ISSN: 0973-7510
E-ISSN: 2581-690X
India is facing a new wave of Lumpy skin disease outbreaks since May 2022, spreading in more than 22 states and causing morbidity to more than 29 lakh animals and mortality to more than 2 lakh animals. Lack of specific antiviral treatment restores symptomatic therapeutic interventions. However, in the advent of large no. of cases and severity of disease, investigations on specific antiviral drugs are imperative. This scientific study was conducted on a group of LSD-affected cattle (n = 40) from the trans-Himalayan region of Kashmir (Jammu and Kashmir). The affected cattle were subjected to different treatments, including acyclovir (n = 27), symptomatic treatment (n = 7), and ivermectin (n = 6), along with supportive drugs. The animals were carefully monitored and compared both within and between groups at various intervals (0-96 hr) using a two-way analysis of variance (ANOVA). Significant (P ≤ 0.05) improvement in regaining rectal temperature, respiration rate, and heart rate was noted 48 hours after treatment in a group of LSD-affected animals (n = 27) treated with acyclovir at a dose rate of 1 mg/kg body weight (small cattle) to 1.5 mg/kg (large cattle) intravenously in 500 ml normal saline along with supportive drugs including enrofloxacin (2.5 mg/kg), combination of meloxicam (0.25 mg/kg) and paracetamol (7.5 mg/kg) and pheniramine maleate (0.5 mg/kg) all intramuscularly, compared to group of LSD affected cattle (n = 6) treated with ivermectin (0.2 mg/kg subcutaneously) and supportive drugs and another group of LSD affected cattle (n = 7) treated symptomatically with only supportive drugs. Acyclovir treated group showed a significant (P ≤ 0.05) decrease in total leucocyte count, lymphocyte, and basophil count from 0 hour to 96 hour of treatment (23.00 ± 0.534 to 5.59 ± 0.208; 13.97 ± 0.310 to 3.43 ± 0.126; 0.11 ± 0.003 to 0.03 ± 0.001 respectively). Significant (P ≤ 0.05) decrease in total oxidative status (TOS: 73.31%) and increase in total antioxidant status (TAS: 59.9%) was observed in acyclovir treated group followed by ivermectin treated group (TOS: 68.05% and TAS: 27.16%) compared to symptomatically treated group (TOS: 42.41% and TAS: 18.75%). Acyclovir being comparatively more specific antiviral agent than ivermectin may have helped in amelioration of clinical severity and regaining of normal physiological, hematological, and oxidative indices in LSD-affected animals. The current study demonstrates expedited recovery, diminished clinical severity, and re-establishment of physiological, hematological and oxidative markers in animals subjected to acyclovir treatment, followed by animals administered with ivermectin, when compared to animals receiving symptomatic treatment. However, further studies are required to investigate safety or adverse effects, if any.
Acyclovir, Antiviral, Cattle, lvermectin, Lumpy Skin Disease, Treatment, Virus
Recent outbreaks of lumpy skin disease in bovines around the globe have brought into the picture this infectious transboundary disease of viral origin and thus becoming World Organization of Animal Health (WOAH)-marked highly contagious vector-borne emerging transboundary pox-viral infection of bovine species.1 Lumpy skin disease (LSD) is a notifiable viral disease caused by Lumpy skin disease virus (LSDV) of the family Poxviridae, subfamily Chordopoxviridae, and genus Capripoxvirus.2-4 Occurrence of LSD outbreaks currently has become a cause of concern and a rise in morbidity and mortality has been noted which ranged from 50-100% and 5-10%, respectively.3,5 The Lumpy skin disease (LSD) virus exhibits a high degree of species specificity, primarily infecting cattle, buffalo, and closely related wildlife. Among these species, cattle display heightened susceptibility to LSD compared to others. While LSD can affect animals of all ages, younger individuals are more prone to the disease.6 The risk of LSD occurrence is subject to various factors that evolve over time. Notably, the incidence of LSD cases escalates significantly with increasing temperatures, particularly when surpassing 35°C, resulting in the highest documented number of disease cases.7 In tropical and subtropical regions cattle are kept outside throughout the year thus increasing the chances of contact with the vector, therefore the disease incidence. In many areas free grazing is practiced which significantly increases the likelihood of encounters with blood-sucking insects.8
The health of afflicted animals has been significantly impacted, resulting in diminished growth and decreased milk production, thereby leading to substantial financial losses for farmers.5,9,10 LSD outbreaks in various states of India resulted in an estimated economic loss of INR 18337.76 crores (USD 2217.26 million) in the current outbreak.5 Milk production has decreased by 26% in affected areas.11 From September 2022, LSD has spread from 251 districts in 15 states (20 lakh infected, 1 lakh dead) to 22 states infecting 29.45 lakh cattle and causing death to more than 2.0 lakh animals.12,13 LSD affected animals exhibited generalized cutaneous nodules along with high fever, inappetence to anorexia, lethargy, reduced milk yield, salivation, lacrimation, nasal discharge, ventral oedema, ocular deformity, dyspnea, and death.5,10,14 Complications included ulceration of nodules, sloughing, suppuration, maggot infestation, corneal opacity, pneumonia, and mastitis.2,5 Prevention strategies include a range of stringent measures such as implementing strict quarantine protocols, imposing confinement and movement restrictions on animals, isolating and carrying out the slaughter of affected animals, administering effective vector control measures, and ensuring proper vaccination of animals.15 Live attenuated and inactivated vaccines have been utilized for the prevention of LSD. Live attenuated LSDV isolates, including the Neethling and KSGP vaccines, as well as vaccinations based on the sheep/goat pox virus (SPPV/GPPV), are currently available options for LSDV immunization. Live attenuated vaccines offer a robust immune response lasting approximately 18 months, surpassing that of inactivated vaccines.
Lack of specific treatment protocol against LSD envisaged symptomatic treatment with the application of supportive drugs including antibiotics for secondary bacterial infections, antipyretics and analgesics for lowering fever and relieving inflammation and pain, antihistamines for allergy, and supplements including liver tonics, antioxidants, vitamin and mineral supplements.5,16 Though supportive treatment provided a bit of symptomatic relief in few cases but disease occurrence has not stopped and frequent mortality in affected animals has been noted which may have contributed to the emergence of recombinant variants having enhanced virulence or pathogenesis.12,17 This envisaged the exploration of specific antivirals against LSDV.
No specific antivirals have been developed against LSD.18 However, some antivirals have scope against poxviruses including nucleoside and nucleotide analogues.19 Cidofovir has proved efficacious against cowpox, camelpox, monkeypox, and orf (sheep pox).19 However, side effects like nephrotoxicity and higher cost may limit its application.20 Acyclovir, (9-{2-hydroxy (ethoxy) methyl} guanine), a purine nucleoside analogue, has been used against poxviruses including human LSD-like conditions and herpes viruses of cattle or buffalo21-24 and horses25; however, there is no report of the use of acyclovir against LSD. Some compounds have been recommended as antivirals against LSDV including methylene blue,26 but we could not get any scientific publication for its use in LSD. Besides having antiparasitic action for prevention of vector borne transmission of LSD, ivermectin has shown in vitro antiviral activity against sheep poxvirus and LSDV.27
There is a paucity of scientific research on the application of antivirals including acyclovir and ivermectin against LSD. Acyclovir has prospects for antiviral chemotherapy in veterinary medicine because of its broad spectrum antiviral activity and is relatively safe for systemic administration.28,29 Ivermectin is reported to have antiviral action against DNA and RNA viruses besides being the common endo-ectoparasiticide.30-32
The present study reports the effect of acyclovir and ivermectin on LSD affected cattle and may serve as the basis for treating animals affected in present outbreaks of LSD.
This study was carried out in the trans-Himalayan region, Kashmir valley of Jammu and Kashmir Union Territory of India, having latitude 33° and 35°N, and longitude 73° and 76°E. The climate is temperate and livestock rearing practices are uniform. The study was carried out during the recent LSD outbreak33 starting from April 2023 to October 2023. A total of forty (n = 40) cattle affected with LSD were treated with acyclovir (n = 27), symptomatically (n = 7) and ivermectin (n = 6) along with supportive drugs. The animals were diagnosed based on clinical signs, polymerase chain reaction (PCR), ultrasonography (USG) and histopathology. The acyclovir treated group (27) included 16 adult cows of above 2 years of age (4 pregnant, 12 nonpregnant) administered at a dose of 1.5 mg/kg body weight, and 11 young cattle of below 2 years of age (4 bullocks, 7 heifers) administered at a dose of 1 mg/kg body weight given intravenously (IV) as a single shot in 500 ml normal saline. Another symptomatically treated group (7) included 3 cows, 3 heifers, and a bullock. The third group (6) was treated with ivermectin at a dose of 0.2 mg/kg given a single shot subcutaneously (SC) included 2 each of heifers, bullocks, and cows. These LSD affected animals were also given supportive drugs including enrofloxacin (2.5 mg/kg), a combination of meloxicam (0.25 mg/kg) and paracetamol (7.5 mg/kg), and pheniramine maleate (0.5 mg/kg), all intramuscularly (IM) once daily for 72 hours (3 days).
All animals were in the initial stages of infection and showing signs of fever, inappetence or anorexia, lethargy, cutaneous nodules on the head, neck, or entire body, swollen lymph nodes, salivation, nasal discharge, lacrimation (7), and oedema on ventral abdomen (9), brisket or legs (4) (Figure 1a-1n).
For ultrasonography, scanning of nodular lesions was done by using a linear probe of the USG machine (Esoate Mylab vet40, 10 MHZ, Esaote, Italy) with slight modifications of procedures described by Tharwat and Oikawa.34 Nodular lesions including skin scrapings were collected for histopathology and PCR.35 For histopathology, tissues were collected from recently developed nodules and fixed in 10% neutral buffered formalin (pH 7.4) for 72 h, washed, dehydrated, embedded in paraffin wax, serially sectioned at 5 µm thickness, and stained with hematoxylin and eosin for histopathological evaluation.36
Molecular confirmation was done by PCR as per Kumar et al.,12 following DNA extraction from nodular lesions by using the snap chill method.37 Briefly, the samples (nodules) were homogenized and centrifuged at 15000 rpm for 5 minutes. The pellet was then suspended in 1x PBS and mixed thoroughly, boiled for 10 minutes followed by chilling in ice for 5 minutes. Mixture was then centrifuged at 15000 rpm for 5 minutes, and supernatant containing DNA was collected in a separate vial and used as a template for PCR. PCR was run as per protocol12 by using ORF12 gene primers (GCC Biotech, India) and master mix (Thermo Fischer, India). The forward sequence for primers (ORF12) is 5′ ATGGAAAAGGAAAAATTATGTAGCG 3′ and reverse sequence is 5′ TTATTGTTTGTCAAAAAAGGTGAGATTTC 3′.12
As milk withdrawal time of acyclovir in cattle is not known, owners were advised for milk withdrawal for about 5 days following start of treatment in case of lactating animals as acyclovir has a short half-life in milk.38,39 Affected animals were not reared for meat purposes.
The animals were observed routinely and clinicopathological parameters were evaluated on a daily basis for 4 days (0 hour to 96 hour) including mortality, clinical signs, vital indices (rectal temperature, heart rate, and respiration rate), and complete blood count (CBC). The oxidative indices were evaluated from 0th hour to 48th hour.
CBC was done on fresh blood samples (2 ml) collected in EDTA vials through jugular vein puncture using an automatic veterinary hematology analyzer (Celltac a MEK-6550; Nihon Kohden, Japan). Total oxidant status (TOS) and total antioxidant status (TAS) were estimated from serum samples as per the method of Erel40,41 respectively.
Statistical analysis
Data was analyzed by two way ANOVA using SPSS (IBM India) for any significance at P ≤ 0.05 between and within the groups for various parameters at different time intervals.
The animals exhibited the characteristic symptoms of LSD, as previously described
(Figure 1). The nodules were firm, slightly raised, oval-round in shape, and well-defined, ranging in size from 0.5 to 5 cm. Ultrasonography revealed the presence of fluid-filled nodular lesions, manifesting as echogenic to hyperechogenic rings or capsules, containing anechoic to hypoechogenic fluid (Figure 2). Some nodules exhibited the presence of echogenic fibrous tissue, while other regions showed hypoechoic pyogenic material. Histopathology revealed tissue degeneration or necrosis, heavy cellular infiltration, and eosinophilic intracytoplasmic inclusion bodies (Figure 3a-3c). Edema, hyperemia, acanthosis, severe hydropic degeneration, and hyperkeratosis in the epidermis and mononuclear cell infiltration, inclusion bodies, and vasculitis in the dermis, were seen.
Figure 1. Clinical signs in LSD affected cattle
Nodular lesions, ocular lesions, lacrimation, corneal discolouration, salivation, nasal discharge, nasal erosions, ulceration, wounds, maggot infestation (a to n).
Figure 2. a). USG of nodular lesions in LSD affected cattle; b). Fluid filled nodule with hyperechogenicity and hyperechoic wall
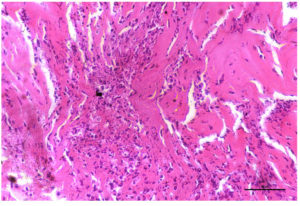 (a). Photomicrograph of skin revealing severe diffuse infiltration with inflammatory cells mainly neutrophils in the superficial and deep dermis. H & E X 40X
(a). Photomicrograph of skin revealing severe diffuse infiltration with inflammatory cells mainly neutrophils in the superficial and deep dermis. H & E X 40X
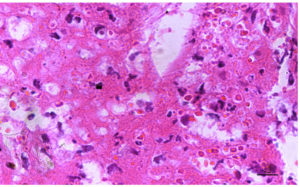 (b). Photomicrograph of skin revealing presence of Intracytoplasmic eosinophilic inclusions caused due to lumpy skin disease virus, H & E X 40X
(b). Photomicrograph of skin revealing presence of Intracytoplasmic eosinophilic inclusions caused due to lumpy skin disease virus, H & E X 40X
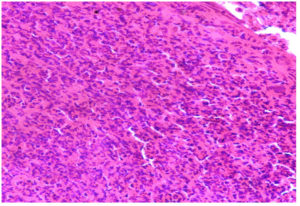 (c). Photomicrograph of skin revealing severe cellular infiltration with presence of Intracytoplasmic inclusion body virus, H & E 40X
(c). Photomicrograph of skin revealing severe cellular infiltration with presence of Intracytoplasmic inclusion body virus, H & E 40X
Figure 3. Histopathology of LSD lesions
On PCR characteristic bands of 636 bp representing the ORF12 gene fragment were noted (Figure 4).
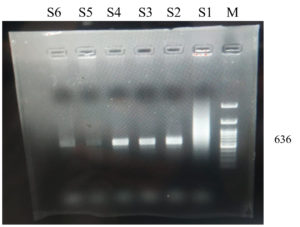 a). M= Marker (100 bp), S= samples (S1=smearing, S2-S4= strong positive, S5, S6=weak positive)
a). M= Marker (100 bp), S= samples (S1=smearing, S2-S4= strong positive, S5, S6=weak positive)
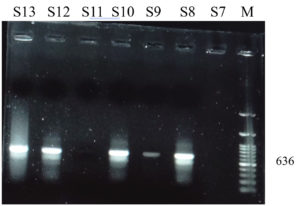 b). M= Marker (100 bp), S= samples (S7, S11=negative, S8-S10, S12, S13= positive)
b). M= Marker (100 bp), S= samples (S7, S11=negative, S8-S10, S12, S13= positive)
Figure 4. PCR products of LSDV showing characteristic bands for ORF12 gene
On receiving acyclovir and ivermectin no mortality was noted in treated animals even beyond the observation period. The effect of various treatment regimens on clinical signs of LSD is given in Table 1. The severity of LSD was reduced in acute febrile animals following intravenous acyclovir infusion along with supportive treatment. Fever, respiratory distress, nasal discharge, salivation, lacrimation, lethargy, and inappetence diminished within 48-72 hours in 22 animals however ventral oedema (11) and nodular lesions (22) in most cases started regressing by 96 hours and in few (5) they persisted for more than 120 hours but without further increasing or aggravating compared to other symptomatically treated group wherein rupturing of nodules, suppuration or maggot infestation was noted. The ivermectin treated group showed a comparatively similar clinical response as acyclovir but with a slight delay as depicted in Table 1. Milk yield showed recovery within 96 hours however owners were advised to discard milk for 5 days. Ivermectin treated animals showed clinical recovery comparable to acyclovir besides none of the LSD affected ivermectin treated animals developed any complications after use unlike symptomatic treated or untreated animals which developed ulceration or wounds and few of them developed maggots (Figure 1).
Table (1):
Effect of treatment on clinical signs
| Clinical sign | Group treated | Hour of treatment | ||||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | ||
| Skin nodules | Acyclovir treated | ++++ | ++++ | +++ | ++ | + |
| Symptomatic treated | ++++ | ++++ | +++++ | ++++ | ++++ | |
| Ivermectin treated | ++++ | ++++ | ++++ | ++ | ++ | |
| Fever | Acyclovir treated | ++++ | ++ | + | – | – |
| Symptomatic treated | ++++ | +++ | +++ | ++ | + | |
| Ivermectin treated | ++++ | ++ | + | – | – | |
| Lethargy | Acyclovir treated | ++++ | +++ | ++ | – | – |
| Symptomatic treated | ++++ | ++++ | +++ | +++ | ++ | |
| Ivermectin treated | ++++ | +++ | +++ | ++ | – | |
| Swollen lymph nodes | Acyclovir treated | ++++ | +++ | ++ | + | – |
| Symptomatic treated | ++++ | ++++ | ++++ | ++++ | +++ | |
| Ivermectin treated | ++++ | +++ | +++ | ++ | – | |
| Ventral oedema | Acyclovir treated | ++++ | ++++ | +++ | + | + |
| Symptomatic treated | ++++ | ++++ | ++++ | ++++ | +++ | |
| Ivermectin treated | ||||||
| Inappetence | Acyclovir treated | ++++ | ++ | + | – | – |
| Symptomatic treated | ++++ | ++++ | +++ | ++ | + | |
| Ivermectin treated | ++++ | ++ | + | – | – | |
| Salivation | Acyclovir treated | ++++ | ++ | + | – | – |
| Symptomatic treated | ++++ | +++ | +++ | ++ | + | |
| Ivermectin treated | ++++ | ++ | + | – | – | |
| Lacrimation | Acyclovir treated | ++++ | ++ | + | – | – |
| Symptomatic treated | ++++ | +++ | +++ | ++ | + | |
| Ivermectin treated | ++++ | ++ | ++ | + | – | |
| Nasal discharge | Acyclovir treated | ++++ | + | + | – | – |
| Symptomatic treated | ++++ | +++ | +++ | ++ | + | |
| Ivermectin treated | ++++ | ++ | + | – | – | |
| Fall in milk yield | Acyclovir treated | ++++ | ++ | ++ | + | – |
| Symptomatic treated | ++++ | +++ | +++ | ++ | ++ | |
| Ivermectin treated | ++++ | +++ | ++ | + | – | |
| Complications | Acyclovir treated | – | – | – | – | – |
| Symptomatic treated | ++ | ++ | + | + | + | |
| Ivermectin treated | + | – | – | – | – | |
Note: Scale: ++++ Prominent signs, +++ mild decrease, ++ moderate decrease, + highly decreased, – diminished
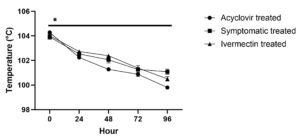
Within 48 hours after starting acyclovir treatment, the affected animals with high temperatures, respiration rates, and heart rates returned to normal values (Figure 5a, 5b, 5c). Between and within the groups significant (P ≤ 0.05) differences were found in rectal temperature. Temperature significantly (P ≤ 0.05) returned to the normal range in acyclovir treated animals over time intervals (Figure 5a). In symptomatic treated animals there was a significant (P ≤ 0.05) fall from 0 to 24 hour and 48 to 72 hour followed by non significant (P > 0.05) change between 24 to 48 hour and 72 to 96 hour. Temperature significantly (P ≤ 0.05) decreased in ivermectin treated animals over time intervals except for non-significant (P > 0.05) fall between 24 to 48 hour.
No significant (P > 0.05) difference in elevated temperature was noted between groups affected with LSD at 0 hour; however, a significant (P ≤ 0.05) decrease in temperature was noted in acyclovir treated group compared to other groups at most of the intervals except 72 hour interval followed by ivermectin group compared to symptomatic treated group.
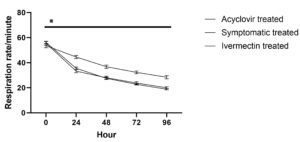
Respiration rate in animals treated with acyclovir and ivermectin showed significant (P ≤ 0.05) return to normal range over different time intervals from 0 to 96 hours. In the symptomatic treated group, significant (P ≤ 0.05) fall was noted upto 72 hours followed by non-significant change (P > 0.05).
Between the groups, no significant (P > 0.05) difference was noted in respiration rate at 0 hour. At each time point, the respiration rate was significantly (P ≤ 0.05) lower in acyclovir treated group followed by ivermectin treated group compared to the symptomatic treated group; however, no significant difference was noted between the acyclovir treated group and ivermectin treated group.
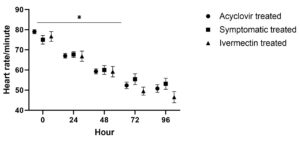
In acyclovir and ivermectin treated group heart rate showed a significant (P ≤ 0.05) decrease upto 72 hour followed by non-significant (P > 0.05) change. In symptomatic treated group significant fall was noted only upto 48 hours followed by non-significant (P > 0.05) change upto 96 hour. No significant (P > 0.05) difference was noted in heart rate between the three groups at various time points.
TLC and DLC were elevated in LSD affected cattle. TLC and lymphocyte counts after 72 and 96 hour of treatment were significantly (P ≤ 0.05) lower in the acyclovir and ivermectin treated group compared to symptomatic group and were comparatively lower in acyclovir treated animals then ivermectin treated animals.
Neutrophil counts were significantly (P ≤ 0.05) lower in acyclovir treated group at 24 and 96 hour compared to ivermectin treated groups and were nonsignificantly (P > 0.05) lower than symptomatic treated group. At 72 hour ivermectin treated groups showed significantly (P ≤ 0.05) lower N counts than symptomatic treated group. Monocyte counts were significantly lower in acyclovir treated animals at 24 hour compared to ivermectin treated group however monocyte counts decreased significantly (P ≤ 0.05) in ivermectin treated group from 48 hour to 96 hour. Eosinophil counts decreased significantly (P ≤ 0.05) in ivermectin treated group at 48 and 96 hour compared to other groups and at 72 hour compared to symptomatic treated group. Basophil counts were significantly (P ≤ 0.05) lower in acyclovir treated group at 24, 72 and 96 hour of treatment compared to other groups.
TLC and DLC values showed significant (P ≤ 0.05) fall from 0 to 96 hour in acyclovir treated group with most of the haematological indices returning to normal range within 72 to 96 hours. TLC, N, E and B decreased significantly (P ≤ 0.05) upto 24 hour, L upto 48 hour in symptomatic group. TLC, L, M, and N decreased significantly (P ≤ 0.05) upto 24 hour in ivermectin treated group whereas E counts decreased significantly upto 72 hour and basophil upto 48 hours.
At 0th hour of treatment, different groups showed non significant (P > 0.05) difference in TAS values (Figure 6a). Significantly (P ≤ 0.05) higher TAS values were noted in acyclovir treated group followed by ivermectin group compared to symptomatic treated group at 24th and 48th hour. The TAS values significantly increased in all the three groups at 24th hour; however, thereafter showed no significant change in acyclovir treated group whereas significant (P ≤ 0.05) fall was noted in symptomatic treated and ivermectin treated group at 48th hour.
No significant (P > 0.05) difference in TOS values was noted in different groups at 0th hour; however, values were significantly (P ≤ 0.05) higher in symptomatic treated group at 48th hour compared to acyclovir treated and ivermectin treated group (Figure 6b). There was a significant decrease in TOS from 0th hour to 48th hour in acyclovir treated and ivermectin treated group however symptomatic group showed non-significant (P > 0.05) decrease from 24th to 48th hour.
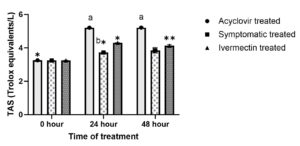 (a). a,bValues with different superscript differ significantly (P ≤ 0.05) between groups at a timepoint.
(a). a,bValues with different superscript differ significantly (P ≤ 0.05) between groups at a timepoint.
*,**Values with different superscript differ significantly (P ≤ 0.05) in a group between differenttime points.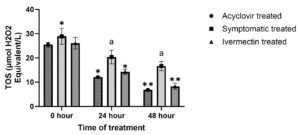 (b). aValues with different superscript differ significantly (P ≤ 0.05) between groups at a time point.*,**Values with different superscript differ significantly (P ≤ 0.05) in a group between different time points.
(b). aValues with different superscript differ significantly (P ≤ 0.05) between groups at a time point.*,**Values with different superscript differ significantly (P ≤ 0.05) in a group between different time points.
Figure 6. Effect on oxidative stress indices
LSD has emerged as an important outbreak in cattle in India including this trans-Himalayan region. This recent outbreak of LSD in cattle has produced characteristic clinical signs along with some unusual lesions including ventral oedema, ocular deformities, erosions and ulcerations. Nodules being the pathognomonic lesions were noted in almost 100% of cases with characteristics typical of LSD as revealed in clinical manifestations, ultrasonography, and histopathology. Anechoic to echoic fluid filled nodule with hyperechoic lining indicate inflammatory fluid accumulated within a fibrous capsule. USG has been used as a routine noninvasive diagnostic technique in cutaneous lesions in humans,42,43 however its application in bovine cutaneous affections is limited.44
Gross and histopathological findings are in corroboration with Kamr et al.45 Edema, hyperemia, acanthosis, severe hydropic degeneration, and hyperkeratosis in the epidermis and mononuclear cell infiltration, inclusion bodies, and vasculitis in the dermis, were seen which is in corroboration with Gharban et al.46 Others have also observed extensive tissue necrosis of epidermal and dermal layers, severe vasculitis, perifollicular edema, mononuclear cell infiltration, giant cells, and eosinophilic intracytoplasmic inclusion bodies in keratinocytes in LSD affected cattle.45,47
PCR helped in the confirmation of LSDV. This is in corroboration with Kumar
et al.12 Genomic evidence on LSDV have also been revealed.33 Characteristic bands of 636 bp of ORF12 gene were noted (Figure 4). PCR has been the routine confirmatory diagnostic procedure for LSD practiced in recent outbreaks.12,45,48
No mortality was noted in treated animals, especially acyclovir and ivermectin even beyond the observation period. This may be due to minimization of severity in affected animals following antiviral therapy. The severity of LSD was reduced in acute febrile animals following intravenous acyclovir infusion along with supportive treatment (Figure 7). Fever, respiratory distress, nasal discharge, salivation, lacrimation, lethargy, and inappetence diminished within 48-72 hours in 22 animals however ventral oedema (11) and nodular lesions (22) in most cases started regressing by 96 hours and in few (5) they persisted for more than 120 hours but without further increasing or aggravating compared to other symptomatically treated group wherein rupturing of nodules, suppuration or maggot infestation was noted. Ivermectin treated group showed a comparatively similar clinical response as acyclovir but with a slight delay as depicted in Table 1. Milk yield showed recovery within 96 hours however owners were advised to discard milk for 5 days for the risk of any residue else acyclovir has short half-life.38,39 Ivermectin treated animals showed clinical recovery comparable to acyclovir besides none of the LSD affected and ivermectin treated animals developed any complications after use unlike symptomatic treated or untreated animals which developed ulceration or wounds and few of them developed maggots (Figure 1). This may be due to added advantage of ivermectin being end-ectoparasiticide along with antiviral action.49,50
Studies on the treatment of LSD are lacking and the effect of treatment on various parameters of LSD affected cattle that could help in current outbreaks has not been evaluated.12,18 Only symptomatic treatment is practiced.51,52 This is helpful in the advent of an outbreak else animals would die of the disease. In our observation 6 of the animals died among 31 LSD affected cattle without any treatment. However, only symptomatic treatment is not effective and investigations on specific antiviral drugs under the prevailing situations of LSD outbreaks and the severity of cases are the need of hour. Acyclovir has potent antiviral action against poxviruses and thus can prevent viraemia and associated pathological alterations in LSD. Further, acyclovir is a purine nucleoside and has proved to be an effective systemic antiviral agent. Besides the first step of phosphorylation is catalyzed by viral, rather than host, thymidine kinase, thus acyclovir is considered relatively safe for systemic administration.29 Similarly, ivermectin has potent action against pox viruses.27 Ivermectin is known for its antiparasitic use but has also proven antiviral against DNA and RNA viruses including pox viruses.49,50
Decrease in fever may be due to the antipyretic action of paracetamol and meloxicam,53 however early returning of normal body temperature in acyclovir treated group followed by ivermectin treated group may be due to additional antiviral action of these drugs countering viraemia induced pyrexia with acyclovir showing prominent effect. Returning of normal respiration rate may be due to amelioration of fever due to antipyretics or antiviral effects of acyclovir and ivermectin preventing from viraemia compared to symptomatic treated groups. Thus enabling early return to normal respiration rate in acyclovir and ivermectin treated groups. Early returning of normal heart rate in acyclovir and ivermectin treated group may be due to antiviral effect of these drugs accompanied by stabilizing of a haemodynamic system by anti-inflammatory, antipyretic or antihistaminic effect of other drugs meloxicam, paracetamol, and pheniramine maleate respectively. Maxwell et al. have also reported similar findings of lowering the rectal temperature and clinical disease in valacyclovir hydrochloride treated horses affected with neuropathogenic equine herpesvirus type-1 infection. However, safety needs to be evaluated.54
Comparatively elevated lymphocyte counts, total leucocyte counts and decreased neutrophil counts were the significant findings in LSD affected cattle. The values returned to normal range within 48-72 hours of acyclovir treatment (Table 2). This may be due to initial stages of viraemia induced lymphocytosis, leukocytosis, and neutropenia. This is against the findings of Shefa et al. who have noted lower lymphocyte counts and total leucocyte counts in LSD affected cattle.48 This may be due to lymphopenia and leucopenia occurring during LSD pathogenesis. Leucopenia and lymphopenia have been noted on 1st-2nd day post-infection and granulocytic leukocytosis and lymphocytosis around 10-14th day of LSD infection.47 These hematological alterations can also be related to secondary bacterial infection which is common in LSD.12
Table (2):
Effect of treatment on total leucocyte count (TLC) and differential leucocyte count (DLC)
| Hematological indices | Group | 0 hour | 24 hour | 48 hour | 72 hour | 96 hour |
|---|---|---|---|---|---|---|
| TLCx103/μl | Acyclovir | 23.0000 ± 0.53376A | 12.2963 ±0.31038B | 11.2222 ± 0.25783C | 6.5556 ± 0.22854D | 5.5926 ± 0.20850E |
| Symptomatic | 23.2857 ± 1.04002A | 12.7143 ±0.71429B | 11.2857 ± 0.42056BC | 10.7143 ± 0.42056aCD | 9.7143 ± 0.28571aCDE | |
| Ivermectin | 23.6667 ± 1.11555A | 12.5000 ±0.76376 | 12.0000 ± 0.57735 | 7.1667 ± 0.60093B | 6.6667 ± 0.66667BC | |
| Lx103/μl | Acyclovir | 13.9693 ± 0.31053A | 7.5204 ±0.19388B | 6.8711 ± 0.15523BC | 4.0159 ± 0.13994aD | 3.4285 ± 0.12612aE |
| Symptomatic | 14.2314 ± 0.62498A | 7.7657 ±0.43650B | 6.8386 ± 0.24752C | 6.5614 ± 0.27055bCD | 6.0386 ± 0.26947bCDE | |
| Ivermectin | 14.3333 ± 0.80277A | 7.4833 ± 0.60093 | 7.0000 ± 0.57735 | 4.8333 ± 0.47726cB | 4.1667 ± 0.47726cBC | |
| Mx103/μl | Acyclovir | 1.0596 ± 0.02811A | 0.5450 ± 0.01511aB | 0.5004 ± 0.01109aBC | 0.2902 ± 0.01053aD | 0.2480 ± 0.00973aDE |
| Symptomatic | 1.0093 ± 0.04591 | 0.5950 ± 0.03620ab | 0.5150 ± 0.01648ab | 0.4821 ± 0.01893b | 0.4364 ± 0.01317b | |
| Ivermectin | 1.0067 ± 0.09888A | 0.6783±0.03167c | 0.4233 ± 0.01406cB | 0.2383 ± 0.01887cC | 0.2200 ± 0.01528acCD | |
| Nx103/μl | Acyclovir | 5.2230 ± 0.12810A | 2.7919 ± 0.07642aB | 2.5859 ± 0.05934BC | 1.4626 ± 0.05691aD | 1.2570 ± 0.04725aDE |
| Symptomatic | 5.2371 ± 0.30341A | 2.9243 ± 0.16429abB | 2.4386 ± 0.19522BC | 2.3886 ± 0.11589bBC | 2.2243 ± 0.07047bC | |
| Ivermectin | 4.9167 ± 0.34392A | 3.2500 ± 0.36401bB | 2.6000 ± 0.32660B | 1.4000 ± 0.16330ac | 1.4167 ± 0.09458a | |
| Ex103/μl | Acyclovir | 2.5026 ± 0.06433 | 1.3367±0.03639 | 1.2126 ± 0.03013a | 0.7126 ± 0.02494aA | 0.6070 ± 0.02242aA |
| Symptomatic | 2.4443 ± 0.17059A | 1.3657 ± 0.08312B | 1.2100 ± 0.04801abBC | 1.1043 ± 0.06986bBC | 1.0400 ± 0.02127bC | |
| Ivermectin | 2.4000 ± 0.15746A | 1.2317 ± 0.10678B | 0.8717 ± 0.02496cC | 0.5933 ± 0.01978ac | 0.5100 ± 0.02191c | |
| Bx103/μl | Acyclovir | 0.1150 ± 0.00267A | 0.0615 ± 0.00155aB | 0.0561 ± 0.00129C | 0.0328 ± 0.00114aD | 0.0280 ± 0.00104aE |
| Symptomatic | 0.1164 ± 0.00520A | 0.0636 ± 0.00357abB | 0.0564 ± 0.00210BC | 0.0536 ± 0.00210bC | 0.0486 ± 0.00143bC | |
| Ivermectin | 0.1183 ± 0.01078A | 0.0767 ± 0.00494cB | 0.0575 ± 0.00440 | 0.0523 ± 0.00406bc | 0.0417 ± 0.00307c |
a,b,cvalues with different superscripts differ significantly in a column
A,B,C,D,Evalues with different superscripts differ significantly in a row
Elevated TOS and decreased TAS were noted in affected cattle. This may be due to LSD induced oxidative stress. Oxidative indices returned to normal range following treatment of 48 hours. This is in corroboration with Kamr et al. and Shefa et al. who have also reported elevated levels of oxidants and decreased levels of antioxidants in LSD affected cattle.45,48 Elevated oxidants may be due to viraemia, inflammatory cascade and disturbances in oxidative system in LSD affected cattle.45,48 Comparatively lower TOS and higher TAS in acyclovir and ivermectin treated group may be due to the amelioration of oxidative stress by antiviral effect of acyclovir and ivermectin countering viraemia induced oxidative stress. In addition to antiviral effect, antioxidant and antiinflammatory roles of ivermectin may also add to its oxidative stress ameliorating effect.55,56
Indian bovine livestock sector is ravaged by the outbreak of severe infectious viral lumpy skin disease. Current outbreaks of LSD have resulted in severely diseased animals, with prominent nodules, high fever, respiratory distress and ventral oedema resulting in high mortality. Supportive treatments though provided a bit of relief but have not proven quite effective under field conditions and disease severity has increased with frequent deaths in affected animals. Hence this necessitated therapeutic intervention of specific antiviral drug acyclovir or ivermectin against LSD affected cattle. In addition to preventing mortality, acyclovir infusion helped in minimizing disease severity and facilitated early recovery compared to LSD affected untreated animals or those treated symptomatically with only supportive drugs. Acyclovir and ivermectin based therapy prevented disease progression especially in acute febrile cases and regressed severe symptoms. Though antiviral drugs may have prospects in combating current outbreak but need to be evaluated for safety or side effects, if any especially acyclovir. For future prevention and control strategies novel drugs and vaccines need to be explored especially in the event of emergence of novel strains as in the current outbreak, higher morbidity and mortality may be due to emergence of recombinant strains or their enhanced virulence and pathogenesis. Thus further monitoring and surveillance of variants of LSDV is imperative.
ACKNOWLEDGMENTS
The authors would like to acknowledge the support and assistance of the authorities of Sher E Kashmir University of Agricultural Sciences and Technology of Kashmir (SKUAST-K) especially the Faculty of Veterinary Sciences and Animal Husbandry (FVSc & AH), Shuhama.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study does not require ethical clearance being of clinical nature involving affected animals requiring therapeutic intervention. Parameters evaluated were required for follow up of the clinical cases.
- Akther M, Akter SH, Sarker S, et al. Global burden of Lumpy Skin Disease, outbreaks, and future challenges. Viruses. 2023;15(9):1861.
Crossref - FAO. 2017. Lumpy skin disease field manual ; Amanual for veterinarians. 20.
- Wilhelm L, Ward MP. The spread of Lumpy Skin Disease Virus across Southeast Asia: Insights from surveillance. Transbound Emerg Dis. 2023;2023(1):3972359.
Crossref - WOAH. 2023. Lumpy skin disease. https://www.woah.org/en/disease/lumpy-skin-disease/. Accessed on 23-09-2023
- Kumar N, Tripathi BN. A serious skin virus epidemic sweeping through the Indian subcontinent is a threat to the livelihood of farmers. Virulence. 2022;13(1):1943-1944.
Crossref - Al-Salihi K). Lumpy skin disease: Review of literature. Mirror of Research in Veterinary Sciences and Animals. 2014;3(3):6-23.
- Gari G, Waret-Szkuta A, Grosbois V, Jacquiet P, Roger F. Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiol Infect. 2010;138(11):1657-1666.
Crossref - Li Y, Zeng Z, Li K, et al. Detection of Culex tritaeniorhynchus Giles and Novel recombinant strain of lumpy skin disease virus causes high mortality in yaks. Viruses. 2023;15(4):880.
Crossref - Sudhakar SB, Mishra N, Kalaiyarasu S, et al. Lumpy skin disease (LSD) outbreaks in cattle in Odisha state, India in August 2019: Epidemiological features and molecular studies. Transbound Emerg Dis. 2019;67(6):2408-2422.
Crossref - Singh A, Kour G, Dhillon SS, Brar PS. Impact of Lumpy Skin Disease in India: Socio-behavioural Analysis, Epidemiology and Economics. 2023.
Crossref - Shagun. Lump skin disease has created a livelihood crisis for India’s small dairy farmers. Down To Earth. 2022. https://www.downtoearth.org.in/news/economy/ground-report-lumpy-skin-disease-has-created-a-livelihood-crisis-for-india-s-small-dairy-farmers-85245 Accessed on 27th January 2023.
- Kumar N, Chander Y, Kumar R, et al. Isolation and characterization of lumpy skin disease virus from cattle in India. PLoS One. 2021;16(1):e0241022.
Crossref - Kateshiya GB. Amid lumpy skin disease outbreak, Centre yet to grant nod to indigenous vaccine for cattle. Indian Express. 2023. https://indianexpress.com/article/cities/rajkot/amid-lumpy-skin-disease-outbreak-centre-yet-to-grant-nod-to-indigenous-vaccine-for-cattle-8651445/. Accessed 23-09-2023.
- Tuppurainen E, Alexandrov T, Beltran-Alcrudo D. Lumpy skin disease field manual – A manual for veterinarians. FAO Animal Production and Health Manual No. 20. Rome. Food and Agriculture Organization of the United Nations (FAO). 2017.
- Tuppurainen E, Dietze K, Wolff J, et al. Review: Vaccines and Vaccination against Lumpy Skin Disease. Vaccines (Basel). 2021;9(10):1136.
Crossref - Spickler AR. Lumpy Skin Disease. 2017. http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php Accessed on 1st January 2023.
- Kumar A, Venkatesan G, Kushwaha A, et al. Genomic characterization of Lumpy Skin Disease virus (LSDV) from India: Circulation of Kenyan-like LSDV strains with unique kelch-like proteins. Acta Tropica. 2023;241:106838.
Crossref - Liang Z, Yao K, Wang S, et al. Understanding the research advances on lumpy skin disease: A comprehensive literature review of experimental evidence. Front Microbiol. 2022;28(13):1065894.
Crossref - De Clercq E, Neyts J. Therapeutic potential of nucleoside/nucleotide analogues against poxvirus infections. Rev Med Virol. 2004;14(5):289-300.
Crossref - Andrei G, Snoeck R. Cidofovir activity against Poxvirus infections. Viruses. 2010;2(12):2803-2830.
Crossref - Kamal SA. Comparative studies on lumpy skin disease virus in human. Med Clin Arch. 2019;3(4):1-8.
Crossref - Lokanadhamu M, Sreedevi B, Reddy TV. Studies on etiology, symptomato logy, diagnosis and therapy in ulcerative thelitis of bufaloes in Andhra Pradesh (India). Buffalo Bulletin (Thailand). 2005;24(3):56-69.
- Firyall S, Ali MM, Muhammad G, et al. Bovine herpes mammillitis (gulwaddee) – a less known disease of cows and buffaloes in Pakistan. Buffalo Bulletin. 2019;38 (4):571-578.
- Shridhar NB. Lithium antimony thiomalate and acyclovir therapy for buffalo mammillitis. The Pharma Innovation Journal. 2020;9:(4):49-52.
- Wong DM, Maxwell LK, Wilkins PA. Use of antiviral medications against equine herpes virus associated disorders. Equine Veterinary Education. 2010;22(5):244-252.
Crossref - Malik P. 2022. https://megahvt.gov.in/notification/LSD_Treatment_Guidelines.pdf.
- Toker EB, Ates O, Yeilbaq K. Inhibition of bovine and ovine capripoxviruses (Lumpy skin disease virus and Sheeppox virus) by ivermectin occurs at different stages of propagation in vitro. Virus Res. 2022;310:198671.
Crossref - Rollinson EA. Prospects for antiviral chemotherapy in veterinary medicine: 2. Avian, piscine, canine, porcine, bovine and equine virus diseases. Antiviral Chemistry and Chemotherapy. 1992;3 (6):311-326.
Crossref - Mercer MA. Purine nucleosides used in animals. In MSD Manual Merck & Co., Inc., Rahway, NJ, USA. 2022.https://www.msdvetmanual.com/pharmacology/antiviral-agents/purine-nucleosides-used-in-animals#. Accessed on 23-09-2023.
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787.
Crossref - Raza S, Shahin F, Zhai W, et al. Ivermectin inhibits Bovine Herpesvirus 1 DNA polymerase nuclear import and interferes with viral replication. Microorganisms. 2020;8(3):409.
Crossref - Nabi-Afjadi M, Mohebi F, Zalpoor H, et al. A cellular and molecular biology-based update for ivermectin against COVID-19: is it effective or non-effective?. Inflammopharmacology. 2023;31 (1):21-35.
Crossref - Bhat S, Chaudry W, Mittal P, et al. Complete genome sequence of the lumpy skin disease virus reported from Jammu and Kashmir, India, during 2022 outbreak. Microbiol Resour Announc. 2023;12(11):e0031723.
Crossref - Tharwat M, Oikawa S. Ultrasonographic characteristics of abdominal and thoracic abscesses in cattle and buffaloes. J Vet Med A Physiol Patholol Clin Med. 2007;54(9):512-517.
Crossref - Mathewos M, Dulo F, Tanga Z. Clinicopathological and molecular studies on cattle naturally infected with lumpy skin diseases in selected districts of Wolaita Zone, Southern Ethiopia. BMC Vet Res. 2022;18(1):297.
Crossref - Bancroft JD, Gamble M. Theory and practice of histological techniques. 5th ed. Edinburgh: Churchill Livingstone Publication. 2002:593-620.
- Parray OR, Yatoo MI, Muheet, et al. Seroepidemiology and risk factor analysis of contagious caprine pleuropneumonia in Himalayan Pashmina Goats. Small Ruminant Research. 2019;171:23-36.
Crossref - Meyer LJ, De Miranda P, Sheth N, Spruance S. Acyclovir in human breast milk. Am J Obstet Gynecol. 1988;158(3):586-588.
Crossref - Bork K, Benes P. Concentration and kinetic studies of intravenous acyclovir in serum and breast milk of a patient with eczema herpeticum. J Am Acad Dermatol. 1995;32(6):1053-1055.
Crossref - Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37(2):112-119.
Crossref - Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103-1111.
Crossref - Nessi R, Betti R, Bencini PL, Crosti C, Blanc M, Uslenghi C. Ultrasonography of nodular and infiltrative lesions of the skin and subcutaneous tissues. J Clin Ultrasound. 1990;18(2):103-109.
Crossref - Almuhanna N, Wortsman X, Wohlmuth-Wieser I, Kinoshita-Ise M, Alhusayen R. Overview of ultrasound imaging applications in dermatology. J Cutan Med Surg. 2021;25(5):521-529.
Crossref - Abouelnasr K, El-Shafaey el-S, Mosbah E, El-Khodery S. Utility of ultrasonography for diagnosis of superficial swellings in buffalo (Bubalus bubalis). J Vet Med Sci. 2016;78(8):1303-1309.
Crossref - Kamr A, Hassan H, Toribio R, Anis A, Nayel M, Arbaga A. Oxidative stress, biochemical, and histopathological changes associated with acute lumpy skin disease in cattle. Vet World. 2022;15(8):1916-1923.
Crossref - Gharban HAJ, Al-Shaeli SJJ, Al-Fattli HHH, Altaee MNK. Molecular and histopathological confirmation of clinically diagnosed lumpy skin disease in cattle, Baghdad Province of Iraq. Vet World. 2019;12(11):1826-1832.
Crossref - Neamat-Allah ANF. Immunological, hematological, biochemical, and histopathological studies on cows naturally infected with lumpy skin disease. Vet World. 2015;8(9):1131.
Crossref - Shefa U, Kim MS, Jeong NY, Jung J. Antioxidant and cell-signaling functions of hydrogen sulfide in the central nervous system. Oxid Med Cell Longev. 2018;2018(1):1873962.
Crossref - Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot (Tokyo). 2020;73(9):593-602.
Crossref - Formiga FR, Leblanc R, de Souza Rebounas J, Farias LP, de Oliveira RN, Pena L. Ivermectin: an award-winning drug with expected antiviral activity against COVID-19. J Control Release. 2021;329:758-761.
Crossref - Salib FA, Osman AH. Incidence of lumpy skin disease among Egyptian cattle in Giza Governorate. Egypt. Veterinary World. 2011;4:162-167.
Crossref - Namazi F, KhodakaramTafti A. Lumpy skin disease, an emerging transboundary viral disease: A review. Vet Med Sci. 2021;7 (3):888-896.
Crossref - Edwards SH. Nonsteroidal anti-inflammatory drugs in animals. In MSD Veterinary Manual. 2021. https://www.msdvetmanual.com/pharmacology/inflammation/nonsteroidal-anti-inflammatory-drugs-in-animals. Accessed on 28th October 2023.
- Maxwell LK, Bentz BG, Gilliam LL, et al. Efficacy of the early administration of valacyclovir hydrochloride for the treatment of neuropathogenic equine herpesvirus type-1 infection in horses. Am J Vet Res. 2017;78 (10):1126-1139.
Crossref - Zhang X, Song Y, Xiong H, et al. Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int Immunopharmacol. 2009;9(3):354-359.
Crossref - DiNicolantonio J, Barroso-Arranda J, McCarty M. Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19. Open Heart. 2020;7(2):e001350.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.