ISSN: 0973-7510
E-ISSN: 2581-690X
Anthropogenic activities have escalated CH4 emissions, exacerbating global warming, yet specialized bacteria known as Methanotrophs play a key role in mitigating atmospheric CH4 levels by consuming 30-70% of emitted methane. This study focuses on exploring the culturable methanotrophic population within Muthukuda mangrove sediments, an unexplored reservoir of methanotrophic diversity. The sediment sample yielded a methanotrophic bacterial count of 1.5 x 103 CFU/g, leading to the selection of three unique bacterial morphotypes (NCT270, NCT271, and NCT272) for in-depth investigation. Optimal growth was observed at pH 8, with peak growth at 30°C, while extreme temperatures of 4°C and 40°C inhibited growth across all isolates. Salinity levels between 20 and 30 ppt supported optimal growth, with strains displaying tolerance to various stressors. Methane served as the sole carbon source for all experiments, with positive urease production noted after 7 days of incubation. Microscopic and biochemical analyses suggested the classification of strains NCT270, NCT271, and NCT272 within Group I methanotrophic genera: Methylomicrobium, Methyloscarcina, and Methylomonas, respectively. BLASTn analysis of 16S rRNA gene sequences shared high similarities with known methanotrophic species Methyloscarcina fibrate (ON834586) with 99.28%, Methylomicrobium album (ON834587) with 98.77% and Methylomonas methanica (ON834588) with 99.15%. The resulting insights enhance our understanding of culturable methanotrophic diversity and underscore its potential for environmental applications.
Methanotrophs, Methane Oxidation, Mangrove Sediments, 16S rRNA Sequencing, CH4 Reduction
Mangrove forests are considered to be one of the most important ecosystems due to its high productivity.1 However, these ecosystems are important sources of greenhouse gases and possesses different groups of aerobic and anaerobic bacteria with varied biogeochemical conditions along spatial and temporal gradients.2 Methane (CH4), a potent greenhouse gas (GHG), alongside carbon dioxide (CO2) and nitrous oxide (N2O), significantly contributes to global warming. Methane’s heat-trapping capacity surpasses that of CO2 by approximately 25 times, underlining its environmental impact. CH4 emissions have increased by 5% since the preceding decade, with anthropogenic emissions accounting for about 60% which results in climate change.3
Almost 30-70 % of the methane that escapes to the atmosphere is consumed by a special group of bacteria the methane oxidizers called Methanotrophs which uses methane as the source of energy and plays a critical role in the carbon cycle. Aerobic methanotrophs belonging to the Gamma proteobacteria are classified into type I (families – Methylococcaceae and Methylothermaceae); Alphaproteobacteria into Type II, with families Methylocystaceae and Beijerinckiaceae; The type I gammaproteobacterial methane oxidizers use the ribulose monophosphate pathway for carbon fixation and the type II alphaproteobacterial methane oxidizers use the serine pathway for carbon fixation. In 1970 Whittenbury et al.,4 started culturing methanotrophs and over the years, though several new genera and species have been identified, there are still several lineages that has not been brought into culture.5,6 Hence it is important to update the understanding of the culture-dependent studies of the methanotrophic world,7 as these methanotrophs are multifunctional with promising environmental bioengineering applications, including removal of methane, biodegradation of toxic compounds etc.8-10
This study endeavors to explore the culturable methanotrophic population within the Muthukuda mangrove sediments, a hitherto unexplored reservoir of methanotrophic diversity. With the multifunctional potential of culturable methanotrophs in mind, this research seeks to isolate methane-oxidizing bacteria and elucidate their growth characteristics and methane oxidation potential. By delving into the environmental applications of methanotrophic isolates, this study aims to contribute to broader ecological and bioengineering endeavors.
Isolation of bacteria
Sediment samples were collected from the mangrove area of Muthukuda, situated near Mimisal in Tamil Nadu’s Pudukottai district, within the Palk Bay region (9° 51’ 38″ N, 79° 8’ 1.57″ E). The sampling site comprises mangroves and a seagrass bed. During low tide surface sediment samples were obtained using a handheld PVC corer (20 cm length, 5 cm diameter) and promptly transported to the laboratory on ice. Upon arrival, samples were sectioned into 0-5 cm intervals for analysis within 3 hours of collection. Careful attention was paid to avoid sampling from the outer edge of the core liner, with samples extracted from the core’s center. Sediment slurries were prepared using autoclaved seawater, followed by appropriate dilutions plated on nitrate mineral salts (NMS) media.4 Autoclaved phosphate buffer solution was added to the media before pouring the plates. Incubation was conducted in gas-tight anaerobic bags at 28 ± 2°C, utilizing a 0.22 µm filtered gas mixture of methane and air (30:70). Plates were observed at regular intervals over 7-14 days. Isolates that grew on the NMS medium were subjected to gram staining analysis.
Bacterial isolates with distinct colony morphological features were chosen and inoculated on nutrient agar plates without methane and on NMS plates with methane. Methane presence was determined by incubating plates in airtight bags containing methane, while plates without methane were incubated under ambient conditions of bacteriological incubator. Isolates that grew on NMS media containing methane were selected for further investigation. Isolates were chosen based on colony morphology (including chromogenesis, opacity, elevation, colony surface, and consistency) and their methane-oxidizing potential. Growth curve experiments were conducted in NMS media in triplicates with methane as the sole source of carbon. Incubation was carried out at 28 ± 2°C, and growth was measuring the optical density (OD) at 620 nm using a UV-VIS Spectrophotometer (UV-1280 Shimadzu Scientific) every 24 hours. Uninoculated broth served as a control. Specific methane oxidizing activity was calculated from the growth curve by averaging triplicate values and determining their standard deviation. Methane leakage was assessed by comparing blank readings from control tubes with those of the samples. Methane oxidation rate was determined by injecting headspace gas from the vials into a gas chromatograph (Shimadzu, GC-2010) chromatography using flame ionization detector (FID) equipped with capillary column (30 m long mega bore) with a detector temperature of 150°C and injector temperature of 100°C. Nitrogen was employed as the carrier gas. Methane oxidation rate was measured by decrease of the gas over time. Methane standard with a concentration of 1-5 ppm was used for reference.
Physiological and Biochemical characteristics of the methanotrophs
Growth of the isolates were assessed at pH 5, 7, and 8 by adjusting the pH of the media using sterile NaOH and HCl solutions. Varied temperatures (4°C, 20°C, and 40°C) were tested for their effect on isolate growth. Tolerance to different stressors was evaluated by exposing the isolates to varying concentrations of NaCl (1 to 5% w/v), 0.01% SDS, 0.001% crystal violet, and 0.001% malachite green incorporated into NMS agar plates.11 Additionally, the isolates’ ability to utilize various substrates (0.1%) such as methanol and ethanol as carbon source in the absence of methane. Likewise Using NMS medium, nitrogen sources were similarly examined in the presence of methane as a carbon source, where yeast extract and D-Glucose was added instead of potassium nitrate at a concentration of 0.1%.10,11 Incubation was conducted at the optimal temperature and pH for each isolate, and growth was monitored for 7 days. Isolates demonstrating growth were categorized as positive, while those showing no growth were deemed negative. Oxidase, Urease activities were determined as described by Smibert and Krieg.12
16s rRNA sequence analysis
DNA was isolated using Bacterial DNA Isolation Kit (Qiagen, USA). 16S rRNA gene was amplified using the universal bacterial primers: forward primer; 27F-52 -AGAGTTTGATCMTGGCTCAG-32 and the reverse primer; 1392R-52 -CTGCTGCSYCCCGTAG-32 containing 10x Taq buffer (2.5 µL), 50 mM MgCl2 (1 µL), 2 mM dNTPs (2.5 µL), 0.2 µL of Platinum® Taq Polymerase (5 µL-1, Invitrogen™), forward primer (5 pmoles), reverse primer (10 pmol), DNA template (8 ng), brought up to a final volume of 25 µL with ultra-pure water. The polymerase chain reactions (PCR) were performed on GeneAmp PCR System 9700 (Applied Biosystems™) thermocycler under the following conditions: 4 min at 96°C, followed by 30 cycles of 30 seconds at 94°C, 30 seconds at 57°C and 1 minutes at 72°C, and a final extension step at 72°C for 10 minutes. Aliquots of 5 µL of each reaction were examined on 1.2% (w/v) agarose gel using Tris Borate ETDA buffer as electrolyte. The PCR amplicons were subjected to 2-way sequencing using ABI 3730xl Genetic Analyzer, Barcode Biotechnologies, Bengaluru, India. The DNA sequences were extracted using ChromasPro (ver. 1.9.9), and the total sequences were subjected to the chimera filter using UCHIME13 and aligned using the CLUSTAL_X program.14 DNA sequences were compared with GenBank sequences using BLAST algorithm.15 The phylogenetic tree was constructed using MEGA X.16
Characterization of Methanotrophic Bacterial isolates
The sediment sample yielded a methanotrophic bacterial count of 1.5 × 103 CFU/g. Fourteen distinct colony morphotypes were selected for further analysis. To confirm methanotrophic activity, a confirmatory test was conducted by streaking isolates on nutrient agar and incubating them in the absence of methane. Three unique bacterial morphotypes (designated as NCT270, NCT271, and NCT272) were selected based on this test for further investigation. These colonies exhibited Gram-negative staining, motility, and appeared round, white, opaque, and flat on NMS agar. Isolates NCT270 and NCT271 were cocci-shaped, while NCT272 was rod-shaped.
Growth Characteristics and Methane Oxidation Potential of Methanotrophic Isolates
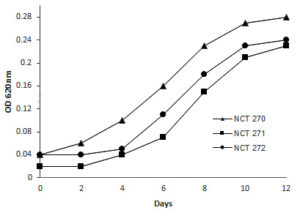
Optimal growth occurred at pH 8, with peak growth observed at 30°C. No growth was detected at extreme temperatures of 4°C and 40°C across all three isolates. Salinity levels between 20 and 30 ppt supported optimal growth. The strains exhibited tolerance to 0.001% SDS, malachite green, and 0.01% crystal violet. Methane served as the sole carbon source for all experiments. Positive urease production was observed after 7 days of incubation. In growth tests with various substrates in the absence of methane, efficient growth was noted with methanol, ethanol. Growth was observed with yeast extract and D-glucose as nitrogen sources (Table). Microscopic and biochemical analysis of strains NCT270, NCT271, and NCT272 suggested their classification within Group I methanotrophic genera: Methyloscarcina, Methylomicrobium, and Methylomonas, respectively. Growth on NMS medium, using methane as the carbon and energy source at 30°C and pH 8 is shown in Figure 1. The methane oxidation potentials of NCT270, NCT271, and NCT272 were measured as 1.04, 2.17, and 3.35 µm of the sample, respectively.
Table:
Characteristics of the bacterial isolates from Muthukuda mangrove sediments with the potential to oxidize methane
Isolate |
NCT270 |
NCT271 |
NCT272 |
|---|---|---|---|
Cell Morphology |
Cocci |
Cocci |
Rod |
Gram stain(±) |
|
|
|
Motility |
+ |
+ |
+ |
Biochemical Reactions |
|||
Oxidase |
+ |
+ |
+ |
Catalase |
+ |
+ |
+ |
Urease |
+ |
+ |
+ |
Substrate utilization |
|||
Carbon source |
|||
Methanol |
+ |
+ |
+ |
Ethanol |
+ |
+ |
+ |
Nitrogen Source |
|||
Yeast Extract |
+ |
+ |
+ |
D Glucose |
+ |
+ |
+ |
Physiological Test |
|||
(a) pH |
|||
pH 6 |
+ |
+ |
+ |
pH 7 |
+ |
+ |
+ |
pH 8 |
+ |
+ |
+ |
(b) Temperature |
|||
4 |
|
|
|
30 |
+ |
+ |
+ |
40 |
|
|
|
(c) Salinity |
|||
0 |
+ |
+ |
+ |
18 |
+ |
+ |
+ |
35 |
+ |
+ |
+ |
Tolerance Test |
|||
0.01% SDS |
+ |
+ |
+ |
0.001% Crystal |
+ |
+ |
+ |
0.001% Malchite Green |
+ |
+ |
+ |
80°C (20 mins) |
|
|
|
16S rRNA sequence analysis
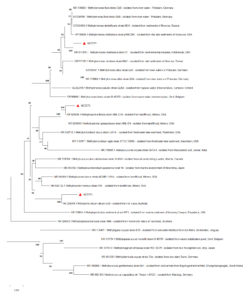
The 16S rRNA gene sequences of the isolates NCT270, NCT271, and NCT272, generated in this study, have been deposited in GenBank under accession numbers ON834586, ON834587, and ON834588, respectively. BLASTn analysis revealed that NCT270, NCT271, and NCT272 exhibited high similarities with known methanotrophic species, specifically Methyloscarcina fibrate (99.28% similarity), Methylomicrobium album (98.77% similarity), and Methylomonas methanica (99.15% similarity), respectively. Similar sequences identified through BLASTn analysis were utilized for constructing a Neighbor Joining tree. The resulting phylogram clearly grouped the NCT strains with their respective species, confirming their taxonomic classification (Figure 2).
Figure 2. The phylogram was constructed using 16S rRNA gene sequences from the strains isolated in this study, along with closely related reference sequences from GenBank. The sequences from this study are marked with red triangles and labeled with the strain names NCT270, NCT271, and NCT272. Reference sequences are identified by their GenBank accession numbers, followed by the genus-species name, strain name, and source of isolation
Isolation of methanotrophic bacteria are considered to be difficult due its slow growth and the growth of non-methane oxidizers as contaminants during cultivation. Due to the above said difficulties isolation of pure culture of methanotrophs are considered as a challenging task and are being carried out only by few researchers in the world.17 Hence our work on the isolation of methanotrophs from the mangrove area gains significance, as there are no reports to date on the same from the mangrove region in south India, to our knowledge. All the three isolates were submitted in the National College Culture Collection Facility. The isolates Methyloscarcina fibrate (ON834586) with a similarity of 99.28% similarity, Methylomicrobium album (ON834587) 98.77%, Methylomonas methanica (ON834588) 99.15% belongs to the type I group following the work of Whittenbery et al.4 Bowman et al.,10 and Wise et al.18
Whittenbury et al., 4 proposed the taxa Methylomonas, Methylobacter, Methylococcus, Methylocystis, and Methylosinus. Later in 1999, Bowman and his colleagues proposed type I methanotrophs, should include the genera Methylococcus, Methylomicrobium, Methylobacter, and Methylomonas and type II methanotrophs, containing closely related groups of species in the genera Methylosinus and Methylocystis. In 1997 Bowman et al.,19 has explained on the inclusion of members of a psychrophilic genus, Methylosphaera and thermophilic genera, Methylocaldum and Methylothermus by Bodrossy et al.20,21 Wise et al.,22 has proposed new genus Methylosarcina gen. nov., describing Methylosarcina fibrata sp. Nov on the basis of characterization, particularly of morphological, fatty acid and phylogenetic data belonging to type I group.
Our work gains evidence from the works of Shiau et al.,23 as their work reports the presence of Type I methanotrophs as the most dominant CH4-oxidizing bacteria and about 50% of populations belonged to Methylosarcina in mangrove soils. Methylosarcina can grow over a temperature range of 4-37°C and pH of 4.0-9.0.23 Earlier works on methanotrophs in tidal mangrove environment has reported the dominance of species belonging to Methylomonas. Among the identified methanotrophs in tidal mangrove Methylosarcina and Methylomonas were found to be dominant.2,23 Type I methanotrophs like Methylomicrobium were dominant of all the others in Taiwan mangrove forests.23 It’s been reported that the methane oxidizers, besides ‘growing on methane and methanol, used other carbon sources as additional organic compounds, which included organic acids, yeast extract, and peptone which stimulated the growth of some methanotrophs.24 In the tidal mangrove soils Methylomonas and Methylosarcina were reported to be more active. Confirmation of similar differences was evident in different locations through high-throughput sequencing of the 16S ribosomal RNA (rRNA) gene. Similarly, Methylomonas, Methylomicrobium and Methylobacter groups involved in CH4 in sediments of Bertioga were reported to growth on methane or methanol as the sole source of carbon. Methanotrophs isolated from various environments like11 Methylosinus trichosporium and Methylosinus sporium had oxidization rates of approximately 0.135 and 0.14 × 10-15 mol methane cell-1 h-1. Higher methane oxidation rates of 64.8 ± 19.5 × 10-16 mol cell-1 h-1 and 13.3±4.6 × 10-16 mol cell-1 h-1 for cell suspensions of methanotrophs which were grown in batch and chemostat cultures have also been reported Benstead et al.25 Various methanotrophic related bacteria are reported to oxidize methane from 0.02 to 1.05 µm in the study conducted in KG basin.24 Though the cultures in the present study fall within the lower range of previously reported rates, our findings contribute to the broader understanding of methanotrophic bacteria which could help in the recycling of organic carbon and in regulating the emission of methane.
This study successfully explored and characterized the culturable methanotrophic bacteria from the Muthukuda mangrove sediments, an area previously unexplored for its methanotrophic diversity. Three distinct bacterial morphotypes, designated NCT270, NCT271, and NCT272, were selected for comprehensive analysis. These isolates demonstrated optimal growth at pH of 8 and a temperature of 30°C. The isolates further proved their ability to grow in the presence of methane and were identified as Methyloscarcina fibrate (ON834586), Methylomicrobium album (ON834587), Methylomonas methanica (ON834588) with molecular identification through 16S rRNA gene sequencing and BLASTn analysis. In conclusion, this study highlights the presence and capabilities of methanotrophic bacteria in Muthukuda mangrove sediments, showcasing their potential in mitigating methane emissions. The characterized strains, with their robust growth characteristics and methane oxidation capabilities, contribute valuable insights into methanotrophic diversity and underscore their potential for environmental applications in reducing methane emissions and combating global warming.
ACKNOWLEDGMENTS
The authors acknowledge the Heads of the Institutes for the facilities provided.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Shiau YJ, Chiu CY. Biogeochemical processes of C and N in the soil of Mangrove Forest ecosystems. Forests. 2020;11(5):492.
Crossref - Shiau YJ, Cai Y, Lin YT, Jia Z, Chiu CY. Community structure of Active Aerobic Methanotrophs in Red Mangrove (Kandelia obovata) Soils under different frequency of tides. Microbial Ecology. 2018;75(3):761-770.
Crossref - Cardaso LOB, Karolski B, Gracioso LH, Borrego BB, Nascimento CAO, Perpetuo EA. Enrichment of Methylosinus – dominant consortia from mangroves for polyhydroxybutyrate (PHB) production. J Environ Chem Eng. 2022;10(5):108490.
Crossref - Whittenbury R, Phillips KC, Wilkinson JF. Enrichment, Isolation and some properties of Methane utilizing bacteria. J Gen Microbiol. 1970;61(2):205-218.
Crossref - Dunfield PF, Dedysh SN. Methylocella: a gourmand among Methanotrophs. Trends Microbiol. 2014;22(7):368-369.
Crossref - Dedysh SN, Knief C. Diversity and Phylogeny of described Aerobic Methanotrophs. Kalyuzhnaya M, Xing XH. (eds) Methane Biocatalysis: Paving the Way to Sustainability. Springer, Cham. 2018:17-42.
Crossref - Cruz SG, Vaksmaa A, Horn MA, Niemann A, Pijuan M, Ho A. Methanotrophs: Discoveries, Environmental relevance, and a perspective on current and future applications. Front Microbiol. 2021;12: 678057.
Crossref - Sullivan JP, Dickinson D, Chase HA. Methanotrophs, Methylosinus trichosporium OB3b, sMMO, and their application to Bioremediation. Crit Rev Microbiol. 1998;24(4):335-373.
Crossref - Pandey VC, Singh JS, Singh DP, Singh RP. Methanotrophs: promising bacteria for environmental remediation. Int J Environ Sci Technol. 2014;11:241-250.
Crossref - Bowman JP, Sly LI, Nichols PD, Hayward AC. Revised taxonomy of the Methanotrophs: Description of Methylobacter gen. nov., Emendation of Methylococcus, Validation of Methylosinus and Methylocystis Species, and a proposal that the family Methylococcaceae includes only the group 1 Methanotrophs. Int J Syst Evol Microbiol. 1993;43(4):735-753.
Crossref - Dianou D, Adachi K. Characterization of methanotrophic bacteria isolated from a subtropical paddy field. FEMS Microbiol Lett. 1999;173(1):163-173.
Crossref - Smibert RM, Krieg NR. General characterization. In:Manual of Methods for General Bacteriology (Gerhardt P, Murray RGE, Costillow RN, Nester EW, Wood WA, Krieg NR, Phillips GB, Eds.). American Society for Microbiology. Washington, DC. 19881:409-443.
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194-2200.
Crossref - Larkin M, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947-2948.
Crossref - Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST:a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389-402.
Crossref - Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X:Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. 2018;35(6):1547-1549.
Crossref - Rahalkar MC, Khatri K, Pandit P, Bahulikar RA, Mohite JA. Cultivation of important methanotrophs from Indian rice fields. Front. Microbiol. 2021;12:669244.
Crossref - Wise MG, McArthur JV, Shimkets LJ. Methylosarcina fibrata gen. nov., sp. nov. and Methylosarcina quisquiliarum sp. nov., novel type 1 methanotrophs. Int J Syst Evol Microbiol. 2001;51(Pt 2):611-621.
Crossref - Bowman JP, Mc-Cammon SA, Skerrat JH. Methylosphaera hansonii gen. nov., sp. nov., a psychrophilic, group 1 methanotroph from Antarctic marine – salinity, meromictic lakes. Microbiol. 1997;143(Pt 4):1415-1459.
Crossref - Bodrossy L, Holmes EM, Holmes AJ, Kovacs KL, Murrell JC. Analysis of 16S rRNA and methane monooxygenase gene sequences reveals a novel group of thermotolerant and thermophilic methanotrophs, Methylocaldum gen. nov. Arch Microbiol. 1997;168(6):493-503.
Crossref - Bodrossy L, Kovacs KL, Mc Donald IR, Murrell JC. A novel thermophilic methane – oxidizing g-Proteobacterium. FEMS Microbiol Lett. 1999;170(2):335-341.
Crossref - Wise MG, Mc Arthur JV, Shimkets LJ. Methanotroph diversity in landfill soil:Isolation of novel type 1 and type II methanotroph whose presence was suggested by culture independent 16S Ribosomal DNA analysis. Appl Environ Microbiol. 1999;65(11):4887-4897.
Crossref - Shiau YJ, Lin CW, Cai Y, Jia Z, Lin YT, Chiu CY. Niche differentiation of Methane – oxidizing Bacteria in Estuarine Mangrove Forest soils in Taiwan. Miroorganisms. 2020;8(8):1248.
Crossref - Sujith PP, Sheba MV, Gonsalves MJBD. Diversity and activity of methanotrophic related bacteria in subsurface sediments of the Krishna- Godavari basin, India. Current Science. 2016;110:1801-1809.
- Benstead J, King GM, Williams HG. Methanol promotes atmospheric methane oxidation by methanotrophic cultures and soils. Appl Environ Microbiol. 1998;64(3):1091-1098.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.