ISSN: 0973-7510
E-ISSN: 2581-690X
Digera muricata Mart, a plant having therapeutic characteristics that has been utilised traditionally, belongs to the Amaranthaceae family, and a promising source of specific natural products utilized as antioxidant, prophylactic, antimicrobial, anthelmintic, anti-diabetic, and allelopathic agent. In the present study, a biologically active phytosterol was isolated from Digera muricata Mart. The isolated compound was characterized by 13C, 1H NMR, FTIR, and HRMS. Characterization of the isolate was done by antimicrobial assay, and molecular docking. The antimicrobial potential of the isolated phytosterol (50 µl) against Streptococcus pyogenes was found to be maximum (ZOI-20.0 ± 1.0), followed by Streptococcus agalactiae (ZOI-11.3±1.5), Candida albicans (ZOI- 09.0 ± 1.0), Klebsiella pneumonia (ZOI-8.6 ± 1.5) and Escherichia coli (ZOI-8.6 ± 1.5). The molecular docking results indicate that the phytosterol binds to the receptor 1AI9 at the 32th and 58th positions; 1KZN receptor at the 76th position, the 5L3J receptor at the 46th (ASN) and 136th (ARG) position; 7WIJ receptor at the 419th (ARG) and 582th (ASP) and 585th (ASN) positions.
Antimicrobial, Antifungal, Phytosterol, Digera muricata, Molecular Docking
Attention has been drawn to natural products based on plants as sustaining medicinal and health ailments due to the cost-effective treatment of chemically synthesized drugs, their toxicities in the sustainability of individual medical aid and development, and the exploitation of plant-mediated new medicines. Some reports on recent investigations and economic expenses, medicinal plants will ultimately continue to have a crucial role in developing as a health aid. These bioactive compounds based on therapeutic plants have a essential role in medical treatment and play a twin role in the innovation of such drugs as they are the backbone for designing such medicine, a natural blueprint for creating novel molecules or; natural products engaged in combating many diseases.1 Therefore, these medicinal plants are acquired for the isolation of such compounds to gain knowledge about their underlying mode of action with reduced toxicity and cost-effective features.2 The enhanced risk and toxicities of chemotherapeutics and the occurrence of multiple drug resistance mutants in pathogenic bacteria forced us to search for such novel drugs having antimicrobial potential.2,3
About experimental plant
As part of the family Amaranthaceae, Digera muricata Mart (D. muricata) grows in the wilderness and is a consumable plant. It is found across India, especially Rajasthan, Maharashtra and Andhra Pradesh.4 D. muricata have many names, including Cancalisoppu, Latamouri or Gungutiya, Latmahuria, Lesua, False Amaranth, Latmahuria, Kunanjara, and Aranya. D. muricata is an annual medicinal plant growing 20-70 cm tall with long, narrowed, and pointed leaf stalks. Flowers grow to 30 cm in height and are spike-like racemes.5 There is typically a greenish-white colour in fruit, with white flowers mixed with pink, carmine, or red. August and September are the months when flowers bloom. The tender shoots and foliage of D. muricata are utilised both medicinally and as a vegetable in the local community for the treatment of gastrointestinal diseases, diabetes, constipation, and bowel issues. Urinary discharges can be treated with flowers and seeds. The application of leaf paste prevented the local production of pus. The mother is given a boiled root infusion after childbirth for induced lactation.6 D. muricata is also used as an expectorant and mild astringent.7,8
D. muricata is regarded as a laxative and a cooling astringent to the bowels in Ayurveda. Fungal infections were treated with the extract of the leaves of the plant.9 People’s health is directly benefited by phytochemicals found in traditional foods.10 A study of D. muricata treatment provides evidence that it has antioxidant properties, is protective against CCl4-induced toxicity, and may help treat diseases caused by free radicals.11 Significant antiproliferative activity against MG-63 cell lines has been shown by the ethanolic leaf extract of D. muricata.12 Recently, two compounds with herbicidal properties were isolated from D. muricata.13 During the growing season, the roots of D. muricata contain sugars and phenols, while the leaves contain proteins, starches, and fats. In addition, the leaf extract has antimicrobial properties against Escherichia coli and Fusarium oxysporum. Kidney stones can be treated with leaf extract.14 For a variety of male hormones in rats, CCl4 disturbances were reversed by D. muricata.15 By use of this herb as a substitute for secondary infertility.16 D. muricata has a well-documented history of being an effective antibacterial.17 The study was intended to extract, isolate and structurally characterize bioactive compound and its antimicrobial potential.
Collection and verification of plant
The herb was gathered from Rajasthan’s Chittorgarh district. The collected plant material was identified and deposited in Botanical Survey of India, Jodhpur, Rajasthan.
Isolation of compound using column chromatography
Column packing
To create an admixture, 16 g of ethyl acetate extract was combined with silica gel (60-120 mesh). The admixture combined with hexane was placed onto a column with a 2.4-inch diameter. Hexane was used as the starting solvent, followed by ethyl acetate to elute the column.
Fractionation of the extract
60 to 120 mesh size activated Silica gel used to fill the glass column. The extract was added to the column and gradually eluted by using 1000 ml of hexane of the ratio 100:0 v/v, 1200 ml of hexane: ethyl acetate mixture in the proportion of 95:5 v/v, 900 ml of hexane:ethylacetate in the proportion of 90:10 v/v, 900 ml of hexane:ethylacetate in the proportion of 85:15 v/v and 800 ml of hexane:ethylacetate in the ratio of 80:20, 400 ml hexane:ethylacetate in the proportion of 75:25 v/v, 800 ml of hexane:ethylacetate in the proportion of 70:30 v/v, 200 ml of ethyl acetate in the proportion of 0:100 v/v.
Thin-layer chromatography (TLC)
Concentrated fraction was placed onto the 20X20 cm activated silica gel TLC plates. The plates were created using various hexane:ethyl acetate ratios, including 90:10, 75:25, 50:50, and 25:75. The plate was subjected to vanillin and iodine fumes in order to identify any spots. A single spot denoted a pure compound with excellent purity and yield was selected for spectral analyses, and structures will be clarified.
Structure elucidation
High-Resolution Mass Spectrometry (HRMS) and Nuclear magnetic resonance (NMR) was exploits to draw the structures of the isolated compound. Thermo Scientific’s Evolution 160 spectrophotometer was used to record UV spectra, while Perkin-Elmer’s FTIR model 1600 spectrophotometer was used to record IR spectra. Tetramethylsilane (TMS) served as the internal standard while spectra of NMR were captured in CDCl3 using a Bruker AMX 500 spectrometer at 500 MHz. Relative to the TMS internal standard, chemical shifts are expressed in ppm, and scalar coupling constants are described in Hz (J).
Antimicrobial susceptibility test
Growth conditions of microorganisms
In the current investigation, test microorganisms included Streptococcus agalactiae (ATCC13813), Escherichia coli (MTCC730), Klebsiella pneumoniae (MTCC432), and Streptococcus pyogenes (MTCC1924). In nutrient broth, the bacterial strains were resurrected. Each bacterium received a fresh inoculum for the antibacterial test, which was performed at 37°C for 24 hours. The nutrient broth was then added to the cell suspension to modify the turbidity until it was close to the 1.5 × 108 CFU/ml McFarland 0.5 standard.
In the current investigation, Candida albicans (MTCC7315) was utilised as a test fungus. It was kept at 4°C on potato dextrose agar (PDA) slants before usage.
Antibacterial susceptibility assay
The conventional disc diffusion technique was used to test the antibiotic sensitivity of bacterial strains to two antibiotics with potencies of 10 μg per disc, including gentamycin, and tetracycline.18 Using the disc diffusion technique, the antibacterial test of isolated compound was conducted. Swabs of the test microorganisms were diluted to a concentration of 100 µl of diluted inoculum (1.5X108 CFU/ml) and added to plates of sterile Mueller Hinton agar (pH 7.2). 1 mg of isolated compound dissolved in DMSO used as stock. From stock 40 µl was added to the 4 mm diameter agar plate well. Pure DMSO used as negative control.
Antifungal susceptibility assay
Candida albicans inoculum was suspended in 5 ml of potato dextrose broth and cultured at 37°C for a day. The experiment was used to evaluate the antifungal activity using disc diffusion principle.18 The inoculum was applied to the PDA medium with a glass spreader aseptically. Separately, 2.5 mm radius paper discs soaked with 40 µl from the stock was put on the infected plates’ surface and kept at 37°C in the incubator for 24 hours. The diameter of the inhibitory zone was used to gauge the antifungal activity. Dimethyl sulfoxide (DMSO) was used as a negative control, and fluconazole served as the positive control. All experiments were run in triplicate.
Minimum inhibitory concentration
The Clinical and Laboratory Standards Institute (CLSI) recommendations and Eloff’s microtitre broth dilution procedure19 were followed to ascertain the isolated compound’s Minimum Inhibitory Concentration (MIC). Initially, isolated compound was prepared by dissolving it in dimethylsulphoxide (DMSO) to achieve a final concentration of 1 mg/ml.
A bacterial suspension with around 5X105 CFU/mL was produced using a 24-hour culture plate and 100 µl of this suspension was introduce into every well of the microplates. For each strain, sterile environment and growth was controlled. The microtiter plates were incubated for 24 hours at 37°C for bacterial species and 48 hours for fungal strains. Following incubation, 40 µl of a 0.4 mg/ml solution of iodonitrotetrazolium chloride was added to every well as a microbial growth indicator. The plates were then incubated at 37°C for 30 min for bacteria and 24 h for fungi, and MIC values were visually determined. The minimum inhibitory concentration was noted as having the least amount of extract with no visible growth, while the MIC value was identified as the concentration completely inhibiting bacterial/yeast growth (the first clear well).
To assess microorganism sensitivity, A negative control test utilizing only DMSO was also conducted and positive control experiments were performed for bacterial strains, gentamycin was used at a starting concentration of 0.10 mg/ml in sterile water, and for fungal strains, fluconazole was used at a starting concentration of 0.10 mg/ml in DMSO and water. Final concentrations for these experiments ranged from 0.1 mg/ml to 0.001 mg/ml.
Molecular docking study
The aim is to explain the behavior of small molecules within the binding site of target proteins. Employing the molecular docking technique, this investigation seeks to comprehend essential biological processes by reproducing the atomic-level interaction with a tiny chemical and a protein.20 Using AutoDock, phytosterol was docked towards the binding site of the proteins. By setting the scaling factor for the nonpolar atoms to 0.8, the flexibility of the proteins was controlled. Default conditions were set for all other parameters. Docking scores were used to measure the binding affinity of protein/ligand complexes.
Receptor information
a) 1AI9: Candida albicans (Yeast)
Protein – Dihydrofolate reductase
Gene – DFR1
AA – 192
b) 1KZN: Escherichia coli (strain K12)
Protein – DNA gyrase subunit B
Gene – gyrB
AA – 804
c) 5L3J: Escherichia coli
Protein- DNA Gyrase b in complex with benzothiazole-based inhibitor
Gene- gyrB, Z5190, ECs4634
AA- 378
d) 7WIJ – Macrophomina phaseolina (strain MS6) (Charcoal rot fungus)
Protein – geranylgeranyl diphosphate synthase
AA – 698
Collection and verification of Plant
Collected herb was identified as D. muricata and deposited in Botanical Survey of India, Jodhpur, Rajasthan.
Isolation of compound using column chromatography
Fractions were collected from column chromatography and concentrated by the rotary evaporator. A pure portion was collected and used for further characterisation at the Hexane:Ethyl acetate (70:30) concentration.
Thin-layer chromatography (TLC)
Isolated pure compounds showing good purity and good yield was taken for spectrum studies and used for structure elucidation.
Characterisation of the isolated compound
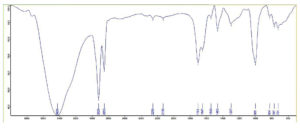
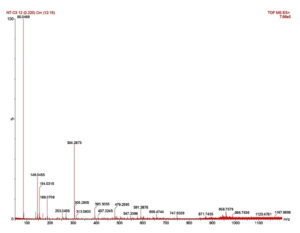
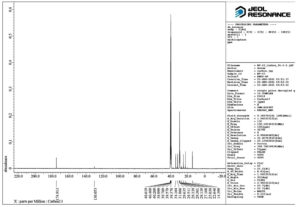
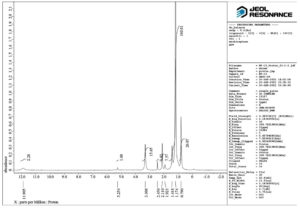
The IR spectrum exhibited a band for H-bonded OH (3246 cm-1), COOH (1701 cm-1) and asymmetric stretching (2920 cm-1), C-H stretching (2853 cm-1), CH2 scissoring (1462 cm-1) and C-O stretching (999 cm-1), C-H in plane and out of plane bending for benzene ring (824-721 cm-1) (Figure 1). The isolated compound’s HRMS, C-13, and proton NMR are presented in Figures 2, 3, and 4, respectively.
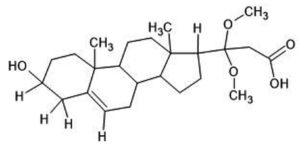
1H NMR (500 MHz, DMSO–d6) δ: 0.74–0.92 (s, 6H, -CH3), 1.12-1.63 (m, 14H, -CH2 of ring), 2.11-2.18 (t, 3H, -CH), 2.45 (s, 2H, -CH2– of open chain), 3.22-3.30 (s, 6H,-OCH3), 3.64-3.81 (m, 1H,-CHOH ), 4.11-4.16 (d, 1H,-C-OH ), 5.17-5.25 (t, 1H, =CH-), 11.91 (1H, s, -COOH) (Figure 5). HRMS; calculated for C24H38O5, 407 (M+ + H). The 1H NMR spectrum revealed the presence of a COOH (11.905 ppm), two methoxy groups (3.308 ppm), secondary methylene (2.45 ppm), one hydrogen directly attached to unsaturated group (5.254 ppm), one hydrogen attached to an electronegative oxygen atom (3.308 ppm) and three hydrogens attached to cyclic group (2.11 ppm) (Figure 4). Figure 5 shows the structure of the isolated compound.
Figure 5. Structure of isolated phytosterol: 3-(2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3-hydroxy-10,13-dimethyl-1H-cyclopenta[a]phenanthren-17-yl)-3,3-dimethoxypropanoic acid
Antimicrobial Susceptibility test
Antimicrobial activity
The antimicrobial potential of the isolated compound (50 µl) against Streptococcus pyogenes was found maximum (ZOI-20.0 ± 1.0) followed by Streptococcus agalactiae (ZOI-11.3 ± 1.5), Candida albicans (ZOI-09.0 ± 1.0) Klebsiella pneumoniae (ZOI-8.6 ± 1.5) and Escherichia coli (ZOI-8.6 ± 1.5) as compared to tetracycline and gentamycin (Table 1).
Table (1):
Antimicrobial activity of the isolated compound
Microorganism |
Compound (50 µl) ZOI (mm) ± SD) |
Compound (25 µl) ZOI (mm) ± SD) |
Tetracycline (mm) ± SD) |
Gentamycin (mm) ± SD) |
|---|---|---|---|---|
Streptococcus agalactiae |
11.3 ± 1.5 |
09.6 ± 0.7 |
14.1 ± 0.7 |
14.2 ± 0.6 |
Streptococcus pyogenes |
20.0 ± 1.0 |
05.8 ± 0.7 |
11.4 ± 0.4 |
14.3 ± 0.7 |
Escherichia coli |
08.0 ± 1.0 |
14.7 ± 0.6 |
14.2 ± 0.7 |
14.9 ± 0.1 |
Klebsiella pneumoniae |
8.6 ± 1.5 |
20.6 ± 0.7 |
19.2 ± 0.6 |
19.2 ± 0.7 |
Candida albicans |
09.0 ± 1.0 |
10.8 ± 0.6 |
– |
– |
MIC of the isolated compound
MIC outcomes for the isolated compounds and fractions are depicted in Table 2. The isolated compound exhibits higher antibacterial potential against Streptococcus pyogenes with MIC of 8 mg/ml as compared to the gentamycin i.e., 4 mg/ml. MIC for Candida albicans found to be 20 mg/ml as compared to the fluconazole i.e., 6 mg/ml.
Table (2):
Minimum inhibitory concentration (MIC) of the isolated compound
| Microorganism | Compound | Gentamycin | Fluconazole |
|---|---|---|---|
| MIC values (mg/ml) | |||
| Streptococcus agalactiae | 10 | 2 | NA |
| Streptococcus pyogenes | 8 | 4 | NA |
| Escherichia coli | 12 | 1 | NA |
| Klebsiella pneumoniae | 16 | 0.5 | NA |
| Candida albicans | 20 | NA | 6 |
Molecular docking
1AI9_C3
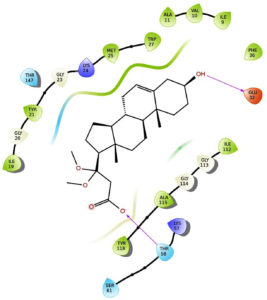
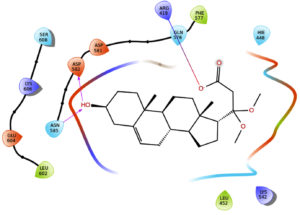
The results indicate that the isolated phytosterol binds to the receptor 1AI9 at the 32nd and 58th position, respectively. GLU at 32th position makes a substrate binding site, while THR at 58th position makes a binding site for NADP+. Both the amino acid forms a helix structure in the protein. The isolated molecule interacts with the side chains of both amino acids, with a docking score of -5.869. The docking score indicates a good interaction between the molecule and the receptor, while gentamycin and streptomycin bind more strongly to the receptor, with docking score of -7.15 and -9.338, respectively (Table 3, Figure 6).
Table (3):
Observation of docking
No. |
Gene |
Electron Donor |
Electron Acceptor |
AA Residue Position |
Role of AA in protein Feature |
Secondary structure of protein |
AA Residue |
Binding site |
Docking Score |
|---|---|---|---|---|---|---|---|---|---|
1 |
1AI9_C3 |
OH |
32 |
Binding site (substrate) |
Helix |
GLU |
Side chain |
-5.869 |
|
O- |
58 |
Binding site (NADP+) |
Helix |
THR |
Side chain |
||||
1AI9_Gentamycin |
NH |
61 |
– |
Helix |
SER |
Side chain |
-7.15 |
||
O |
115 |
Binding site (NADP+) |
Helix |
ALA |
Back bone |
||||
O |
115 |
Binding site (NADP+) |
Helix |
ALA |
Back bone |
||||
NH |
118 |
Binding site (substrate) |
Helix |
TYR |
Side chain |
||||
1AI9_Streptomycin |
OH |
11 |
Binding site (NADP+) |
Beta strand |
ALA |
Back bone |
-9.338 |
||
OH |
11 |
Binding site (NADP+) |
Beta strand |
ALA |
Back bone |
||||
NH |
19 |
Binding site (NADP+) |
Beta strand |
ILE |
Back bone |
||||
NH |
112 |
Binding site (substrate) |
– |
ILE |
Back bone |
||||
OH |
112 |
Binding site (substrate) |
– |
ILE |
Back bone |
||||
OH |
115 |
Binding site (NADP+) |
Helix |
ALA |
Back bone |
||||
2 |
1KZN_C3 |
O |
76 |
ATPase domain |
– |
ARG |
Side chain |
-3.95 |
|
O- |
76 |
– |
– |
ARG |
Side chain |
||||
1KZN_Gentamycin |
NH |
46 |
Binding site (ADP) |
Helix |
ASN |
Back bone |
-3.068 |
||
NH |
50 |
– |
Helix |
GLU |
Side chain |
||||
1KZN_Streptomycin |
OH |
46 |
Binding site (ADP) |
Helix |
ASN |
Side chain |
-7.039 |
||
OH |
46 |
Binding site (ADP) |
Helix |
ASN |
Side chain |
||||
OH |
46 |
Binding site (ADP) |
Helix |
ASN |
Side chain |
||||
OH |
90 |
– |
Helix |
ILE |
Side chain |
||||
OH |
96 |
– |
Helix |
ALA |
Back bone |
||||
OH |
120 |
Binding site (ATP) |
Helix |
VAL |
Back bone |
||||
3 |
5L3J_C3 |
OH |
46 |
ASN |
Side chain |
-3.627 |
|||
O |
136 |
ARG |
Side chain |
||||||
O (Covenant bond) |
136 |
ARG |
Side chain |
||||||
5L3J_Gentamycin |
NH |
46 |
ASN |
Side chain |
-6.238 |
||||
NH |
50 |
GLU |
Side chain |
||||||
OH |
50 |
GLU |
Side chain |
||||||
NH |
73 |
ASP |
Side chain |
||||||
5L3J_Streptomycin |
OH |
46 |
ASN |
Side chain |
-6.282 |
||||
OH |
46 |
ASN |
Side chain |
||||||
OH |
50 |
GLU |
Side chain |
||||||
NH |
97 |
VAL |
Back bone |
||||||
NH |
97 |
VAL |
Back bone |
||||||
4 |
7WIJ_C3 |
O- (Covalent bond) |
419 |
ARG |
Side chain |
-4.507 |
|||
OH |
582 |
ASP |
Side chain |
||||||
OH |
585 |
ASN |
Side chain |
||||||
7WIJ_Gentamycin |
OH |
451 |
SER |
Side chain |
-5.052 |
||||
OH |
517 |
GLN |
Side chain |
||||||
O |
542 |
LYS |
Side chain |
||||||
O |
578 |
GLN |
Side chain |
||||||
O |
578 |
GLN |
Side chain |
||||||
NH |
581 |
ASP |
Side chain |
||||||
7WIJ_Streptomycin |
OH |
451 |
SER |
Side chain |
-8.896 |
||||
OH |
517 |
GLN |
Side chain |
||||||
OH |
520 |
ASP |
Side chain |
||||||
NH |
542 |
LYS |
Side chain |
||||||
NH |
578 |
GLN |
Side chain |
||||||
OH |
578 |
GLN |
Side chain |
||||||
NH |
581 |
ASP |
Side chain |
||||||
NH |
581 |
ASP |
Side chain |
*More docking score indicates a more binding affinity with the receptor molecule.
1KZN_C3
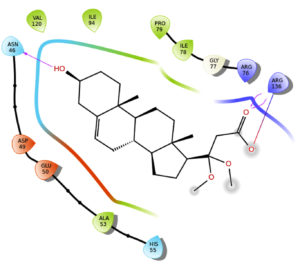
The results indicate that the isolated phytosterol binds to the receptor 1KZN at the 76th position. ARG at 76th position makes an ATPase domain region. Two different oxygen atoms in the isolated molecule interact with the side chains of both amino acids, with a docking score of -3.95, which is more than the docking score of gentamycin (-3.068) and less than the docking score of streptomycin (-7.039). More docking scores indicate more binding affinity with the receptor (Table 3, Figure 7).
5L3J_C3
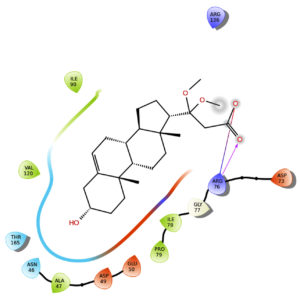
The results indicate that the isolated phytosterol binds to the receptor 5L3J at the 46th (ASN) and 136th (ARG) positions, respectively. The isolated molecule interacts with the amino acid’s side chains, with a docking score of -3.627. The docking score of the isolated compound is less than gentamycin (-6.238) and streptomycin (-4.507). Besides, all three molecules compound, Gentamycin, and Streptomycin interacts with the 5L3J rectors at the same position 46th amino acid residue ASN but the binding affinity of compound is less (Table 3, Figure 8).
7WIJ_C3
The results indicate that the isolated phytosterol binds to the receptor 7WIJ at the 419th (ARG) and 582th (ASP) and 585th (ASN) positions, respectively. The isolated molecule interacts with the side chains of all three amino acid, with a docking score of -4.507. The docking score of compound is less than the docking score of gentamycin (-5.052) and streptomycin (-8.896) (Table 3, Figure 9).
Digera muricata Mart. is a plant with a long history of traditional medicinal use in various cultures. Recently, there has been growing interest in exploring its bioactive compounds for potential therapeutic applications. One such compound that has garnered attention is a novel phytosterol found in Digera muricata Mart., which exhibits promising antimicrobial properties. The antimicrobial potential of phytosterols extracted from the leaf of Annona squamosa, Adenocalymma alliaceum, and Amaranthus tricolor was examined. These phytosterol was isolated, purified, characterized, and assessed for their antibacterial effects on both gram-positive bacteria, like Staphylococcus aureus, Staphylococcus albus, and Streptococcus viridians, and gram-negative bacteria, including Escherichia coli, Pseudomonas pyocyanea, and Klebsiella.21 The study by Monu et al. in 2008 also investigated the antimicrobial potential of phytosterols in milk.22
Furthermore, a bacteriostatic experiment involving phytosterols derived from pumpkin seeds revealed significant antagonistic actions against E. coli, Bacillus subtilis, Staphylococcus aureus, and Salmonella. Complete inhibition of bacterial at a dosage of 3.0 mg/mL, proliferation was seen.23 Numerous phytochemicals contained in D. muricata have been shown to have potent anti-inflammatory and powerful free radical scavenging effects.24 D. arvensis (a synonym of D. muricata) demonstrates notable antibacterial activity against Ralstonia solanacearum (previously known as Pseudomonas solanacearum), with a mean zone of inhibition measuring 6.14 mm.25
Present evidence from studies demonstrating the antimicrobial activity of the novel phytosterol. The discovery of a novel phytosterol with antimicrobial potential in Digera muricata Mart. opens up exciting possibilities for harnessing its health-promoting effects. The antimicrobial properties of this phytosterol hold promise for addressing oxidative stress-related conditions, and further research may unveil its full therapeutic potential. Integrating this compound into functional foods and pharmaceuticals could offer innovative strategies for promoting health and preventing diseases associated with oxidative damage. However, ongoing exploration is necessary to fully recognize its mechanisms of action, assess safety, and improve its applications for human health.
The isolated compound was characterized by 13C, 1H NMR, FTIR, and HRMS and subjected to antimicrobial assay, and molecular docking study. The antimicrobial potential of the isolated phytosterol against Streptococcus pyogenes was found to be maximum (ZOI-20.0 ± 1.0). The molecular docking results indicate that the phytosterol binds to the receptor 1AI9 at the 32th and 58th positions; 1KZN receptor at the 76th position, the 5L3J receptor at the 46th (ASN) and 136th (ARG) position; 7WIJ receptor at the 419th (ARG) and 582th (ASP) and 585th (ASN) positions. This study showed that isolated phytosterols might exploits as potential antibacterial agents.
The novel phytosterol isolated from Digera muricata Mart. represents a promising avenue for the development of antimicrobial agents. Its unique properties and demonstrated efficacy against various microorganisms make it a valuable candidate for further exploration. However, more research is required to know its mechanisms, assess its safety, and optimize its potential applications in clinical settings. The discovery of this novel phytosterol opens up new possibilities for combating microbial infections and addressing the challenges of antibiotic resistance in modern medicine. Propose future research directions, including further investigations into the specific molecular pathways affected by the phytosterol and optimization of delivery methods.
ACKNOWLEDGMENTS
The authors sincerely thank Suresh Gyan Vihar University, Jaipur, for providing a platform for this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SM conceptualized and designed the study. NT, AKS, HN, RD, AS performed analysis. ZZ performed data interpretation. MIA and MM wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Jain SK. Dictionary of Indian folk medicine and ethnobotany. 1991.
- Bozovic M, Mladenovic M, Ragno R. Chemical composition and antimicrobial activity of essential oils. Front Pharmacol. 2023;14:1120756.
Crossref - Rolnik A, Stochmal A, Beata O. The In vitro antiplatelet activities of plant extracts from the Asteraceae family. Biomed Pharmacother. 2022;149:112809.
Crossref - Aravindhan V, Rajendran A. Diversity of invasive plant species in boluvampatti forest range, the southern Western Ghats, India. Am-Eurasian J Agric Environ Sci. 2014;14:724-731.
- Jagatha G, Senthilkumar N. Evaluation of anti-diabetic activity of methanol extract of Digera muricata (l) mart in alloxan induced diabetic rats. Int J Pharm Sci Res. 2011;2(6):1525-1529.
Crossref - Miah MM, Das P, Mridha SA, Kuddus MR, Rashid MA. Bioactivities of Digera muricata (L.) Mart. Available in Bangladesh. Dhaka Univ J Pharm Sci. 2017;16(2):251-254.
Crossref - Parrotta JA. Healing plants of peninsular India. CABI Publishing, Wallingford, UK. 2001.
Crossref - Shah A, Marwat SK, Gohar F, et al. Ethnobotanical study of medicinal plants of semi-tribal area of Makerwal and Gulla Khel (lying between Khyber Pakhtunkhwa and Punjab Provinces), Pakistan. Am J Plant Sci. 2013;4:98-116.
Crossref - Chandra M, Mahesh NM. Antifungal activity of medicinal plant extracts against seed-borne pathogenic fungi, Acta Biol Indica, 2013;2:481-483. http:// bioscipubl.com/journals/abi/pdf/481-483.pdf
- Palozza P, Krinsky, NI. b-Carotene and a-tocopherol are synergistic antioxidants. Arch Biochem Biophys. 1992;297(1):184-187.
Crossref - Usmani S, Hussain A, Farooqui AHA. Pharmacognostical and phytochemical analysis of Digera muricata Linn. growing as a weed in fields of Uttar Pradesh region of India. Int J Pharm Sci. 2013;5(suppl 1):142-145.
- Selvi VP, Roy A, Jagadeswaran D. Anticancer Property of Digera muricata Leaf Extract: An In vitro Study. Cureus. 2023;15(11):e49276.
Crossref - Akbar M, Raza A, Khalil T, Yasin NA, Nazir Y, Ahmad A. Isolation of herbicidal compounds, quercetin and b-caryophyllene, from Digera muricata. Arab J Chem. 2023;16(5):104653.
Crossref - Sharma N, Tanwer BS, Vijayvergia R. Study of medicinal plants in Aravali regions of Rajasthan for treatment of kidney stone and urinary tract troubles. Int J Pharmtech Res. 2011;3(1):110-113.
- Khan MR, Ahmed D. Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem Toxicol. 2009;47(6):1393-1399.
Crossref - Chettleborough J, Lumeta J, Magesa S. Community use of non-timber forest products: A case study from the Kilombero Valley Frontier Tanzania. Kilombero Valley Integrated Environmental Management Programme. The Society for Environmental Exploration, UK & The University of Dar es Salaam. 2000. https://www.easternarc.or.tz/wp-content/uploads/2019/12/FT-KV-NTFP-report.pdf
- Mathad P, Mety SS. Phytochemical and Antimicrobial Activity of Digera Muricata (L.) Mart. E J Chem. 2010;7(1):275-280.
Crossref - Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am J Clin Pathol. 1966;45(4):493-496.
Crossref - Eloff J. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64(8):711-713.
Crossref - McConkey BJ, Sobolev V, Edelman M. The performance of current methods in ligand-protein docking. Curr Sci. 2002;83(7):845-856.
- Sharm RK. Phytosterols: Wide-spectrum antibacterial agents. Bioorganic Chem. 1993;21(1):49-60.
Crossref - Monu E, Blank G, Holley R, Zawistowski J. Phytosterol effects on milk and yogurt microflora. J Food Sci. 2008;73(3):M121-M126.
Crossref - Xu YQ, Pang LP, Qi HJ, Yang Y, Yang YL. Antioxidant activity and antibacterial effect of phytosterol from pumpkin seeds. Acad. Periodical Farm Prod. Process. 2012;5:14-16.
- Ravi S, Duraisamy P, Krishnan M, Martin LC, Manikandan B, Ramar M. Sitosterol-rich Digera muricata against 7-ketocholesterol and lipopolysaccharide-mediated atherogenic responses by modulating NF-ֺB/iNOS signalling pathway in macrophages. 3 Biotech. 2023;13(10):331.
Crossref - Taran SNU, Ali SA, Haq NU, et al. Antioxidant and antimicrobial activities, proximate analysis and nutrient composition of eight selected edible weeds of Peshawar region. J Xi’an Shiyou Univ, Nat Sci. 2022;18(9):517-545.
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.