ISSN: 0973-7510
E-ISSN: 2581-690X
Staphylococcus aureus (S. aureus) is a very common human pathogenic microorganism that can cause a variety of infectious diseases, including skin and soft tissue infections, endocarditis, osteomyelitis, bacteremia, and lethal pneumonia. About one-third of the common population is colonized with S. aureus. MRSA is a formidable pathogen known to cause high mortality & morbidity, that poses a significant threat to public health worldwide. Presence of MRSA strains, resistant to multiple antibiotics especially in hospital stay, has complicated the management of infections caused by this bacterium. The aim of this study was to shed light on the prevalence and antimicrobial sensitivity pattern of MRSA among patients in a tertiary care center located in Faridabad, Haryana. This cross-sectional observational study was conducted in the Department of Microbiology, ESIC Medical College & Hospital, a 510 bedded tertiary care teaching hospital in Faridabad, Haryana, India. All wound samples including pus, exudates, wound swab and tissue samples received for aerobic culture and antimicrobial sensitivity from various clinical departments from January 2019 to July 2019 were included in this study. A total of 747 samples were received from January 2019-July 2019. Mean age of this study population was found to be 50.7 ± 14.8 years. Out of 747 samples, 226 (30.25%) were culture positive. Among the S. aureus isolates, methicillin resistance was seen amongst 39 (58.2%). Antibiotic Susceptibility results of S. aureus showed 100% resistance to Penicillin along with 100% resistance to Fluoroquinolones in both MRSA and MSSA. High prevalence of MRSA amongst patients highlights the importance of continued surveillance and implementation of antimicrobial stewardship program to control the menace of antimicrobial resistance. Strict adherence to Infection Control practices its regular follow up to assess the effectiveness of any hospital infection control measures taken is the key.
Resistance, MRSA, MSSA, Penicillin, Antibiotics, Healthcare, Hospital
Infectious diseases are the second leading cause of death for people worldwide.1 Staphylococcus aureus (S. aureus) is a very common human pathogenic microorganism that can cause a variety of infectious diseases, including skin and soft tissue infections, endocarditis, osteomyelitis, bacteremia, and lethal pneumonia. About one-third of the common population is colonized with S. aureus.1 Prior colonization of S. aureus in moist areas like skin & anterior nares predisposes the wound to get infected with the same. It is an efficient pathogen and quite easily adapts to varied conditions.
The glycan chains of N-acetyl-glucosamine and N-acetylmuramic acid that make up the cell wall of Gram-positive bacteria, such as S. aureus, are heavily cross-linked by pentapeptides (UDP-MurNAc-penta). Each precursor component is synthesized in the cytoplasm and delivered to the growing cell’s division septum. The structural similarity between penicillin and the D-alanyl-D-alanine (D-ala-D-ala) residues of newly synthesized UDP-MurNAc-penta allows penicillin to inhibit peptidoglycan crosslinking by acting as a substrate analogue.2
Beta-lactams bind to penicillin-binding proteins (PBPs), forming long-lasting acyl-enzyme complexes that block the transpeptidase activity of these enzymes. This inhibition reduces peptidoglycan cross-linking, leading to a mechanically weakened cell wall incapable of withstanding the high internal osmotic pressure, which results in cell lysis. In staphylococci, the killing mechanism is highly cell cycle dependent, and it is proposed that mis-coordination in the spatial and temporal deposition of peptidoglycan during cell division leads to cell death. It is also suggested that cell lysis and cell death may be two separate events.3-5
Similar to other bacteria, S. aureus possesses several PBPs that, in addition to providing transpeptidase activity, seem to fulfil distinct functions in coordinating peptidoglycan synthesis. Different classes of beta-lactams target individual PBPs with variable affinity, which may explain why different beta-lactams introduce various morphological changes.6 Thus, the molecular events underlying the bactericidal effects of beta-lactam antibiotics depend on the affinity of the specific compounds for the various PBPs.
A notable feature of most Methicillin-resistant Staphylococcus aureus (MRSA) isolates is the heterogeneous expression of resistance to beta-lactams.7,8 In these strains, populations derived from a single cell exhibit varying resistance levels, with the majority of cells showing low resistance and a minority displaying high resistance. Hospital-acquired MRSA (HA-MRSA) isolates typically demonstrate high-level, homogeneous methicillin resistance, whereas community-acquired MRSA (CA-MRSA) isolates often exhibit low-level, heterogeneous resistance. Understanding the molecular mechanisms behind this phenomenon has been facilitated by identifying mutations that convert strains with low, heterogeneous resistance into homogeneous, highly resistant strains.8
Methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) represent the two primary clinical forms of S. aureus.9 Methicillin resistance is defined as resistance to penicillinase-stable, anti-staphylococcal drugs. MRSA is a significant pathogen associated with high mortality and morbidity, posing a substantial threat to global public health. The presence of multi-drug resistant MRSA strains, particularly in hospital settings, complicates the management of infections caused by this bacterium. Effective control of MRSA spread requires understanding its prevalence and antimicrobial sensitivity patterns within specific healthcare environments.
Antimicrobial resistance (AMR) and infectious disease prevalence are critical public health concerns globally, with heightened significance in regions like India.10 Although Gram-negative organisms exhibit higher resistance rates compared to Gram-positive organisms, it is essential to assess the extent of this issue comprehensively. The primary sources of MRSA transmission are hospital staff and patients who are infected or colonized with MRSA strains, predominantly through contact transmission. Risk factors that enhance the emergence and spread of MRSA include prolonged and repeated hospitalization, indiscriminate antibiotic use, lack of awareness, misuse of intravenous drugs, and the presence of indwelling medical devices.11
Comprehensive AMR surveillance, including MRSA screening, is crucial for identifying trends and patterns in antimicrobial resistance and emerging pathogens at various levels—from local hospitals to provincial, national, and global scales. AMR surveillance aids not only in developing and updating measures against antimicrobial resistance but also in refining guidelines for treating bacterial infections. This, in turn, improves patient outcomes and reduces the length of hospital stays.12,13
Faridabad, a major city in the northern Indian state of Haryana, serves as a hub for tertiary medical care, catering to a diverse population. The aim of this study was to shed light on the prevalence and antimicrobial susceptibility pattern of MRSA among patients in a tertiary care center located in Faridabad, Haryana. By conducting surveillance studies, this research provides valuable insights into the local epidemiology of MRSA, enabling healthcare providers to devise effective strategies for infection control and appropriate antibiotic therapy.
This cross-sectional observational study was conducted in the Department of Microbiology, ESIC Medical College & Hospital, a 510 bedded tertiary care teaching hospital in Faridabad. All wound samples including pus, exudates, wound swab and tissue samples received for aerobic culture and sensitivity from various clinical departments from January 2019 to July 2019 were included in this study.
The samples were processed for aerobic culture using standard methods. The samples were cultured on 5% Sheep Blood agar and MacConkey’s agar and was inoculated into Tryptic Soy broth.14 The culture plates were looked for any bacterial growth after 18-24 hours of incubation. If there was no growth observed after 24 hours of incubation the plates were further looked for growth at 48 -72 hours of incubation .
Suspected bacteria that were beta-hemolytic on blood agar, Gram-positive, and catalase-positive were identified using an automatic identification instrument (Vitek-2 Compact system, bioMerieux Inc., France).
Antimicrobial susceptibility testing was performed by Vitek 2 AST-P628 card (bioMerieux Inc., France), according to the updated Clinical and Laboratory Standards Institute guidelines.
Data analysis
The demographic and laboratory data were entered in Microsoft Excel sheets and analysed using the same. A chi-square test was conducted to compare the differences in proportion among groups. P values <0.05 were considered statistically significant.
This study was approved by the IEC, ESIC Medical College, Faridabad.
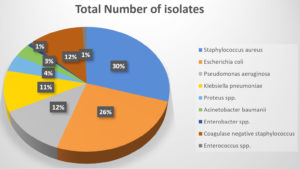
A total of 747 samples were received from January 2019-July 2019. Mean age of the study population was 50.7 ± 14.8 years. Out of 747 samples, 226 (30.25%) were culture positive. The distribution of bacterial isolates is given in Table 1 and in Figure 1. Most of the samples were reported from the male patients (466/747, 62.3%) as compare to the females (281/747, 37.7%).
Table (1):
Distribution of all bacterial isolates from the culture growth
Isolated Organism |
Total no. of isolates |
Percentage |
|---|---|---|
Staphylococcus aureus |
67 |
29.6% |
Escherichia coli |
58 |
25.6% |
Pseudomonas aeruginosa |
28 |
12.5% |
Klebsiella pneumoniae |
24 |
10.6% |
Proteus spp |
10 |
4.6% |
Acinetobacter baumanii |
8 |
3.5% |
Enterobacter spp |
2 |
0.8% |
Coagulase Negative Staphylococcus |
27 |
12% |
Enterococcus spp. |
2 |
0.8% |
Total |
226 |
100% |
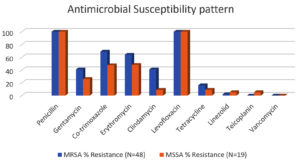
Amongst all isolates, S. aureus was the most common and retrieved from 67 (29.6%) patients, followed by Escherichia coli, Pseudomonas aeruginosa (Table 1). Among the S. aureus isolates, methicillin resistance was seen amongst 39 (58.2%). MRSA strain were mostly isolated from surgical ward and OPD 28 (71.9%), followed by Gynecology ward. Similar pattern of distribution was observed with MSSA isolates (Table 2). Antibiotic Susceptibility results of S. aureus showed 100% resistance to Penicillin along with 100% resistance to Fluoroquinolones in both MRSA and MSSA (Figure 2). Resistance to Vancomycin was not observed (Table 3).
Table (2):
Distribution of MRSA and MSSA isolates from the different wards
| Location | MRSA | MSSA | ||
|---|---|---|---|---|
| Total no. of isolates (n) | % | Total no. of isolates (n) | % | |
| Surgical OPD and Ward | 28 | 71.9% | 10 | 35.7% |
| Gynecological OPD and Ward | 8 | 20.5% | 12 | 42.8% |
| ENT OPD and ward | 3 | 7.6% | 6 | 21.5% |
| Total | 39 | 100% | 28 | 100% |
Table (3):
Antimicrobial Susceptibility pattern among the MRSA and MSSA isolates
Antibiotic |
MRSA % Resistance (N=48) |
MSSA % Resistance (N=19) |
|---|---|---|
Penicillin |
100 |
100 |
Gentamycin |
41 |
26 |
Co-trimoxazole |
68.8 |
47.5 |
Erythromycin |
64 |
48 |
Clindamycin |
41 |
9 |
Levofloxacin |
100 |
100 |
Tetracycline |
16 |
9 |
Linezolid |
2 |
5.2 |
Teicoplanin |
0 |
5.2 |
Vancomycin |
0 |
0 |
S. aureus continues to be the leading cause of skin and soft tissue related infections (SSTI) in the community as well as infections in the hospitalized patients and MRSA is also a major health problem worldwide, the prevalence of which has increased over these years.14 Data on MRSA transmission trends requires continued surveillance especially in developing nations like India. The cell envelope, ribosomes, and nucleic acids are the three targets of antibiotics developed to treat S. aureus. Methicillin is a member of the beta lactamase class of enzymes, which targets the cell membrane. Genes that are less sensitive to the effects of antibiotics are acquired, which leads to the development of methicillin resistance.15
The results of this study provide valuable insights into the prevalence, distribution, and antibiotic susceptibility pattern of S. aureus isolates, particularly MRSA, among patients in various wards of the healthcare facility.
In this study we observed that, from the total 747 samples, maximum number of samples were from males (62.3%, 466/747) as compare to females (37.7%. 281/747). This result was found similar to many studies done by Kaur K et al., Adhikari P et al. and Mandal M et al.16-18 The higher representation of male patients in the study population may reflect specific risk factors associated with gender or variations in healthcare-seeking behavior.
Overall culture positivity rate was 30.25% amongst the 747 samples received over six months. Culture positivity rate of 45-90% has been reported in various other studies.12,19,20 The high percentage of positive culture results indicates the urgent need for effective measures to control drug resistant infections.
The data revealed that S. aureus was the most commonly isolated bacterium, found in 67 (29.6%) patients, followed by Escherichia coli (25.6%), Pseudomonas aeruginosa (12.30%) and Klebsiella pneumoniae (10.6%). This finding emphasizes the importance of S. aureus as a significant pathogen responsible for infections in the studied population. Similarly, a study done by Dhungel S et al. also found that S. aureus (27.1%; 39/144) was the predominant bacteria.20 The data can help develop a local antibiogram for formulating an empirical antibiotic panel for different set of samples.
The findings of this study reveal a substantial prevalence of MRSA (58.2%) among patients attending the tertiary care center, while the remaining 41.79% were MSSA. This high prevalence of MRSA raises alarming concerns regarding the potential complications and challenges associated with treating infections caused by multidrug-resistant strains. More than 50% prevalence of MRSA was observed from different states of India, which support to the above findings of the study.16,21,22 Other investigations have found a significant low incidence of MRSA in various regions of the nation, such as 32% and 31.1% in a study by Bilal Ahmad et al. and Rajaduraipandi et al.23,24
The variation in prevalence rates can be attributed to disparities in antibiotic prescription practices and infection control measures implemented across various locations. For instance, at Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, the MRSA prevalence was 43%, while at Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, it stood at 35%.25 This could be attributed to the fact that since our hospital is a tertiary healthcare facility, the patients are most of the times referred from primary or secondary level healthcare settings. The common practice of taking over the counter antibiotics with wrongful dose and duration can add to this issue.
The distribution of MRSA and MSSA isolates across different wards of the healthcare facility was also examined. As expected, the surgical ward and OPD were identified as the primary sources of MRSA isolates, accounting for 51% of the cases. Similar patterns were observed with MSSA isolates. This result was supported by other studies done by Kaur K, Dhungel S, and Raut S et al., in which they also reported that a higher proportion of MRSA was derived from IPD wards as compare to OPD.16,20,26 This observation suggests that both MRSA and MSSA infections are prevalent in surgical and outpatient settings, emphasizing the need for rigorous infection control practices in these areas.
Furthermore, the study investigated the sources of MRSA isolates. Pus exudates were the most common sources, accounting for 47.92% of MRSA isolates and indicating the presence of localized infections. Abscesses were the second most frequent source, representing 25% of isolates followed by Ulcer (16.66%) and then wound swab (10.42%). Another study also reported maximum isolation from pus and wound swabs.16,26 These findings highlight the importance of appropriate wound management and infection control measures to prevent the spread, particularly in settings where surgical procedures are performed.
The antibiotic susceptibility pattern of S. aureus isolates, including both MRSA and MSSA, was assessed. Notably, all S. aureus isolates exhibited 100% resistance to Penicillin, underlining the inefficacy of this antibiotic against S. aureus infections. Fluoroquinolones one of the most commonly prescribed oral drug also demonstrated 100% resistance in both MRSA and MSSA isolates. This highlights the limited therapeutic options available in OPD setting for treating S. aureus infections in the studied population. Fortunately, no resistance to Vancomycin was observed, suggesting that this antibiotic remains a viable treatment option for severe MRSA infections.
The study was conducted in a single healthcare facility, which may limit the generalizability of the findings to other settings. Future research should involve multiple healthcare facilities and larger sample sizes to obtain more comprehensive and representative data on the prevalence and antibiotic susceptibility of MRSA and MSSA infections.
The importance of continued surveillance and practical implementation of antimicrobial stewardship program is need of the hour. To control the menace of antimicrobial resistant, a combined and comprehensive approach is required. The data needs regular follow up to assess the effectiveness of any hospital infection control measures taken. Serious input from hospital administration can help to bring down the prevalence of resistance and eventually help in patient management.
ACKNOWLEDGMENTS
The authors would like to acknowledge Mrs. Chand, Senior Technical Staff, ESIC Medical College and Hospital, Faridabad, for her constant technical support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, ESIC Medical College and Hospital, Faridabad, India, with EC file number 134X/11/13/2023-IEC/DHR/129.
- Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol. 2020;10:107.
Crossref - Tipper DJ, Strominger JL. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965;54(4):1133-1141.
Crossref - Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113-137.
Crossref - Bayles KW. The bactericidal action of penicillin: new clues to an unsolved mystery. Trends Microbiol. 2000;8(6):274-278.
Crossref - Maidhof H, Johannsen L, Labischinski H, Giesbrecht P. Onset of penicillin-induced bacteriolysis in staphylococci is cell cycle dependent. J Bacteriol. 1989;171(4):2252-2257.
Crossref - Giesbrecht P, Kersten T, Maidhof H, Wecke J. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol Mol Biol Rev. 1998;62(4):1371-1414.
Crossref - Tomasz A, Nachman S, Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991;35(1):124-129.
Crossref - de Lencastre H, Chung M, Westh H. Archaic strains of methicillin-resistant Staphylococcus aureus: molecular and microbiological properties of isolates from the 1960s in Denmark. Microb Drug Resist. 2000;6(1):1-10.
Crossref - Antimicrobial Resistance C, Ikuta KS, Sharara F. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655.
Crossref - Dayan GH, Mohamed N, Scully IL, et al. Staphylococcus aureus: the current state of disease, pathophysiology and strategies for prevention. Expert Rev Vaccines. 2016;15(11):1373-1392.
Crossref - European Antimicrobial Resistance C. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health. 2022;7(11):e897-e913.
Crossref - Nikita, Mohan S, Kakru DK. Prevalence Pattern, Microbial Susceptibility and Treatment of MRSA in A Tertiary Care Hospital. Int J Curr Adv Res. 2022;11(03):388-391.
- Veeraraghavan B, Walia K. Antimicrobial susceptibility profile & resistance mechanisms of Global Antimicrobial Resistance Surveillance System (GLASS) priority pathogens from India. Indian J Med Res. 2019;149(2):87-96.
Crossref - Collee JG. Mackie & McCartney Practical Medical Microbiology. 14th ed. 2016.
- Kumar P, Gupta G, Gupta GK, Mishra V, Gupta G. Prevalence of MRSA and Antimicrobial Susceptibility Staphylococcus aureus in Clinical Samples in National Capital Region, India. J Pharm Res Int. 2021;33(59A):209-215.
Crossref - Kaur K, Gill AK, Kaur M. Methicillin Resistance, Vancomycin intermediate and vancomycin resistance Staphylococcus aureus prevalence in a tertiary care hospital of Punjab, India. NJLM. 2019;8(3):MO01-MO0324.
- Adhikari P, Basyal D, Rai JR, et al. Prevalence, antimicrobial susceptibility pattern and multidrug resistance of methicillin-resistant Staphylococcus aureus isolated from clinical samples at a tertiary care teaching hospital: an observational, cross-sectional study from the Himalayan country, Nepal. BMJ Open. 2023;13(5):e067384.
Crossref - Mandal M, Dey S, Kumar D, Biswas PP, Nandan K, Sen A. Determination of vancomycin and linezolid resistance in Staphylococcus aureus isolated from Katihar district of Bihar. India J Evolution Med Dent Sci. 2017;6(16):1244-1247.
Crossref - Arunkumar V, Prabhagaravarthanan R, Bhaskar M. Prevalence of methicillin-resistant Staphylococcus aureus infections among patients admitted in critical care units in a tertiary care hospital. Int J Res Med Sci. 2017;5(6):2362-2366.
Crossref - Dhungel S, Rijal KR, Yadav B, et al. Methicillin-Resistant Staphylococcus aureus (MRSA): Prevalence, Antimicrobial Susceptibility Pattern, and Detection of mecA Gene among Cardiac Patients from a Tertiary Care Heart Center in Kathmandu, Nepal. Infect Dis (Auckl). 2021;14:11786337211037355.
Crossref - Chatterjee A, Rai S, Guddattu V, Mukhopadhyay C, Saravu K. Is methicillin-resistant Staphylococcus aureus infection associated with higher mortality and morbidity in hospitalized patients? A cohort study of 551 patients from South Western India. Risk Manag Healthc Policy. 2018;11:243-251.
Crossref - Kulshrestha A, Anamika V, Mrithunjay K, Himanshu V, Manish K, Dalal AS. A prospective study on the prevalence and antibiotic sensitivity pattern of methicillin-resistant Staphylococcus aureus isolated from various clinical specimens at a tertiary care postgraduate teaching institute. Int J Curr Microbiol Appl Sci. 2017;6(3):1859-1869.
Crossref - Mir BA, Srikanth. Prevalence and antimicrobial susceptibility of Methicillin-Resistant Staphylococcus aureus and Coagulase-negative staphylococci in a tertiary care hospital. Asian J Pharm Clin Res. 2013;6(3):231-234.
- Rajaduraipandi K, Mani KR, Panneerselvam K, Mani M, Bhaskar M, Manikandan P. Prevalence and Antimicrobial Susceptibility Pattern of Methicillin-Resistant Staphylococcus aureus: A Multicentre Study. Indian J Med Microbiol. 2016;24(1):34-38.
Crossref - Rajkumar S, Sistla S, Manoharan M, et al. Prevalence and genetic mechanisms of antimicrobial resistance in Staphylococcus species: A multicentre report of the Indian Council of Medical Research Antimicrobial Resistance Surveillance Network. Indian J Med Microbiol. 2017;35(1):53-60.
Crossref - Raut S, Bajracharya K, Adhikari J, Pant SS, Adhikari B. Prevalence of methicillin-resistant Staphylococcus aureus in Lumbini Medical College and Teaching Hospital, Palpa, Western Nepal. BMC Res Notes. 2017;10(1):187.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.