological zone,28,29 soil properties,30-32 vegetation type33 and agricultural management pratice.34
In Senegal, fonio millet is cultivated under various agricultural management practices across different climatic zones.35 However, little is known about the AMF density, diversity and distribution across the fonio millet agroecosystems. We hypothesized that the type of fonio millet agroecosystem and pedoclimatic conditions might influence the AMF density and distribution in soils. We therefore, assessed in this study the inoculum potential, density and diversity of AMF spores, and soil enzyme activities in five fonio millet agroecosystems belonging to three climatic zones (Sudanian, Sudano-Sahelian and Sudano-Guinean).
Study site and soil sampling
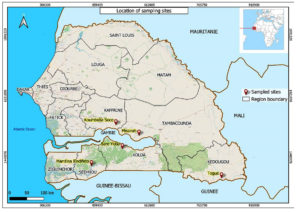
Rhizosphere soils were collected after the growing season from fonio millet fields located in five sites (Koumbidia Soce, Missirah, Togue, Sare Yoba and Mandina Findifeto) (Figure 1). Koumbidia Soce (13°54’22’’N and 14°52’0’’W) and Missirah (13°31’34’’N and 13°30’53’’W) are located in Kaffrine and Tambacounda region, respectively. They belong to the Sudano-Sahelian zone characterized by mean annual rainfall varying between 600 and 800 mm, temperatures between 30°C and 35°C and leached soil, without ferruginous spots or weakly stained on sandy-clay sandstones. The vegetation is a savannah in Missirah. Togue (12°30’0’’N and 12°1’0’’W) is located in the region of Kedougou in the Sudano-Guinean zone with mean annual rainfall of 1103 mm and temperatures varying between 30°C and 33°C. Sare Yoba (12°55’59’’N and 14°07’59’’W) and Mandina Findifeto (12°48’32’’N and 15°31’53’’W), are located in Sedhiou and Kolda region, respectively, in the Sudanian zone. In this zone, the mean annual rainfall is 1048 mm, the temperatures fluctuate between 27°C and 38°C and the soil is leached with pseudo-gley and ferruginous concretions on shale or weakly ferralitic on sandy-clay sandstones.
In each fonio millet agroecosystem, a sampling area of 100 × 100 m was delimited, soil was collected from 6 points at a depth of 0 to 25 cm and then the soil samples were pooled together in plastic bags and brought to the lab. The soil samples were sieved to <4 mm and kept at 4°C.
Characterization of soil properties
Physical (sand, silt, clay) and chemical (C, N, P, P2O5, C/N) characteristics of the five soils were analyzed at the Laboratory of Soil, Water and Plant of ISRA/CNRA at Bambey (Senegal) using standard methods. The soil physical characterization was carried out as described in Disale et al.36 The soil samples were placed in a mechanical shaker and sieved for 5 min through a series of sieves to determine the size of different soil particles. The combustion system Thermo-Finnigan Flash EA 1112 (ThermoFinnigan, France) was used to quantify the total amount of soil carbon and nitrogen.37 The amount of soil total and available phosphorus was evaluated as described by Bibi and colleagues.38 Soil organic matter (OM) was determined from organic carbon as follows:
OM (%) = organic carbon (%) × 1724. Soil pH was measured in soil-water (1:2,5) suspensions.37
Determination of mycorrhizal inoculum potential
The mycorrhizal inoculum potential in each soil sample was evaluated by the dilution technique.39 Briefly, a quarter-fold dilution series (1, 1/4, 1/16, 1/64, 1/256 and 1/1024) was prepared by thoroughly mixing defined proportions of non-sterilized and sterilized soil. Then, 50 g of each diluted soil sample were placed in 5 pots, and 3 seeds of Zea mays L. (a highly mycotrophic plant) were sown per pot. The seedlings were thinned to one per pot and all plants were kept in glasshouse and watered with demineralized water. After 45 days, roots of all plants from the dilution ratios were harvested and stained with Trypan blue as described in Founoune-Mboup et al.40 The presence of mycorrhizal infection in stained root segments was observed by light microscope at a magnification of 100X. The most probable number (MPN) of AMF propagules that can colonize plant roots was calculated as follows: Log MPN = (x log a)–K, where x represents mean of mycorrhized plants for all dilution ratios, a (factor of dilution) = 4, K=constant given by the table of Fisher & Yates.41,42
Identification and enumeration of AMF spores from field-collected soils
The extraction of AMF spores from each field-collected soil was carried out using the wet sieving and decanting method.43 Briefly, 100 g of soil were mixed with 1 L of water and decanted in a series of 400-50 µm sieves. Then, the material of 200, 100 and 50 µm pore-sieves was re-suspended in water and collected in tubes. Two solutions of sucrose at 20% and 60% were successively added and centrifugation was done at 3000 g/min for 3 minutes. After that, the supernatant containing AMF spores was poured in a 50 µm mesh and rinsed with tap water. AMF spores were grouped and counted according to their morphological characters and using a dissecting microscope. The International Culture Collection of Arbuscular and Vesicular-Mycorrhizal Fungi was used for AM fungi description (https://invam.wvu.edu/methods/spores/enumeration-of-spores). The AMF spore density and abundance of each AMF species were expressed per 100 g of soil. Three replicates were made for each composite soil from each sampled site.
Determination of AMF species composition from trap culture
The trap culture method allows to confirm AMF spore identification (spores are sometimes damaged) from the field-collected soils. On the other hand, this method induces emergence of AMF that would not naturally sporulate.44 The trap culture was performed with field-collected soils using mays (Zea mays L.) for 3 months under glasshouse conditions. For this purpose, each field-collected soil was mixed with an autoclaved nutrient-poor sandy soil from Sangalkam (1:2 v/v) to serve as culture substrate. For each agroecosystem, 9 pots of 1 kg were filled with the culture substrate and 3 fonio seeds were sown per pot (9 replicates x 5 soil sites). Plants were watered every two days for three months. At the end of experiment, plants were harvested and AMF spores were isolated from soils, enumerated and identified as previously described.43
Soil enzymatic activities
Enzymatic activities were determined from field-collected soils as described in Ndoye et al.28,45 The activity of FDA (3’.6’-diacetylfluorescein) hydrolysis was measured according to Patle et al.46 For this test, to 1 g of the soil, 15 ml of 60 mM potassium phosphate buffer (pH 7.6) and 0.2 ml of 1000 µg FDA ml1 were added (with 3 replicates per soil origin). A blank enzyme without FDA and a blank substrate without soil were included. After 1h of shaking on an orbital incubator at 30°C, the flasks were removed and 1 ml of each suspension were transferred into Eppendorf tube and mixed with 1 ml of acetone to stop the reaction. After centrifugation (10000 rpm/min for 5 min), 1 ml of the supernatant was measured at 490 nm on a spectrophotometer (Ultrospec 3000 Pharmacia Biotech). The concentration of fluorescein was calculated using the calibration graph standard and expressed as µg FDA/g of soil/h.
Acid and alkaline phosphatase activities were quantified using a colorimetric determination of p-nitrophenol released after soil incubation with p-nitrophenyl phosphate as substrate (pNPP, 5mM).47,48 Briefly, 25 mg of soil sample was mixed with 400 µl of buffered sodium p-nitrophenyl phosphate solution (pH 6 and pH 11 for acid and alkaline phosphatase, respectively) and 100 µl pNPP (p-Nitrophenyl Phosphate, 5 mM). A blank enzyme without pNPP and a blank substrate without soil were included. After incubation at
37°C for 1 h, the reaction was complexed with 100 µl of CaCl2 (0,5 M) then stopped by adding 400 µl of NaOH (0,5 M) solution. After centrifugation (10000 rpm/min for 5 min), 1 ml of the supernatant was measured at 400 nm on a spectrophotometer (Ultrospec 3000 Pharmacia Biotech). The amount of released p-nitrophenol was determined at 400 nm and expressed as µg pNPP/g of soil/h.
Statistical analysis
The Shapiro–Wilk and Levene tests were used to checked the normality and homogeneity of variance, respectively. So, comparisons of means were performed by Kruskal-Wallis test instead of one-way ANOVA when the test was significant. Statistical analyses were performed using rcompanion, FSA, TH.data, pgirmess packages in R software.49,50 The probability threshold “p value” was set at 0.05 in order to establish statistically significant differences between groups.
For each agroecosystem, AMF species richness, Shannon and Wiener diversity index (H’) and Simpson Dominance index (D) were determined. The Shannon and Wiener diversity index was calculated as H’ = -pi (ln pi) where pi represents the proportion of individuals found in the ith species, estimated as ni /N, ni being the number of individuals in the ith species and N, the total number of individuals. The inverse of the Simpson dominance index was evaluated using the following formula: 1-D = 1-[ni (ni-1)/N (N-1)]; where ni represents the number of the ith types and N the number of individuals in the population.
Pearson correlation coefficients were determined to investigate the relation between AMF spore diversity and density, soil enzyme activities, and soil physicochemical characteristics. All statistical analyses were conducted in R v4.3.1.
Soil physicochemical characteristics
Our results showed that the sampled soils were sandy silt clay in Missirah and Mandina Findifeto and sandy clay silt in the other three sites with pH ranging from 5.84 to 6.98 (Table 1). For soil C, N, P, P2O5, and organic matter (OM) contents, the highest values were obtained in Togue, and the lowest values in Mandina Findifeto. On the contrary, soil pH follows an opposite trend, showing the lowest value in Togue and the highest in Mandina Findifeto. Considering the climatic zones, the Sudanian zone had the lowest C, N, P, P2O5 and OM contents in soil and the highest value of soil pH, whereas the Sudano-Sahelian zone had intermediate values, as compared to the Sudano-Guinean zone (Table 1).
Table (1):
Physicochemical characteristics of the field-collected soils
| Sites (climatic zone) | Soil properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clay (%) | Silt (%) | Sand (%) | C (%) | N (%) | P (‰) | P2O5 (‰) | C/N | OM (%) | pH(H2O) | |
| Koumbidia Soce (Sudano-Sahelian zone) | 5.13 | 4.87 | 90.00 | 0.66 | 0.05 | 1.08 | 2.46 | 12.10 | 1.13 | 6.15 |
| Missirah (Sudano-Sahelian zone) | 5.42 | 10.58 | 84.00 | 0.77 | 0.06 | 0.61 | 1.34 | 12.45 | 1.33 | 6.09 |
| Togue (Sudano-Guinean zone) | 3.86 | 5.66 | 90.48 | 1.71 | 0.14 | 3.374 | 7.72 | 12.58 | 2.95 | 5.84 |
| Sare Yoba (Sudanian zone) | 5.56 | 5.56 | 88.89 | 0.55 | 0.05 | 0.538 | 1.23 | 12.01 | 0.94 | 6.45 |
| Mandina Findifeto (Sudanian zone) | 3.74 | 10.73 | 85.53 | 0.49 | 0.04 | 0.410 | 1.19 | 12.17 | 0.84 | 6.98 |
Inoculum potential, AMF species diversity and spore density in field-collected soils
Mycorrhizal soil infectivity of the 5 field-collected soils ranged from 5 to 71 propagules in 50 g of dry soil (Table 2). Mandina Findifeto soil showed higher MPN value (70.90 propagules per 50 g of soil) as compared to those of other field-collected soils (ranging from 5.20 to 12.60 propagules per 50 g of soil). Those latter soils did not differ significantly in terms of MPN values. The lowest MPN was obtained in Sare Yoba located in the same climatic zone with Mandina Findifeto.
Table (2):
Mycorrhizal inoculum potential (MPN*) in the field-collected soils
| Climatic zone | Sites | MPN | MPNi | MPNs |
|---|---|---|---|---|
| Sudano-Sahelian | Koumbidia Soce | 12.59b | 4.66b | 25.84b |
| Missirah | 9.14b | 4.28b | 19.53b | |
| Sudano-Guinean | Togue | 12.10b | 5.89b | 26.90b |
| Sudanian | Sare Yoba | 5.20b | 2.43b | 11.10b |
| Mandina Findifeto | 70.90a | 33.18a | 151.47a |
*MPN= Most probable number. MPNi: MPN minimal. MPNs: MPN maximal
In column, values followed by the same letter are not significantly different according to the Kruskal-Wallis test (P < 0.05)
In addition, a total of 12 morphotypes of AMF belonging to 7 genera (Scutellospora, Gigaspora, Racocetra, Dendiscutata, Acaulospora, Glomus and Sclerocystis) was recorded from the 5 field-collected soils (Figure 2 and 3). Nine (Scutellospora sp. aff. dipurpurascens, Gigaspora sp.1, Gigaspora sp.2 aff. gigantea, Racocetra gregaria, Dendiscutata sp aff. heterogama, Acaulospora sp.1, Glomus sp.2, Glomus sp.3, Glomus sp.4) out of the 12 AMF species were common to the 5 sites, two (Gigaspora sp.3 and Glomus sp.1) were found only in two sites (Koumbidia Soce and Sare Yoba) and one (Sclerocystis sp) in three sites (Koumbidia Soce, Missirah and Togue).
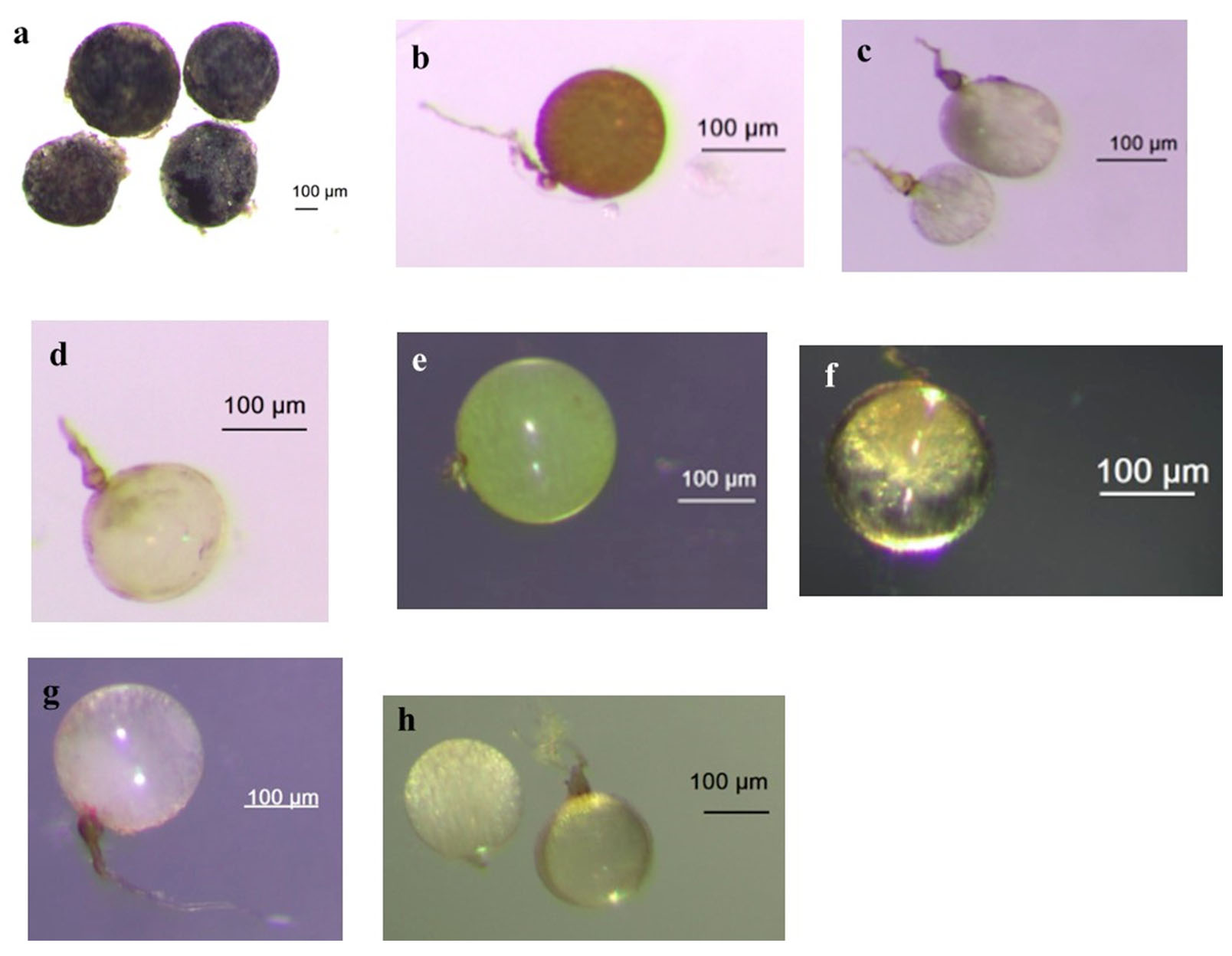
Figure 2. Some AMF species found from field-collected soils and trap culture
(a) Racocetra sp aff. gregaria (b) Dendiscutata sp. (c) Scutellospora sp. (d) Gigaspora sp.1 (e) Gigaspora sp.2 aff. gigantea (f) Gigaspora sp.3 aff. albida (g) Gigaspora sp.4 aff. rosea (h) Gigaspora sp.5
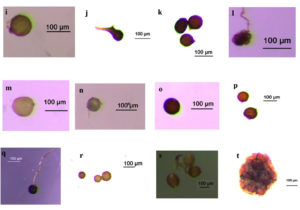 Figure 3. Some AMF species found from field-collected soils and trap culture
Figure 3. Some AMF species found from field-collected soils and trap culture
(i) Acaulospora sp.1 (j) Acaulospora sp.2 (k) Ambispora sp. (l) Sclerocystis sp. (m) Glomus sp.1 (n) Glomus sp.2 (o) Glomus sp.3 (p) Glomus sp.4 (q) Glomus sp.5 (r) Glomus sp.6 (s) Glomus sp.7 (t) Sporocarpe of Glomus sp.8
The spore density of AMF species varied depending on soil origin (Table 3). For instance, Glomus sp.2 and Glomus sp.3 displayed their highest density in Koumbidia Soce (1017.33 and 770.67 spores/100 g of soil, respectively), while Scutellospora sp. aff. dipurpurascens and Dendiscutata sp. aff. heterogama had their highest density in Mandina Findifeto (97.67 and 920.67 spores/100 g of soil, respectively). The lowest AMF spore densities were observed in Koumbidia with Scutellospora sp, Gigaspora sp.1 and Gigaspora sp.2; in Missirah with Dendiscutata sp and Glomus sp.2; in Togue with Racocetra sp and Glomus sp.3; in Sare Yoba with Glomus sp.4; and in Mandina Findifeto with Acaulospora sp.1.
Table (3):
Density of arbuscular mycorrhizal fungal spores in the field-collected soils
| Sites | |||||
|---|---|---|---|---|---|
| Sudano-Sahelian zone | Sudano-Guinean zone | Sudanian zone | |||
| AMF species | Koumbidia Soce | Missirah | Togue | Sare Yoba | Mandina Findifeto |
| Scutellospora sp. aff. dipurpurascens | 4.67d | 51.33b | 19.33c | 55.00b | 97.67a |
| Gigaspora sp.1 | 8.67b | 41.67a | 10.67b | 46.33a | 42.33a |
| Gigaspora sp.2 aff. gigantea | 12.67b | 20.67a | 12.00b | 5.67c | 10.67b |
| Gigaspora sp.3 aff. albida | 8.33b | 0.00c | 0.00c | 12.00a | 0.00c |
| Dendiscutata sp. aff. heterogama | 59.67b | 15.67c | 18.33c | 67.00b | 920.67a |
| Racocetra sp. aff. gregaria | 3.33a | 3.33a | 2.00a | 4.00a | 4.67a |
| Acaulospora sp.1 | 31.67a | 18.33b | 31.33a | 15.00b | 8.00c |
| Sclerocystis sp. | 1.67a | 1.00a | 1.00a | 0.00a | 0.00a |
| Glomus sp.1 | 4.67b | 0.00c | 0.00c | 12.00a | 0.00c |
| Glomus sp.2 | 1017.00a | 145.67e | 663.67c | 226.33d | 824.33b |
| Glomus sp.3 | 770.33a | 557.33b | 312.67c | 350.67c | 709.33a |
| Glomus sp.4 | 397.67b | 305.67c | 308.67c | 163.67d | 548.00a |
| Total density of AMF spores | 2320.33b | 1160.67d | 1379.67c | 957.67e | 3165.33a |
| AMF species richness | 12 | 10 | 10 | 11 | 9 |
| Diversity index | |||||
| Shannon-Weiver (H’) | 1.29a | 1.44ab | 1.32ab | 1.63b | 1.56ab |
| Simpson (1-D) | 0.67a | 0.68a | 0.67a | 0.77a | 0.77a |
| Hill (1-Hill) | 0.59a | 0.65a | 0.60a | 0.75a | 0.73a |
In row, values followed by the same letter are not significantly different according to the Kruskal-Wallis test (P < 0.05)
The total spore density of AMF also differed significantly between the five sites, ranging from 957 to 3166 spores per 100 g of dry soil (Table 3). The density of AMF spores was significantly higher in Mandina Findifeto (3165.33 spores/100 g of soil) than in other sites. It was followed by those of Koumbidia Soce, then Togue (2320.33 and 1379.67 spores/100 g of soil, respectively). The lowest AMF density was recorded in Sare Yoba (957.67 spores/100 g of soil).
Shannon index ranged from 1.63 to 1.29, while Simpson index varied from 0.77 to 0.67 and Hill index from 0.75 to 0.59. The highest diversity indices were observed in Sare Yoba in the Sudanian zone, whereas the soil from Koumbidia Soce in the Sudano-Sahelian zone showed the lowest diversity indices (Table 3).
Composition of AMF species from trap culture
A total of 20 AMF species belonging to 8 genera (Racocetra, Dendiscutata, Scutellospora, Gigaspora, Acaulospora, Ambispora, Glomus and Sclerocystis) were recorded from trap culture (Table 4). Of the 20 AMF species, 17 were found in Koumbidia Soce, 16 in Missirah, 15 in Togue, 15 in Sare Yoba and 11 in Mandina Findifeto. Only 10 out of the 20 AMF species were shared by the 5 sites, while two AMF species, Glomus sp.5 and Glomus sp.6, were recorded exclusively in Sare Yoba. Besides, 8 of the 20 AMF species revealed by trap culture were not detected by spore identification from field-collected soils (Tables 3 & 4).
Table (4):
Species of AMF associated with Digitaria exilis stapf in trap culture
| Families | Genera | Species | Sites | ||||
|---|---|---|---|---|---|---|---|
| Sudano-Sahelian zone | Sudano-Guinean zone | Sudanian zone | |||||
| Koumbidia Soce | Missirah | Togue | Sare Yoba | Mandina Findifeto | |||
|
Gigasporaceae |
Racocetra | Racocetra sp. aff. gregaria | + | + | + | + | + |
| Dendiscutata | Dentiscutata sp. aff. heterogama | + | + | + | + | + | |
| Scutellospora | Scutellospora sp. aff. dipurpurascens | + | + | + | + | + | |
|
Gigaspora |
Gigaspora sp.1 | + | + | + | + | + | |
| Gigaspora sp.2 aff. gigantea | + | + | + | + | + | ||
| Gigaspora sp.3 aff. albida | + | – | + | + | – | ||
| Gigaspora sp.4 aff. rosea | + | + | – | – | – | ||
| Gigaspora sp.5 | – | + | + | + | – | ||
| Acaulosporaceae | Acaulospora | Acaulospora sp.1 | + | + | + | + | + |
| Acaulospora sp.2 | + | + | – | – | – | ||
| Ambisporaceae | Ambispora | Ambispora sp. | + | + | + | – | – |
| Glomeraceae | Glomus | Glomus sp.1 | + | + | + | + | – |
| Glomus sp.2 | + | + | + | + | + | ||
| Glomus sp.3 | + | + | + | + | + | ||
| Glomus sp.4 | + | + | + | + | + | ||
| Glomus sp.5 | – | – | – | + | – | ||
| Glomus sp.6 | – | – | – | + | – | ||
| Glomus sp.7 | + | + | – | – | – | ||
| Glomus sp.8 | + | – | + | – | + | ||
| Sclerocystis | Sclerocystis sp. | + | + | + | + | + | |
| 4 families | 8 genera | 20 AMF species | 17 | 16 | 15 | 15 | 11 |
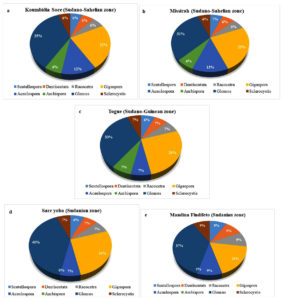
The abundance of AMF genera in each soil was presented in Figure 4 (a, b, c, d, e). Glomus was the most diverse genus with 8 AMF species, and accounted for around 35%, 35%, 31%, 33%, 40% and 36% of total abundance in Koumbidia Soce (Figure 4a), Missirah (Figure 4b), Togue (Figure 4c), Sare Yoba (Figure 4d) and Mandina Findifeto (Figure 4e), respectively. It was followed by Gigaspora with 5 AMF species accounting for 18.18% to 26.65% of total abundance across sites. Acaulospora was represented by 2 AMF species accounting for 6.67% to 12.5% of total abundance across sites. Although being each represented by one AMF species, the genera Dendiscutata, Racocetra, Scutellospora and Sclerocystis were found in all sites. Ambispora represented by one AMF species was not found in Sare Yoba and Mandina Findifeto, the two sites located in the Sudanian zone (Table 4). Meanwhile, it contributed from 6% to 7% of total abundance in each site where it was found (Figures 4d and 4e).
Thus, the trapping culture revealed 8 more AMF species (Gigaspora sp.4 aff. rosea, Gigaspora sp.5, Acaulospora sp.2, Ambispora sp., Glomus sp.5, Glomus sp.6, Glomus sp7, Glomus sp.8) than direct observation from field-collected soils.
Soil enzyme activities
Soil FDA-hydrolytic activity in Koumbidia Soce (0.53 µg FDA/g of soil/h) was significantly higher than those in Togue, Sare Yoba and Mandina Findifeto. The lowest FDA-hydrolytic activity was obtained in the Mandina Findifeto soil with 0.27 µg FDA/g of soil/h (Figure 5A). The activity of acid phosphatase was significantly higher in soils from Koumbidia Soce (188.98 µg pNPP /g of soil/h) and Missirah (187.14 µg pNPP /g of soil/h), the two sites located in the Sudano-Sahelian zone, as compared to other sites (Figure 5B). There were no statistically significant differences in acid phosphatase activity between soils collected from Togue, Sare Yoba and Mandina Findifeto. However, the greatest alkaline phosphatase activity was obtained in soil collected from Togue (276.67 µg pNPP /g of soil/h), followed by that from Missirah, Sare Yoba, Mandina Findifeto and Koumbidia Soce (Figure 5C).
 Figure 5. Enzyme activities of field-collected soils
Figure 5. Enzyme activities of field-collected soils
(A) FDA (Fluorescein diacetate. μg FDA/g of soil/h). (B) PHA and (C) PHB (Phospatasis acid and Basis. μg pNPP/g of soil/h)
Boxes followed by the same letter are not significantly different according to the Kruskal-Wallis test (P < 0.05)
Correlation matrix between density and diversity of AMF, soil physicochemical properties and soil enzyme activities
Soil N, P, P2O5, C and OM had significant positive correlations between them (Figure 6). Soil pH was strongly positively correlated with AMF spore density (r2 = 0.650, P-value = 0.235) and soil mycorrhizal potential (r2 = 0.820, P-value = 0.089); and negatively correlated with the diversity of AMF from field-collected soil (r2 = -0.419, P-value = 0.235) and the diversity of AMF from trap culture (r2 = -0.810, P-value = 0.096). Soil C, N, P and available P were negatively correlated with soil MPN, spore density and the diversity of AMF from field-collected soils; and positively correlated with the diversity of AMF from trap culture although those correlations were not significant.
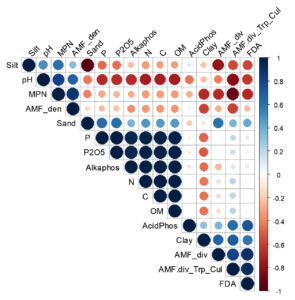 Figure 6. Correlation matrix of the different variables (AMF density and diversity. soil physicochemical properties and soil enzyme activities)
Figure 6. Correlation matrix of the different variables (AMF density and diversity. soil physicochemical properties and soil enzyme activities)
Positive correlations are displayed in blue and negative correlations in red color. Color intensity and size of circle are proportional to correlation coefficients. In the right side of the correlogram. the legend color shows the correlation coefficients and the corresponding colors
In addition, soil alkaline phosphatase was positively and significantly correlated with total C (r2 = 0.989, P-value = 0.001), total N (r2 = 0.988, P-value = 0.002), total P (r2 = 0.945, P-value = 0.015), available P (r2 = 0.989, P-value = 0.015), and OM (r2 = 0.990, P-value = 0.01). A positive and significant relationships between soil FDA activity and the diversity of AMF from field-collected soils in one hand (r2 = 0.893, P-value = 0.041); and the diversity of AMF from trap culture (r2 = 0.918, P-value = 0.028) were noted. The correlation between the diversity of AMF from trap culture and MPN was significantly negative (r2 = -0.906, P-value = 0.034).
Understanding the microbial community diversity and structure in the soil-plant continuum is essential to harness beneficial plant-microbe interactions in agricultural ecosystems.51-53 Indeed, it would help in developing efficient inoculants and sustainable strategies for the successful manipulation of microbial communities to improve crop yields and soil resilience.54-56
Here, we analyzed the density and diversity of AMF; and the enzyme activities in soils from 5 fonio millet agroecosystems in Senegal. Our results revealed that agroecological conditions influence AMF spore density and diversity. These findings might be partially explained by differences in physicochemical characteristics of soils and rainfall. Previous study from Ndoye et al.28 reported the influence of environmental factors on soil AMF spore density. Also, a significant difference in AMF spore density between three agroecological zones of the Central African Republic was observed by Djasbe and colleagues.57 This is consistent with the results of Maffo and coworkers58 obtained from two agroecological zones in Cameroon.
On the other hand, it has been reported that AMF inoculum potential has a major influence on mycorrhizal effectiveness and early root infection.59 In this study, the observed high AMF spore density and mycorrhizal inoculum potential in Mandina Findifeto might be partially attributed to its lower clay, nutrient and organic matter contents; and higher pH and silt content as compared to other sites. Similarly, Swarnalatha and colleagues60 had obtained a higher AMF spore density in a silty sandy loam soil compared to a silty clay loam soil. Moreover, the presence of clay might reduce the production of AMF spores.60 These findings indicate the influence of soil type on AMF density.
One of the objectives of the present work was to determine the AMF diversity in soils from five fonio millet agrosystems. A total of 12 species from field-collected soils and 20 species from trap culture was recorded with differences between sites. Those site effects could be linked to soil physicochemical properties and environmental conditions.60,61 The negative correlations obtained between soil nutrient contents and AMF spore density and diversity collaborate other findings.61,62 In fact, it is reported that soil mineral nutrients, specially P might influence AMF diversity and density.63 For example, in North China, study of Lang and colleagues64 in a long-term field experiment revealed that AMF alpha diversity gradually decreased as the P fertilizer rate increased. On the other hand, Delroy and colleagues51 found that the diversity of AMF tends to expand at optimal P. However, evidence points out that P supply does not necessarily have a detrimental effect on AMF diversity.65 Those results suggested that besides nutrient contents, other parameters (organic matter, humidity, pH, etc) might influence soil AMF parameters.63 Previous studies have shown the influence of soil pH and rainfall on AMF sporulation,66,67 spore density and richness.68-70 In this respect, Zhao and colleagues71 reported that increase in temperature and precipitation can promote mycelia and spore development by allowing the plant to supply more photosynthetic products to AMF.
Furthermore, several studies have focused on how geographic distance and the local environment affect the structure of AMF communities.72-74 Congruently, our results as well as those obtained from other agroecosystems in Senegal75,76 showed site effects on AMF diversity. In the present work, AM fungi species richness in field-collected soils and trap culture was higher in Koumbidia Soce site which contains higher amounts of N, P, C, OM, sand and clay than Mandina Findifeto site. It is apparent that abiotic factors, particularly soil chemical properties, can influence the AMF community structure and abundance.76,77 Results of Song et al.78 on Sephora flavescens Ait in China also supports the hypothesis that soil chemicals exert a selective effect of soil AMF population.
On the other hand, due to their effects on several ecosystem processes such as soil geochemical cycles, plant diversity and productivity, and soil composition, Glomeromycota communities have a wide environmental impact.19 Glomus was the dominant genus in our sites as observed in various environments.74-76,79 This might be related to their greater environmental adaptability and capacity to colonize plant roots more widely because of their efficient production of mycelia and spores.80 Moreover, Glomus species have been reported to promote fonio growth and yield under glasshouse conditions.9 In the present study, the low spore density and diversity of Scutellospora and Racocetra might be explained by their huge spores which take longer to mature than small spores81 and/or by the ability to grow only from an intact mycelium or with live spores.82,83
In addition, Glomus, Dendiscutata and Scutellospora dominated in Mandina Findifeto site which contained the lowest amounts of nutrient contents and OM compared to other sites. In contrast, Acaulospora has greater abundance in Togue site and lower abundance in Mandina Findifeto. Songachan and Kayang83 noted that Glomus species dominated in natural sites and Acaulospora species in cultivated ones, due probably to the failure of hyphal network disturbance in environments that might have benefited Glomus species.
In this work, some AMF species (belonging to Ambispora, Glomus and Gigaspora) revealed by trap culture were not detected by spore identification from field-collected soils. Similar findings were reported by Leal et al.,84 Chairul et al.85 and Rodriguez-Morelos et al.86 This demonstrated that cryptic AMF spores that are invisible during sampling or in field conditions can be encouraged to germinate through trap culture.44,87 This shows the importance of the combination of spore identification from trap culture and field-collected soils in AMF analysis.86
Furthermore, it was shown that soil pH might affect directly or indirectly AMF community composition by impacting P availability.80,88 Our results revealed positive relationships between soil mycorrhizal inoculum potential, AMF density and soil pH even if that was not significant. Bainard et colleagues77 found a negative correlation between some AMF species and phosphate concentrations in the soil. Thus, the main factors influencing the spatial variation in the AMF community across the site appeared to be soil pH or pH-driven changes in soil chemistry and Electrical conductivity.70,89
The lower AMF diversity observed in Mandina Findifeto compared to Togue and other sites might partially be related to soil OM and carbon contents as reported by Zhang et al.90
Moreover, many studies have shown correlations between soil nutrients and enzymatic activities,91,92 as confirmed by our study. Also, we found that soil FDA activity was positively and significantly correlated with AMF diversity and negatively with soil MPN and soil pH. This is consistent with the study of Cheng and coworkers93 showing a positive correlation between AMF diversity and soil enzyme activities. However, the correlation between soil alkaline phosphatase and soil nutrient contents was positive except for soil pH. In this way, Moradi et al.94 observed a positive correlation between acid and alkaline phosphatase activity, soil OM and N.
Our study shows an appreciable AMF density and diversity in the five tropical soils in Senegal. The results in field-collected and trap culture samples, respectively, revealed 12 and 20 species of AMF belonging to 8 genera and 4 families from 5 fonio millet agroecosystems into three climatic zones (Sudanian, Sudano-Sahelian and Sudano-Guinean). The AMF diversity increases with the trap culture. In both field-collected and trap culture soils, Glomus was the dominant genus in term of spore density and diversity in the five agroecosystems. The abundance and diversity of AMF species and FDA-hydrolytic and phosphatase activities varied between fonio agroecosystems as well as between climatic zones. Thus, abiotic factors like soil physicochemical properties might influence AMF spore density and diversity. Furthermore, Soil pH and texture were the variables that best explained the distribution of AMF spores.
This work contributes to our understanding of diversity and ecology of AMF in fonio millet agroecosystems. It therefore contributes to paving the way towards the development of microbial engineering approaches for agronomic improvement of fonio millet. However, more studies are necessary to better identify and explain the main driving factors of AMF community at different locations.
ACKNOWLEDGMENTS
The authors would like to thank the LMI LAPSE (IRD) and the WAAPP Fonio project (CERAAS/ISRA) for the financial support. The authors are also grateful to the reviewers for their relevant comments and suggestions which helped us to improve the quality of the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This work was funded by the LMI LAPSE (Laboratoire International Adaptation des Plantes et microorganismes associés aux Stress Environnementaux, IRD) and the WAAPP/PPAAO 2A (West Africa Productivity Program, CERA58I06 SE).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Abdul SD, Gayaunan D, Sawa FB, et al. Proximate composition of Digitaria species (exilis and iburua) in Bogoro LGA of Bauchi State. Madridge J Food Technol. 2023; 8(1):210-213.
Crossref - Wilma SS, Emeses BO, Rambo EK. Prospects and constraints to Ache production and processing In Bogor local Government area of Bache State: Implication to Relevant Technology Transfer. IOSR J Agr Vet Sci. 2018;11(7):58-64
- Adegbola RQQ, Otitodun GO, Jimoh MO, et al. Digitaria species (Acha): panacea for malnutrition and food insecurity in Nigeria. Int J Agr App Sci. 2023;4(2):17-26.
Crossref - Zhu F. Fonio grains: Physicochemical properties, nutritional potential, and food applications. Compr Rev Food Sci Food Saf. 2020;19(6):3365-3389.
Crossref - Deriu AG, Vela AJ, Ronda F. Techno-Functional and Gelling Properties of Acha (Fonio) (Digitaria exilis stapf) Flour: A Study of Its Potential as a New Gluten-Free Starch Source in Industrial Applications. Foods. 2022;11(2):183.
Crossref - Talabi AO, Vikram P, Thushar S, et al. Orphan Crops:A Best Fit for Dietary Enrichment and Diversification in Highly Deteriorated Marginal Environments. Front Plant Sci. 2022;13:839704.
Crossref - Wang X, Chen S, Ma X, et al. Genome sequence and genetic diversity analysis of an under-domesticated orphan crop, white fonio (Digitaria exilis). Giga Sci. 2021;10(3)
Crossref - Kanlindogbe C, Sekloka E, Kwon-Ndung EH. Genetic Resources and Varietal Environment of Grown Fonio Millets in West Africa:Challenges and Perspectives. Plant Breed Biotech. 2020;8:77-88.
Crossref - Ndoye F, Diedhiou AG, Gueye M, et al. Reponse du fonio (Digitaria exilis Stapf) a l’inoculation avec des champignons mycorhiziens a arbuscules en conditions semi-controlees. J Appl Biosc. 2016;103:9783-9799
- Abrouk M, Ahmed HI, Cubry P, et al. Fonio millet genome unlocks African orphan crop diversity for agriculture in a changing climate. Nat Com. 2020;11(1):4488.
Crossref - Enyiukwu DN, Bassey IN. Fonio (Digitaria spp.):The Good Tasting, Potential Food Security Cereal for Africa begs for Research Attention. Direct Res J Agr Food Sci. 2020;8(7):231-238.
- Diop BM, Gueye MC, Leclerc C, et al. Ethnolinguistic and genetic diversity of fonio (Digitaria exilis) in Senegal. Plants Peop Plan. 2023:1-13.
Crossref - Soumare A, Diedhiou AG, Kane A. Bambara groundnut:a neglected and underutilized climate-resilient crop with great potential to alleviate food insecurity in sub-Saharan Africa. J Crop Impr. 2022;36(5):1-21.
Crossref - Shi Z, Zhang J, Lu S, Li Y, Wang F. Arbuscular mycorrhizal fungi improve the performance of sweet sorghum grown in a Mo-contaminated soil. J Fungi. 2020;6(2):44.
Crossref - Yan P, Hou H, Lv Y, et al. Diversity characteristics of arbuscular mycorrhizal fungi communities in the soil along successional altitudes of Helan Mountain, arid, and semi-arid regions of China. Front Microbiol. 2023;14:1099131.
Crossref - Bello TT, Fabiyi OA. Arbuscular Mycorrhizal Fungi and Attainment of Food Security. Ansari RA, Rizvi R, Mahmood I. (eds). Myc Symb Agroec Rest. 2024:31-50.
Crossref - Fokom R, Eke P, Adamou S, Therese NO, Fabrice FB, Dieudonne N. Arbuscular mycorrhizal fungi symbiosis and food security. Developments in Applied Microbiology and Biotechnology.. 2023:227-244.
Crossref - Sheikh-Assadi M, Khandan-Mirkohi A, Taheri MR, Babalar M, Sheikhi H, Nicola S. Arbuscular mycorrhizae contribute to growth, nutrient uptake, and ornamental characteristics of Statice (Limonium sinuatum [L.] Mill.) subject to appropriate inoculum and optimal phosphorus. Hort. 2023;9(5):564.
Crossref - Wahab A, Muhammad M, Munir A, et al. Role of Arbuscular Mycorrhizal Fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants. (Basel), 2023;12(17):3102.
Crossref - Mbodj D, Effa-Effa B, Kane A, et al. Arbuscular mycorrhizal symbiosis in rice:establishment, environmental control and impact on plant growth and resistance to abiotic stresses. Rhizosphere. 2018;8:12-26.
Crossref - Mulyadi, Jiang L. Combined Application of Arbuscular Mycorrhizal Fungi (AMF) and nitrogen fertilizer alters the physicochemical soil properties, nitrogen uptake, and rice yield in a polybag experiment. Agric. 2023;13(7):1364.
Crossref - Thomopoulos S, Elsgaard L, Juhl Munkholm L, Ravnskov S. Evaluation of the relation between soil biomass of arbuscular mycorrhizal fungi and glomalin-related soil protein in conservation agriculture. Soil Biol Biochem. 2023;187:109222.
Crossref - Fall AF, Nakabonge G, Ssekandi J, et al. Roles of arbuscular mycorrhizal fungi on soil fertility:Contribution in the improvement of physical, chemical, and biological properties of the soil. Front Fung Biol. 2022;3:723892.
Crossref - Diedhiou AG, Mbaye FK, Mbodj D, et al. Field Trials Reveal Ecotype-Specific Responses to Mycorrhizal Inoculation in Rice. PLoS ONE. 2016;11(12):e0167014.
Crossref - Sun Z, Song J, Xin X, Xie X, Zhao B. Arbuscular mycorrhizal fungal proteins 14-3-3- are involved in arbuscule formation and responses to abiotic stresses during AM symbiosis. Front Microbiol. 2018;5:9-19.
Crossref - Diagne N, Ngom M, Djighaly PI, et al. Roles of Arbuscular Mycorrhizal Fungi on lant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity. 2020;12(10):370.
Crossref - Jamiolkowska A, Thanoon AH, Skwarylo B, Patkowska E, Mielniczuk E. Mycorrhizal inoculation as an alternative in the ecological production of tomato (Lycopersicon esculentum mill.). Int Agrophys. 2020;34(2):253-264.
Crossref - Ndoye F, Kane A, Ngonkeu E, et al. Changes in land use system and environmental factors affect Arbuscular Mycorrhizal Fungal density and diversity, and enzyme activities in rhizospheric soils of Acacia senegal (L.) Willd. Int Scho Res Net ISRN Ecol. 2012:563191
- Diop I, Ndoye F, Diedhiou AG, et al. Diversity and spore density of arbuscular mycorrhizal fungi in the rhizosphere of Cowpea (Vigna unguiculata [ L .] Walp.) cultivated in different soils in Senegal. J Anim Plant Sci. 2021;48(1):8552-8565.
- Dhumal KC, Shinde BP. Impact of chemical properties of soil on spore density, colonization and distribution of native arbuscular mycorrhizal fungi associated with Capsicum annuum L. J Appl Biol Biot. 2020;8(05):059-067.
Crossref - Zhang J, Quan C, Ma L, Chu G, Liu Z, Tang X. Plant community and soil properties drive arbuscular mycorrhizal fungal diversity:A case study in tropical forests. Soil Ecol Lett. 2021;3(1):52-62.
Crossref - Rasmussen PU, Abrego N, Roslin T, et al. Elevation and plant species identity jointly shape a diverse arbuscular mycorrhizal fungal community in the High Arctic. New Phytol. 2022:236(2):671-683.
Crossref - Rodriguez-Echeverria S, Teixeira H, Correia M, et al. Arbuscular mycorrhizal fungi communities from tropical Africa reveal strong ecological structure. New Phytol. 2017;213(1):380-390.
Crossref - Katja Kozjek, Dominika Kundel, Kushwaha SK, et al. Long-term agricultural management impacts arbuscular mycorrhizal fungi more than short-term experimental drought. Appl Soil Ecol. 2021;168:104140.
Crossref - Diop BM, Gueye MC, Agbangba CE, et al. Fonio (Digitaria exilis (Kippist) Stapf): A socially embedded cereal for food and nutrition security in Senegal. Ethno Letters. 2018;9(2):150-165.
Crossref - Disale AS, D P Chavan DP, Alameen AS, Undre P. Soil Characterization Using Physical and Chemical Properties. J Phys Conf Ser. 2020;1644(1):012026.
Crossref - Okolo CC, Gebresamuel G, Zenebe A, et al. Soil organic carbon, total nitrogen stocks and CO2 emissions in top and subsoils with contrasting management regimes in semi arid environments. Sci Rep. 2023;13(1):1117.
Crossref - Bibi S, Irshad M, Ullah F, et al. Phosphorus extractability in relation to soil properties in different fields of fruit orchards under similar ecological conditions of Pakistan. Front Ecol Evol. 2023;10:1077270.
Crossref - Gagou E, Chakroune K, Abbas M, Lamkami T, Hakkou A. Evaluation of the mycorrhizal potential of date palm (Phoenix dactylifera L.) rhizosphere soils in the Figuig Oasis (Southeastern Morocco). J Fungi. 2023;9(9):931.
Crossref - Founoune-Mboup H, Diallo B, Adigoun RFR, Kane A, Fall AF. Contribution of arbuscular mycorrhizal fungi to the bioavailability of micronutrients (iron and zinc) in millet accessions. Front Plant Sci. 2024;15:1364469.
Crossref - Fisher RA Yates F. Statistical tables for biological agriculture and medical research, sixth ed. Hafner Publ Comp, Davien. 1948.
- Fisher RA, Yates, F. Statistical Tables for Biological and Medical Research. 6th edition. Hafner Publ Comp Davier. 1970.
- Boya S, Puttaswamy P, Mahadevappa N, Sharma B, Othumbamkat R. Enumerating Indigenous Arbuscular Mycorrhizal Fungi (AMF) Associated with Three Permanent Preservation Plots of Tropical Forests in Bangalore, Karnataka, India. Bacteria. 2023;2(1):70-80.
Crossref - Makdoh K, Kayang K. Diversity of Arbuscular Mycorrhizal Fungi in trap cultures prepared from abandoned coalmine overburden spoils. J Pure Appl Microbiol. 2019;13(1):629-636.
Crossref - Ndoye F, Kane A, Bakhoum N, et al. Response of Acacia senegal (L.) Willd. to inoculation with arbuscular mycorrhizal fungi isolates in sterilized and unsterilized soils in Senegal. Agrof Syst J. 2013;87(4):941-952.
Crossref - Patle PN, Navnage NP, Barange PK. Fluorescein Diacetate (FDA): Measure of Total Microbial Activity and as Indicator of Soil Quality. Int J Curr Micr App Sci. 2018;7(6):2103-2107.
Crossref - Margalef O, Sardans J, Fernandez-Martinez M, et al. Global patterns of phosphatase activity in natural soils. Sci Rep. 2017;7(1):1337.
Crossref - Azene B, Zhu R, Pan K, et al. Land use change alters phosphatase enzyme activity and phosphatase-harboring microbial abundance in the subalpine ecosystem of southeastern Qinghai-Tibet Plateau, China. Ecol Ind. 2023;153:110416.
Crossref - Alvarez-Lopeztello J, del Castillo RF, Robles C, et al. Arbuscular mycorrhizal fungi improve the growth of pioneer tree species of tropical forests on savanna and tropical rainforest soils under nursery conditions. Sci Fung. 2021; 51:1296.
Crossref - De Benedetto D, Montemurro F, Diacono M. Repeated geophysical measurments in dry and wet soil conditions to describe soil water variability. Sci Agric. 2020;77(5):e20180349.
Crossref - Delroy B, Zhang HY, Bissett A, Powell JR. Divergent responses between lineages of arbuscular mycorrhizal fungi to soil phosphorus and nitrogen availability. Pedol-J Soil Ecol. 2024;103:150934.
Crossref - Hartman K, van der Heijden MGA, Wittwer RA , Banerjee S, Walser JC, Schlaeppi K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome. 2018;6(1):14.
Crossref - Mofini MT, Diedhiou AG, Simonin M, et al. Cultivated and wild pearl millet display contrasting patterns of abundance and co-occurrence in their root mycobiome. Sci Rep. 2022;12(1):207.
Crossref - Cheng YT, Zhang L, He SY. Plant-microbe interactions facing environmental challenge. Cell Host Microbe. 2019;26(2):183-192.
Crossref - Singh BK, Trivedi P, Egidi E, Macdonald CA, Delgado-Baquerizo M. Crop microbiome and sustainable agriculture. Nat Rev Microbiol. 2020;18(11):601-602.
Crossref - Dondjou DT, Diedhiou AG, Mbodj D, et al. Rice developmental stages modulate rhizosphere bacteria and archaea co-occurrence and sensitivity to long-term inorganic fertilization in a West African Sahelian agro-ecosystem. Env Microbiome. 2023;18(1):1-17.
Crossref - Djasbe MD, Elian HDB, Diop TA, Ndoumou DO. Diversity of Arbuscular mycorrhizal fungi in the three agroecological zones of the Central African Republic. Afr J Biotec. 2021;21(1):26-34.
Crossref - Maffo AF, Ngonkeu ELM, Chaintreuil C, et al. Morphological and molecular diversity of arbuscular mycorrhizal fungi associated to Carica papaya L. rhizosphere in two agro-ecological zones in Cameroon. Afr J Agric Res. 2022;18(8):632-646.
Crossref - Jia T, Zhang Y, Yao Y, et al. Effects of AMF inoculation on the eco-physiological characteristics of Imperata cylindrica under differing soil nitrogen conditions. Front Plant Sci. 2023;14:1134995.
Crossref - Swarnalatha K, Trimurtulu N, Ammani K, Ashok S. AM fungal spore diversity in different agroclimatic zones of Andhra Pradesh, India. Int J Curr Microbiol App Sci. 2017;6(3):1496-1505.
Crossref - Egboka NT, Agim LC, Okon MA, Okolia NH, Afangidea AI, Okonjob PN. Population density of arbuscular mycorrhizal fungi and physico-chemical properties of soils as affected by cropping systems. J CleanWas. 2022;6(1):27-32.
Crossref - Lin C, Wang Y, Liu M, et al. Effects od nitrogen deposition and phosphorus addition on arbucular mycorrhizal fungi of Chinese fir (Cunninghamia lanceolata). Sci Rep. 2020; 10:12260.
Crossref - Zhang S, Luo P, Yang J, et al. Responses of Arbuscular Mycorrhizal Fungi Diversity and Community to 41-Year Rotation Fertilization in Brown Soil Region of Northeast China. Front Microbiol. 2021;12:742651.
Crossref - Lang M, Zhang C, Su W, Chen X, Zou C, Chen X. Long-term P fertilization significantly altered the diversity, composition and mycorrhizal traits of arbuscular mycorrhizal fungal communities in a wheat-maize rotation. App Soil Ecol. 2022;170:104261.
Crossref - Wang XZ, Sui X, Liu Y, et al. N-P fertilization did not reduce AMF abundance or diversity but alter AMF composition in an alpine grassland infested by a root hemiparasitic plant. Plant Diver. 2018;40(3):117-126.
Crossref - Feng Z, Liu X, Qin Y, et al. Cooperation of arbuscular mycorrhizal fungi and bacteria to facilitate the host plant growth dependent on soil pH. Front Microbiol. 2023;14:1116943.
Crossref - Neupane J, Guo W, Cao G, Zhang F, Slaughter L, Deb S. Spatial patterns of soil microbial communities and implications for precision soil management at the field scale. Precis Agric. 2022;23:1008-1026.
Crossref - Janowski D, Leski T. Factors in the distribution of mycorrhizal and soil fungi. Diversity. 2022;14:1122.
Crossref - Dickey JR, Fordyce JA. Greater change in arbuscular mycorrhizal fungal richness as a response to short-term rainfall exclusion across the North American monsoon season. Elem Sci Anth. 2024;12(1):1-15.
Crossref - Zhou Y, Chen K, Muneer MA, et al. Soil moisture and pH differentially drive arbuscular mycorrhizal fungal composition in the riparian zone along an alpine river of Nam Co watershed. Front Microbiol. 2022;13:994918.
Crossref - Zhao LL, Zhang KX, Sun X, He X. Dynamics of arbuscular mycorrhizal fungi and glomalin in the rhizosphere of Gymnocarpos przewalskii in Northwest Desert China. Appl Soil Ecol. 2022;170:104251.
Crossref - Thanni B, Merckx R, De Bauw P, et al. Spatial variability and environmental drivers of cassava-arbuscular mycorrhiza fungi (AMF) associations across Southern Nigeria. Mycorrhiza. 2022;32(2):1-13.
Crossref - Ma X C, Geng QH, Zhang HG, et al. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multi-functionality. New Phytol. 2021;229(5):2957-2969.
Crossref - Jiang S, Hu X, Kang Y, et al. Arbuscular mycorrhizal fungal communities in the rhizospheric soil of litchi and mango orchards as affected by geographic distance, soil properties and manure input. Appl Soil Ecol. 2020; 152:103593.
Crossref - Samba-Mbaye RT, Anoir CM, Diouf D, et al. Diversity of arbuscular mycorrhizal fungi (AMF) and soils potential infectivity of Vachellia nilotica (L.) Hurter PJH, Mabb. rhizosphere in Senegalese salt-affected soils. Afr J Biotech. 2020;19(7):487-499.
Crossref - Thiocone KMO, Soumare A, Charahabil MM et al. Density and diversity of arbuscular mycorrhizal fungi in Anacardium occidentale L. plantations in Senegal. Afr J Micr Res. 2023; 17(9):221-229.
Crossref - Vieira LC, da Silva DKA, da Silva IR, et al. Ecological aspects of arbuscular mycorrhizal fungal communities in different habitat types of a Brazilian mountainous area. Ecol Res. 2019;34(1):182-192.
Crossref - Song J, Han Y, Bai B, Jin S, He Q, Ren J. Diversity of arbuscular mycorrhizal fungi in rhizosphere soils of the Chinese medicinal herb Sophora flavescens Ait. Soil Tillage Res. 2019a;195:104423.
Crossref - Song J, Chen L, Chen F, Ye J. Edaphic and host plant factors are linked to the composition of arbuscular mycorrhizal fungal communities in the root zone of endangered Ulmus chenmoui Cheng in China. Ecol Evol. 2019b;9(15):8900-8910.
Crossref - Zhao H, Li XZ, Zhang ZM, Zhao Y, Yang J, Zhu Y. Species diversity and drivers of arbuscular mycorrhizal fungal communities in a semi-arid mountain in China. Peer J. 2017;5:e4155.
Crossref - Mukhongo RW, Ebanyat P, Masso C, Tumuhairwe JB. Composition and spore abundance of arbuscular mycorrhizal fungi in sweet potato producing areas in Uganda. Front Soil Sci. 2023;3:1152524.
Crossref - Biermann BJ, Linderman RG. Use of vesicular-arbuscular mycorrhizal roots, intraradical vesicles and extra-radical vesicles as inoculum. New Phytol. 1983;95(1):97-105.
Crossref - Songachan LS, Kayang H. Diversity of arbuscular mycorrhizal fungi associated with Flemingia vestita Benth. ex Baker. Mycology. 2013;4(2):85-95.
Crossref - Leal PL, Carvalho TS, Siqueira JO, Moreira FMS. Assessment of the occurrence and richness of arbuscular mycorrhizal fungal spores by direct analysis of field samples and trap culture-a comparative study. An Acad Bras Cienc. 2017;90(Suppl 1):2359-2373.
Crossref - Chairul, Zozy AN, Suwirmen, Syamsuradi, Reini. Exploration of Indigenous Arbuscular Mycorrhizal Fungi on Post Mining Soil as Rehabilitation Strategy. J Biol Sci. 2019;19:218-223.
Crossref - Rodriguez-Morelos VH, Soto-Estrada A, Perez-Moreno J, Franco-Ramirez A, Pablo DR. Arbuscular mycorrhizal fungi associated with the rhizosphere of Swietenia macrophylla (Magnoliophyta: Meliaceae), in Los Tuxtlas, Veracruz, Mexico. Chil J Nat Hist. 2014;87:9.
Crossref - Covacevich F, Hernandez Guijarro K, Crespo EM, Lumini E, Mega MSR, Lugo MA. Arbuscular Mycorrhizal Fungi from Argentinean Highland Puna Soils Unveiled by Propagule Multiplication. Plants. 2021;10(9):1803.
Crossref - Davison J, Moura M, Semchenko M, et al. Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phyto. 2021;231(2):763-776.
Crossref - Li X, Qi Z, Yu X, et al. Soil pH drives the phylogenetic clustering of the arbuscular mycorrhizal fungal community across subtropical and tropical pepper fields of China. App Soil Ecol. 2021;165:103978.
Crossref - Zhang L, Xu M, Liu Y, et al. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016;210(3):1022-1032.
Crossref - Wang L, Hamel C, Lu P, et al. Using enzyme activities as an indicator of soil fertility in grassland-an academic dilemma. Front Plant Sci. 2023;14:1175946.
Crossref - Jian S, Li J, Chen J, et al. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization:a meta-analysis. Soil Biol Biochem. 2016;101:32-43.
Crossref - Cheng Y, Xu X, Zhang Y, et al. Intercropping of Echinochloa frumentacea with leguminous forages improves hay yields, arbuscular mycorrhizal fungi diversity, and Soil enzyme activities in saline-alkali soil. Agro. 2023;13(9):2356.
Crossref - Moradi M, Shirvany A, Matinizadeh M, et al. Arbuscular mycorrhizal fungal symbiosis with Sorbus torminalis does not vary with soil nutrients and enzyme activities across different sites. IForest. 2015;8(3):308-313.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.