ISSN: 0973-7510
E-ISSN: 2581-690X
Multidrug-resistant (MDR) Gram-negative bacterial infections have emerged as a major public health concern. The aim of the present study was to detect the rate of infections due to MDR Gram-negative bacteria (GNB) in a tertiary care hospital, the rate of Carbapenemases and AmpC-β-lactamases production and the Antimicrobial susceptibility test pattern (AST) among MDR GNB. The rate of MDR GNB during the study period was 25.70%. Urine samples showed the highest contribution to the total MDR GNB. Among the total MDR GNB isolates, 166 were randomly selected and included in the present study. A higher rate of MDR GNB was reported among male patients (61.5%) compared to the females (38.5%) and most of them were from the patients aged between 61-70 years (30.7%). The most prevalent MDR GNB was Klebsiella pneumoniae 80 (48.12%), followed by Escherichia coli 43 (25.9%). AST of MDR GNB revealed their significant resistance to β-lactamases/β-lactamases inhibitors, cephalosporins, fluoroquinolones and carbapenem drugs (98%). Of 123 MDR Enterobacterales, 83% of them were found to be Metallo β-lactamase (MBLs) producers by mCIM and eCIM methods. Of 43 MDR non-fermenters, 29 (67.4%) of them were found to be carbapenemase producers by MHT. About 29.51% of MDR GNB isolates were found to be AmpC producers by AmpC disk test. A reliable and rapid phenotypic method to detect carbapenemases and AmpC β-lactamases among MDR GNB in a routine microbiology laboratory method is clinically important to guide antibiotic therapy and implementation of effective infection control practices.
Multidrug-resistant, Gram-negative Bacteria, Hospital Acquired Infections, Carbapenemase, AmpC β-lactamase, Modified Hodge Test, Carbapenemase Inactivation Method
Antimicrobial resistance (AMR) has emerged as a pivotal concern of the 21st century.1,2 It presents a critical and escalating challenge to the world, with an increased risk of morbidity and mortality, and it is recognized by the World Health Organization (WHO) as a public health concern.3,4 Multidrug-resistance (MDR) is defined as non-susceptibility to at least one agent in three or more antimicrobial groups. Infections caused by MDR Gram-negative bacteria (GNB) constitute a significant concern due to limited therapeutic options. MDR-GNB, such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia, are responsible for major hospital-acquired infections (HAIs), including urinary tract infections (UTIs), ventilator-associated pneumonia (VAP), intra-abdominal infections (IAIs), surgical site infections (SSIs), and bacteremia.5-7
The Centers for Disease Control and Prevention (CDC) in the USA have classified Carbapenem-resistant(CRE) and ESBL-producing Enterobacteriaceae, Carbapenem-resistant Acinetobacter baumannii (CRAB), and MDR and ESBL-producing Pseudomonas aeruginosa as urgent threats.8 The root causes of the rise in MDR infections are multifaceted, including a lack of antibiotic stewardship program, irrational use of existing antimicrobial agents in hospitalized patients, and the use of antibiotics in veterinary feeds, which contribute to the development of adaptive resistance mechanisms.9
Gram-negative bacteria (GNB) exhibit a high degree of adaptability to various antimicrobial agents through the utilization of diverse mechanisms. These mechanisms may either be intrinsic to a given species or acquired. Intrinsic resistance mechanisms arise from mutations in chromosomal genes and include enzymes that inactivate drugs, increased efflux of antimicrobials, altered outer membrane porins (OMPs), and modifications to target sites. On the other hand, acquired mechanisms result from the transfer of resistance genes via mobile genetic elements (MGEs), most notably the beta-lactamase genes encoded on plasmids, or mechanisms that do not involve enzymes, such as Qnr, which confers resistance to fluoroquinolones in Enterobacterales.7,10,11
ESBLs are plasmid-encoded β-lactamases that confer resistance to extended-spectrum b-lactam antibiotics in GNB.12 AmpC β-lactamases, like cephalosporinases can be both plasmid and chromosomally encoded, and they confer resistance to most b-lactam drugs in GNB.13 Carbapenemase genes provide a persistent and transferable form of resistance, enabling their dissemination among naive bacteria.14,15 It is important to consider both carbapenemase-producing bacteria and those employing alternative carbapenem resistance mechanisms.16
The effective management of infections caused by MDR GNB necessitates a comprehensive approach. Reliable and rapid phenotypic methods for detecting carbapenemases, AmpC b-lactamases, and extended-spectrum β-lactamases in MDR GNB in routine microbiology laboratory is of clinical significance. Such methods guide antibiotic therapy and facilitate the implementation of effective infection control practices, ultimately improving the care of critically ill patients at high risk of harboring MDR GNB.6,14 The present study aims to provide insights into the incidence of infections caused by MDR GNB, their resistance mechanisms, and the prevalence of AmpC β-lactamases and carbapenemase-producing MDR GNB among patients at a tertiary care hospital in Mysuru.
This was a laboratory based prospective study conducted in the Department of Microbiology, for a period of one year (May 2022 – April 2023). All MDR GNB isolated from clinical samples were included in this study and processed according to the standard protocol. The sociodemographic details of the patients and microbiological records were obtained from the digital data stored online at the Hospital.
Species identification, AST & storage
Identification of GNB and AST was done by Automated Vitek 2 system (bioMerieux Vitek). The isolates which were found to be resistant for at least one agent in three or more antimicrobial classes were considered as MDR GNB. The pure cultured growth of these MDR GNB isolates on MacConkey agar were sub-cultured onto nutrient agar vials and stored at 4°C.
A randomly selected 166 MDR GNB isolates were screened for Carbapenemase and AmpC β-lactamase production.
Phenotypic detection of carbapenemase production
All the MDR GNB isolates were brought to room temperature and sub-cultured onto MacConkey agar before conducting the phenotypic testing.
The MDR Enterobacterales isolates were screened for Carbapenemase production by Modified Carbapenemase Inactivation method (mCIM), Ethylene diamine tetra acetic acid (EDTA)Carbapenemase Inactivation method (eCIM). Non-fermenters were screened for Carbapenemase production by Modified Hodge test (MHT).
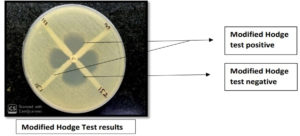
Screening for Carbapenemase production by MHT17
Test procedure
- Lawn culture of ATCC 25922 E. coli was done on Muller Hinton Agar (MHA) plates
- Meropenem disk (10 µg) was placed in the center of each plate, 3-5 colonies of each test organism inoculated in the form of a straight line from the meropenem disk edge to the end of the plate and incubated at 37°C for 16-18 hours.
Interpretation
MHA plate were checked for the growth of ATCC E. coli around each test organism at the intersection of the inoculated line and inhibition zone. Positive growth indicates Carbapenemase production and no growth indicates no Carbapenemase production.
mCIM and eCIM test for suspected carbapenamse production in Enterobacterales according to CLSI guidelines17
Test procedure for mCIM
- For each isolate to be tested, 1 µL loopful of bacteria from an overnight pure culture plate was inoculated into 2 mL of Tryptic soy broth (TSB) and vortexed for 10-15 seconds.
- 10 µg meropenem disk was added to the tube using sterile forceps so that entire disk was immersed and incubated at 37°C for 4 hours.
- MHA plate was lawn cultured with E. coli ATCC 25922.
- Meropenem disk from each TSB-meropenem disk suspension was removed using the flat side of a sterile 10 µL loop and excess broth was drained by pressing the loop along the inside edge of the tube. The disk was removed from the tube and placed on the MHA plate inoculated with the E. coli ATCC 25922. The MHA plates were inverted and incubated at 37°C for 18-24 hours.
- Zones of inhibition was measured as routine disk diffusion method.
Test procedure for eCIM
- 20 µL 0.5 M EDTA was added to a 2 mL TSB tube to obtain a final concentration of 5 mM EDTA.
- For each isolate to be tested, 1 µL loopful of bacteria from an overnight pure culture plate was inoculated into EDTA-TSB broth and vortexed for 10-15 seconds.
- 10 µg meropenem disk was added to each tube using sterile forceps so that entire disk was immersed and incubated at 37°C for 4 hours.
- Meropenem disk from the EDTA-TSB- meropenem disk suspension was removed as in mCIM test and placed on the MHA plate which was inoculated with ATCC E. coli 25922.
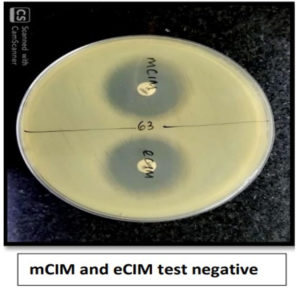
Test interpretation: mCIM
- mCIM positive: Zone diameter of 6-15 mm or presence of pinpoint colonies within a 16-18 mm zone
- mCIM negative: Zone diameter of ≥19 mm (clear zone)
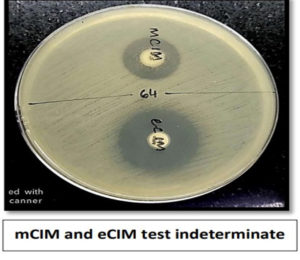
- mCIM indeterminate: Zone diameter of 16-18 mm or zone diameter of ≥19 mm with the presence of pinpoint colonies within the zone.
eCIM – Interpret only when mCIM test is positive
- eCIM positive: A ≥5 mm increase in zone diameter for eCIM vs zone diameter for mCIM. For eCIM test, ignore pinpoint colonies within any zone of inhibition.
- eCIM negative: A ≤4 mm increase in zone diameter for eCIM vs zone diameter of mCIM
- The test was reported as depicted in the Table 1.
Table (1):
Reporting of mCIM and eCIM test
mCIM |
eCIM |
Report |
|---|---|---|
Negative |
Do not interpret |
Carbapenemase not detected |
Positive |
Negative |
Serine carbapenemase detected |
Positive |
Positive |
Metallo-β-lactamase detected |
Indeterminate |
Do not interpret |
Testing inconclusive for the presence of carbapenemase |
mCIM- modified carbapenemase inactivation method, eCIM- EDTA Carbapenemase inactivation
Phenotypic detection of AmpC production
Screening for AmpC production by AmpC disk test (Disk Diffusion method)
All the MDR GNB isolates (166) were tested for AmpC production.
Test procedure
- A lawn culture of Escherichia coli ATCC 25922 indicator strain was made on the MHA plate.
- Sterile disk (6 mm) was moistened with sterile saline (10 µl) and inoculated with a suspension of the test organism.
- The cefoxitin disk (30 µg) and sterile disk inoculated with test organism were placed adjacent to each other on the MHA plate and incubated overnight at 37°C.
Interpretation
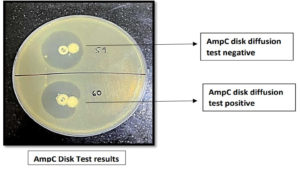
- Positive result: flattening or distortion of the cefoxitin inhibition zone
- Negative result: undistorted cefoxitin inhibition zone.18
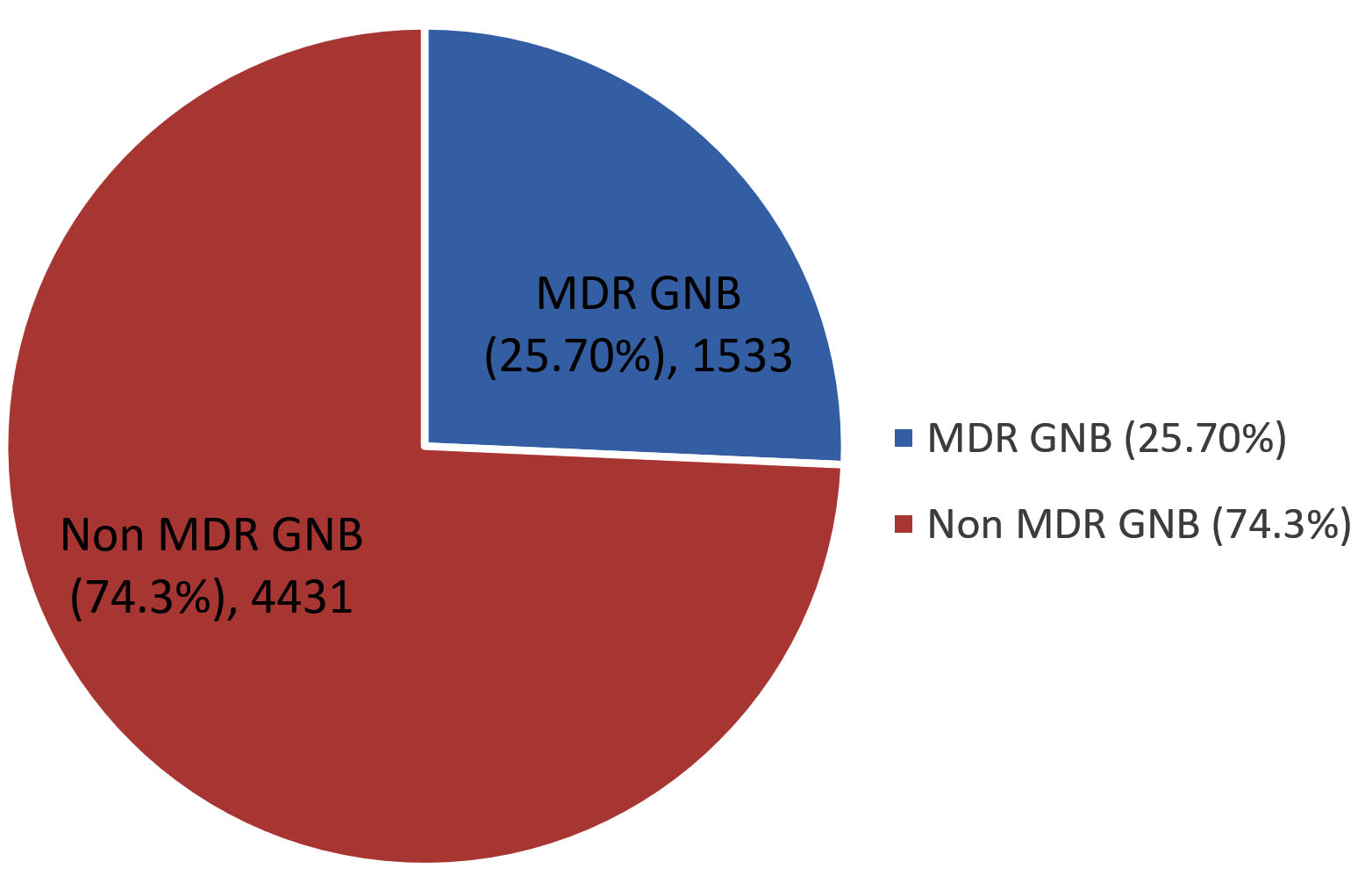
Of the 26775 samples received in the microbiology laboratory for culture and sensitivity, 8997 (33.60%) samples were culture positive, of which 5964 (22.27%) samples yielded growth of GNB and among them, 1533 (25.70%) isolates were found to be MDR. The rate of MDR GNB during the study period was 25.70% (Figure 1).
Urine samples contributed highest 476 (31.05%) to the total MDR GNB, followed by endotracheal aspirate 220 (14.35%), blood 116 (7.56%), sputum 113 (7.37%), bronchoalveolar lavage 91 (5.93%), bile 23 (1.5%), tissue 19 (1.23%), ear swab 8 (0.52%), vaginal swab 5 (0.32%) and synovial fluid 1 (0.06%) (Table 2).
Table (2):
Contribution of each sample to the total MDR GNB during the study period
| No. of MDR GNB | Percentage of MDR | |
|---|---|---|
| Pus | 461 | 30.07% |
| Blood | 116 | 7.56% |
| ET | 220 | 14.35% |
| Sputum | 113 | 7.37% |
| Urine | 476 | 31.05% |
| BAL | 91 | 5.9% |
| Bile | 23 | 1.5% |
| Synovial fluid | 1 | 0.06% |
| Tissue | 19 | 1.23% |
| Ear swab | 8 | 0.52% |
| Vaginal Swab | 5 | 0.32% |
| Total | 1533 | |
ET-endotracheal aspirate, BAL- bronchoalveolar lavage
Among the 1533 MDR GNB isolates reported during the study period, 166 isolates were randomly selected and included in the present study to detect the various resistant mechanisms. Of the 166 randomly selected isolates, 65 (39.15%) of them were reported among the patients in the age group 61-80 years. Higher rate of MDR GNB were isolated from male patients (61.5%) as compared to the female patients (38.5%). The maximum number 106 (63.8%) of MDR GNB were isolated from the wards, followed by ICUs 36 (21.68%) and outpatient department 24 (14.45%).
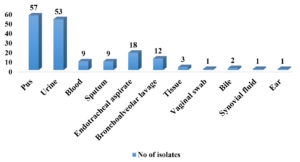
Maximum number of isolates were selected from pus samples 57 (34.33%), followed by urine 53 (31.92%), endotracheal aspirate 18 (10.84%), bronchoalveolar lavage 12(7.22%), blood 9 (5.42%), sputum 9 (5.42%), tissue 3 (1.80%), 2 (1.02%) from bile samples and 1 (0.60%) each from vaginal swab, synovial fluid & ear (Figure 2).
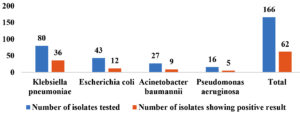
Out of 166 MDR GNB isolates, 123 (74.09%) were Enterobacterales and 43 (25.90%) were non-fermenters. Most prevalent MDR GNB was Klebsiella pneumoniae 80 (48.12%), followed by Escherichia coli 43 (25.9%), Acinetobacter baumannii 27 (16.2%) and Pseudomonas aeruginosa 16 (9.6%). The antimicrobial susceptibility pattern of MDR GNB showed that the most of the isolates were resistant to β-lactamases/β-lactamases inhibitors, cephalosporins, fluoroquinolones and carbapenem drugs (98%). AST results of MDR Enterobacterales and Non-fermenters are shown in Table 3 and Table 4, respectively.
Table (3):
Overall susceptibility of MDR Enterobacterales
| Organism | Total isolates | Antibiotics resistant to | Number and Percentage of Resistance | Organism | Total isolates | Number and Percentage of Resistance |
|---|---|---|---|---|---|---|
| Klebsiella pneumoniae | 80 | Amoxicillin clavulanate | 78 (97.5%) | E. coli | 43 | 42 (97.67%) |
| Piperacillin tazobactam | 80 (100%) | 43 (100%) | ||||
| Cefuroxime | 80 (100%) | 42 (97.67%) | ||||
| Cefuroxime axetil | 78 (97.5%) | 42 (97.67%) | ||||
| Ceftriaxone | 78 (97.5%) | 43 (100%) | ||||
| Cefaperazone sulbactum | 77 (96.25%) | 41 (91.34%) | ||||
| Cefipime | 78 (97.5%) | 43 (100%) | ||||
| Ertapenem | 80 (100%) | 42 (97.67%) | ||||
| Imepenem | 75 (93.75%) | 43 (100%) | ||||
| Meropenem | 79 (98.75%) | 41 (91.34%) | ||||
| Amikacin | 19 (23.75%) | 23 (53.5%) | ||||
| Gentamicin | 69 (86.25%) | 28 (65.11%) | ||||
| Ciprofloxacin | 80 (100%) | 42 (97.67%) | ||||
| Tigecycline | 5 (6.25%) | 0 | ||||
| Fosfomycin | 4 (5%) | 0 | ||||
| Colistin | 2 (2.5%) | 1 (2.32%) | ||||
| Trimethoprim sulfamethoxazole | 61 (76.25%) | 37 (86.04%) | ||||
| Nitrofurantoin | 14 (17.5%) | 6 (27.27%) |
Table (4):
Overall susceptibility of MDR Non-fermenters
| Organism | Total isolates | Antibiotics resistant to | Number and Percentage of Resistance | Organism | Total isolates | Number and Percentage of Resistance |
|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 27 | Piperacillin tazobactam | 27 (100%) | Pseudomonas aeruginosa | 16 | 16 (100%) |
| Ceftazidime | 27 (100%) | 14 (87.25%) | ||||
| Cefaperazone sulbactum | 22 (81.5%) | 13 (81.25%) | ||||
| Cefipime | 23 (85.18%) | 13 (81.25%) | ||||
| Imepenem | 27 (100%) | 15 (93.75%) | ||||
| Meropenem | 27(100%) | 16 (100%) | ||||
| Amikacin | 22 (81.5%) | 13 (81.25%) | ||||
| Gentamicin | 25 (92.6%) | 16 (100%) | ||||
| Ciprofloxacin | 27 (100%) | 16 (100%) | ||||
| Levofloxacin | 4 (14.81%) | 16 (100%) | ||||
| Minocycline | 7 (25.92%) | – | ||||
| Tigecycline | 0 | – | ||||
| Colistin | 0 | 0 | ||||
| Trimethoprim sulfamethoxazole | 24 (88.9%) | – | ||||
| Nitrofurantoin | 4 (14.8%) | – | ||||
| Aztreonam | – | 16 (100%) |
The 123 Enterobacterales were subjected to Modified carbapenemase Inactivation method (mCIM) & EDTA carbapenemase Inactivation method (eCIM) to detect carbapenemase production according to the standard protocol recommended by CLSI guidelines (M100) 2022, of which 83% (102) of them were found to be Metallo β-lactamase (MBLs) producers.
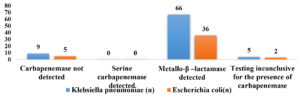
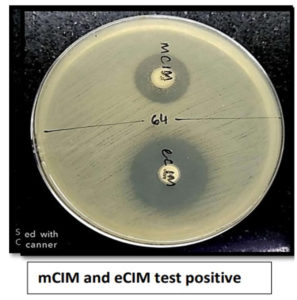
Of 80 Klebsiella pneumoniae, 66 (82.5%) were found positive for mCIM and eCIM and were considered positive for Metallo-β-lactamase production, 5 (6.25%) were found to be indeterminate and were considered inconclusive and remaining 9(11.25%) isolates were found negative for mCIM and were considered as negative for carbapenemase production. Of 43 Escherichia coli, 36 (83.72%) were found positive for mCIM and eCIM and were considered positive for Metallo-β-lactamase production, 2(4.65%) was found to be indeterminate and was considered inconclusive and remaining 5 (11.62%) isolates were found negative for mCIM and were considered as negative for carbapenemase production as shown in Figure 3. Figures 4,5, and 6 show the positive, negative, and indeterminate results of mCIM and eCIM test, respectively
Non-fermenters (n=43) were not screened by mCIM and eCIM since both the tests are not recommended by CLSI for testing carbapenemase production in A. baumannii and eCIM test is not recommended CLSI for testing carbapenemase production in P. aeruginosa.
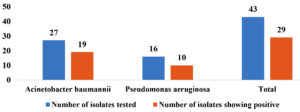
The Non-fermenters (43) were screened for carbapenemase detection by Modified Hodge Test, of which 29 (67.4%) of them were found to be carbapenemase producers. Of 27 A. baumannii 70.37% (19) of them were MHT positive, of 16 P. aeruginosa, 62.5% (10) of them were MHT positive (Figure 7 and 8).
All the isolates were subjected to AmpC detection by AmpC disk test, of which 29.51% (49) of them were found to be AmpC producers. AmpC β-lactamases were more frequently found among A. baumannii (33.33%) followed by P. aeruginosa (31.25%), K. pneumoniae (28.75%) and E. coli (27.9%) as depicted in the Figure 9 and 10.
Antimicrobial resistance has been a vital issue of 21st century. The emerging Antimicrobial resistance (AMR) among GNB poses an alarming threat to world healthcare and presents a substantial challenge in choosing empiric antibiotic therapy in seriously ill patients.5
The rate of MDR GNB in the present study was 25.7%, however, Silpi Basak et al., at Jawaharlal Nehru Medical College, Wardha, India have reported higher prevalence 37.1% of MDR GNB.19 Aisha et al., from Eastern Saudi Arabia,13 Meng et al., from People’s Hospital, Jining, Shandong Province, China.20 and Vasant et al from Mumbai21 have also reported higher prevalence of MDR GNB i.e., 60.4%, 42.5% and 30.8%, respectively. Ours being a teaching hospital, adherence to infection control practices and antibiotic stewardship program is in place resulting in lesser rate of MDR GNB.
In a study conducted by Nicolas Francisco Fernandez-Martinez et al., at Reina Sofםa University Hospital, Spain have reported a higher isolation rate of MDR GNB among male patients (61.5%) compared to the female patients (38.5%)22 and this is found consistent with the current study where we have also reported the higher isolation rate of MDR GNB among males (72.89%) compared to females (27.10%).
In our study, the maximum number of MDR GNB were reported in the patients aged between 61-70 years (30.7%) which is consistent with the study by Aurora E Pop-Vicas, et al., at Beth Israel Deaconess Medical Center (Boston, MA) who have also reported that patients aged >=65 years contributed to the highest MDR GNB.23 Same study mentioned above23 has also reported that urine samples have the highest contribution (55%) to the total MDR GNB which correlates with our study where urine samples (31.05%) has contributed to highest number of MDR GNB.
In a study conducted by Silpi Basak, Wardha, et al., the highest number of MDR GNB strains were isolated from surgery wards (32.3%) followed by different ICUs (18.3%),19 however in the current study, we have reported a slightly higher rate in ICUs (30.4%) than Surgical wards (14.45%). This high incidence of MDR isolation in ICUs in the present study can be justified by the fact that MDR isolates are a common nosocomial pathogen which are present in ICU environment and also, ours being a tertiary care hospital where patients admitted to ICUs are referred from other hospitals and are previously exposed to antibiotics.
Silpi Basak et al,19 Wardha, Vasant et al.,21 Mumbai, reported E. coli (38%) to be the most prevalent MDR GNB followed by K. pneumoniae (28.4%), A. baumannii (19%), P. aeruginosa (19%), but, in the current study we have reported Klebsiella pneumoniae 80 (48.12%), as the most prevalent MDR GNB followed by Escherichia coli 43 (25.9%), Acinetobacter baumannii 27 (16.2%) and Pseudomonas aeruginosa 16 (9.6%). Our results were consistent with the study conducted by Mutasim et al.13, at a Referral Hospital, Saudi Arabia in which the most prevalent MDR GNB reported was K. pneumoniae (34.69%) followed by E. coli (30.20%), A. baumannii (20%) and P. aeruginosa (15.10%). A study by Ching Jou, et al., Australia, reported that the antimicrobial susceptibility of MDR GNB shows the most common three-drug resistance pattern to β-lactamases/β-lactamases inhibitors, third-generation cephalosporin and fluoroquinolones,24 this correlates with the present study, also our isolates were found to be resistant to carbapenem drugs (98%).
We subjected 123 MDR Enterobacterales to Modified carbapenemase Inactivation method (mCIM) & EDTA carbapenemase Inactivation method (eCIM) to detect carbapenemase production, of which 83% of them were found to be Metallo β-lactamase (MBLs) producers which was slightly higher than that reported by Dalia El. Nobi, et al., Assiut University, Egypt, who have reported 75.6% of MDR Enterobacterales to be metallo β-lactamase (MBLs) producers by mCIM and eCIM method.25
In our study, the 43 MDR non-fermenters were screened for carbapenemase detection by Modified Hodge Test, of which 29 (67.4%) of them were found to be carbapenemase producers which include, 70.39% of A. baumannii and 62.5% of P.aeruginosa, whereas in a study by Hala B. Othman, et al., Ain Shams University Hospitals, Cairo, Egypt, have reported 54% of their P. aeruginosa isolates were MHT positive26 and a study by Zahra Moulana, et al., Babol University of Medical Sciences, Babol, Iran27 showed 84% of A. baumannii isolates to be MHT positive.
Jennifer et al. at US Hospitals have reported 31% of their MDR GNB isolates to be AmpC β-lactamases producers,28 which correlates with the current study where we have reported 29.51% of MDR GNB isolates to be AmpC producers by AmpC disk test. The present study showed that A. baumannii (33.33%) and P. aeruginosa (31.25%) were the most common AmpC β-lactamases producers followed by K. pneumoniae (28.75%) and E. coli (27.9%), this result correlates with a study conducted by Mutasim et al., at a Referral Hospital, Saudi Arabia.13
Ours being a tertiary care hospital, patients would have been previously exposed to antibiotics there by facilitating the development of drug resistance including carbapenem resistance.
Therefore, it is imperative to develop a methodological approach that can expedite the comprehensive analysis and verification of resistance mechanisms within Multi-Drug Resistant (MDR) bacteria.
The increased cases of MDR GNB infections have become a global threat in hospitals and acute healthcare settings. Infections with MDR E. coli, P. aeruginosa, K. pneumoniae and A. baumannii contribute to the mortality of the most vulnerable populations.
The burden of drug resistance should be addressed with effective infection control practices like contact precautions to prevent further transmission of these MDR GNB within the hospital and in the community, implementation of evidence-based antibiotic stewardship programs, strict law for dispensing of antibiotics and awareness about rational use of antibiotics, public awareness program and hospital staff education.
Reliable phenotypic screening methods for carbapenemases and AmpC β-lactamases among MDR isolates will help in selecting appropriate antimicrobial therapy. This approach will not only advance our scientific understanding of antibiotic resistance but also have immediate clinical implications, enabling healthcare providers to facilitate timely therapeutic interventions and infection control strategies more effectively.
Limitations
In the present study, confirmation of different resistant mechanisms was not done using molecular methods, which makes way for future research directions.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RPM conceptualized and designed the study. VK and ANJ performed data collection. VK and RD performed the experiments. VK, RPM, RD and ANJ performed data analysis and interpretation of results. VK designed the figures. RPM supervised the project. VK, RPM, RD and ANJ drafted the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This work was funded by the JSS Academy of Higher Education and Research (JSSAHER), Mysuru, Karnataka, India, under JSSAHER Research grants: JSSAHER/REG/RES/URG/54/2011-12/7541.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethical Committee, JSS Medical College, JSS Academy of Higher Education & Research, Karnataka, India, with reference number JSS/MC/PG/36/2022-23.
- Pulingam T, Parumasivam T, Gazzali AM, et al. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci. 2022;170:106103.
Crossref - Nadeem SF, Gohar UF, Tahir SF, et al. Antimicrobial resistance: more than 70 years of war between humans and bacteria. Crit Rev Microbiol. 2020;46(5):578-599.
Crossref - Oliveira VD, Rubio FG, Almeida MT, Nogueira MC, Pignatari AC. Trends of 9,416 multidrug-resistant Gram-negative bacteria. Rev Assoc Med Bras (1992). 2015;61(3):244-249.
Crossref - Kanj SS, Bassetti M, Kiratisin P, et al. Clinical data from studies involving novel antibiotics to treat multidrug-resistant Gram-negative bacterial infections. Int J Antimicrob Agents. 2022;60(3):106633.
Crossref - Kaye KS, Pogue JM. Infections Caused by Resistant Gram-Negative Bacteria: Epidemiology and Management. Pharmacotherapy. 2015;35(10):949-962.
Crossref - Cerceo E, Deitelzweig SB, Sherman BM, Amin AN. Multidrug-Resistant Gram-Negative Bacterial Infections in the Hospital Setting: Overview, Implications for Clinical Practice, and Emerging Treatment Options. Microb Drug Resist. 2016;22(5):412-431.
Crossref - Ruppe E, Woerther PL, Barbier F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann Intensive Care. 2015;5(1):61.
Crossref - CDC. Antibiotic-resistant Germs: New Threats. Centers for Disease Control and Prevention. 2020. http://www.cdc.gov/DrugResistance/Biggest-Threats.html
- Morris S, Cerceo E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics (Basel). 2020;9(4):196.
Crossref - Teklu DS, Negeri AA, Legese MH, Bedada TL, Woldemariam HK, Tullu KD. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob Resist Infect Control. 2019;8:39.
Crossref - Muntean D, Horhat FG, Badioiu L, et al. Multidrug-Resistant Gram-Negative Bacilli: A Retrospective Study of Trends in a Tertiary Healthcare Unit. Medicina (Kaunas). 2018;54(6):92.
Crossref - Rawat D, Nair D. Extended-spectrum β-lactamases in Gram-negative Bacteria. J Glob Infect Dis. 2010;2(3):263-274.
Crossref - Ibrahim ME, Abbas M, Al-Shahrai AM, Elamin BK. Phenotypic Characterization and Antibiotic Resistance Patterns of Extended-Spectrum β-Lactamase- and AmpC b-Lactamase-Producing Gram-Negative Bacteria in a Referral Hospital, Saudi Arabia. Can J Infect Dis Med Microbiol. 2019;2019:6054694.
Crossref - Cordeiro-Moura JR, Fehlberg LCC, Nodari CS, et al. Performance of distinct phenotypic methods for carbapenemase detection: The influence of culture media. Diagn Microbiol Infect Dis. 2020;96(1):114912.
Crossref - Tamma PD, Simner PJ. Phenotypic Detection of Carbapenemase-Producing Organisms from Clinical Isolates. J Clin Microbiol. 2018;56(11):e01140-18.
Crossref - Codjoe FS, Donkor ES. Carbapenem Resistance: A Review. Med Sci. (Basel). 2017;6(1):1.
Crossref - M100: Antimicrobial Susceptibility Testing Standards. Clinical & Laboratory Standards Institute. 2019. https://clsi.org/standards/products/microbiology/documents/m100/
- Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161-182.
Crossref - Basak S, Singh P, Rajurkar M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J Pathog. 2016;2016:4065603.
Crossref - Wang M, Wei H, Zhao Y, et al. Analysis of multidrug-resistant bacteria in 3223 patients with hospital-acquired infections (HAI) from a tertiary general hospital in China. Bosn J Basic Med Sci. 2019;19(1):86-93.
Crossref - Nagvekar V, Sawant S, Amey S. Prevalence of multidrug-resistant Gram-negative bacteria cases at admission in a multispeciality hospital. J Glob Antimicrob Resist. 2020;22:457-461.
Crossref - Fernandez-Martinez NF, Carcel-Fernandez S, De la Fuente-Martos C, et al. Risk Factors for Multidrug-Resistant Gram-Negative Bacteria Carriage upon Admission to the Intensive Care Unit. Int J Environ Res Public Health. 2022;19(3):1039.
Crossref - Pop-Vicas AE, D’Agata EM. The rising influx of multidrug-resistant gram-negative bacilli into a tertiary care hospital. Clin Infect Dis. 2005;40(12):1792-1798.
Crossref - Lim CJ, Cheng AC, Kennon J, et al. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: a nested case-control study. J Antimicrob Chemother. 2014;69(7):1972-1980.
Crossref - El. Nobi D, Elgendy SG, Bakry R, Hassan AS, El-Sabaa EMW. Phenotypic and genotypic characterization of carbapenemases in carbapenem-resistant gram-negative bacilli isolated from adult cancer patients. Microbes Infect Dis. 2023;4(3):853-870.
Crossref - Othman HB, Halim RMA, Abdul-Wahab HEE, Atta HA, Shaaban O. Pseudomonas aeruginosa – Modified Hodge Test (PAE-MHT) and ChromID Carba Agar for Detection of Carbapenemase Producing Pseudomonas aeruginosa Recovered from Clinical Specimens. Open Access Maced J Med Sci. 2018;6(12):2283-2289.
Crossref - Moulana Z, Babazadeh A, Eslamdost Z, Shokri M, Ebrahimpour S. Phenotypic and genotypic detection of metallo-beta-lactamases in Carbapenem resistant Acinetobacter baumannii. Caspian J Intern Med. 2020;11(2):171-176.
Crossref - Black JA, Moland ES, Thomson KS. AmpC disk test for detection of plasmid-mediated AmpC beta-lactamases in Enterobacteriaceae lacking chromosomal AmpC beta-lactamases. J Clin Microbiol. 2005;43(7):3110-3113.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.