ISSN: 0973-7510
E-ISSN: 2581-690X
Compost is a natural and sustainable way to improve soil fertility and enhance plant growth. Moringa leaves have high mineral, cytokinin, and vitamin content which are useful for growth so that they can be used as organic fertilizer. Azotobacter and Streptomyces are from soil and have many biological activities. This study aimed to detect the importance of bioagents formula with Moringa Compost (MC) to enhance plant growth in poor sterile soil and plants were irrigated with half strength of Hoagland nutrient solution. Moringa leaves were collected and cleaned, and organic compost was prepared and analyzed for microbial and chemical composition. The prepared MC was rich in nitrogen and minerals and had high content of bacteria and fungi. The two bioagents used were isolate MB5 and MB11 which were characterized and molecular identified as Azotobacter chroococcum MB5 and Streptomyces griseus MB11. The free-living A. chroococcum can fix atmospheric nitrogen while Streptomyces is a filamentous bacterium with a high ability to produce secondary metabolites. The addition of 20% MC to soil increased soil EC and microbial counts compared to MC-free soil. Moreover, inoculation of soil with either AZ or ST increased the microbial counts and soil EC and the clearest increase was in the case of inoculation of soil with MC+AZ+ST. It also found that MC extract alone with the bacterial filtrates increases seed germination of Phaseolus vulgaris L. (common bean), which is a herbaceous annual worldwide plant, grown for its edible dry seeds or green unripe pods. In this regard, inoculation of soil with inoculum of both A. chroococcum MB5, and S. griseus MB11, in the presence of MC has the most pronounced effect and enhances both the growth, fresh and dry weights, leaf number, plant height, and root length of P. vulgaris grown under greenhouse conditions for one month and chemical content of the plant protein carbohydrates, P, N, Ca++ and K+. In conclusion, the combined application A. chroococcum MB5 and S. griseus MB11, as a biofertilizers with Moringa compost is recommended to enhance P. vulgaris growth. The use of these biofertilizers can reduce the use of chemical fertilizers, which can have detrimental effects on soil and the environment. Therefore, further research on the inoculation and application of these microorganisms with MC is essential for sustainable agriculture.
Moringa, Compost, Inoculum, Phaseolus vulgaris, Azotobacter chroococcum, Streptomyces griseus
To deliver the nutrients required to feed the world’s population, which is expected to increase from 7.3 to 9.8 billion by 2050, new environmentally friendly uses must be developed.1 Enhancing growth could be performed in one of three ways: directly by adding plant growth-promoting bacteria, which can directly boost growth, decrease pathogen activity, and adjust soil microbial balance.2 Several microorganisms have been introduced into the soil to boost plant tolerance to various environmental factors. These bacteria can be employed in conjunction with other microbial agents to boost growth due to plant growth-stimulating substances and the production of potential antagonistic agents.3
The production of crops for food, fuel, and other industrial purposes is essential for human survival. However, the quality of the soil is a vital factor in crop production. Soil fertility is a complex process that depends on several factors, including the presence of organic matter, beneficial microorganisms, and nutrients. Chemically, using fertilizers is expensive and severely harms the environment and the beneficial soil microorganisms that live there in addition, it poses a risk to human health and can create situations that help in the rapid emergence of chemical-resistant pathogens. To increase plant growth, the application of bioagents with a high degree of safety and minimal environmental impact is recommended.4 The application of organic fertilizers is a common practice to improve soil fertility and increase crop yield.
Moringa (Moringa oleifera) is a drought-tolerant tree native to some parts of Asia and arid regions and has been used traditionally as a source of food and medicine. It produces huge quantities of leaves and composting their wastes can be an excellent way to create nutrient-rich organic matter for soil improvement and sustainable agriculture. Many researchers documented the preparation of compost and organic fertilizer from Moringa leaves which can be used as organic fertilizer and this compost has gained popularity due to its high nutrient content like nitrogen, phosphorus, potassium, and other essential nutrients which improve soil fertility and increase crop yield.5 Kartika6 reported that Brassica rapa growth and development were increased by Moringa compost which was rich in protein, vitamins A, B, C, D, E, and K, folic acid, biotin, and cytokinin in addition to Ca+2, Fe+3, K+1, Zn+2 ions.
Primarily, bacteria are the smallest and the most numerous living organisms in the compost, which account for 80-90 % of cells found in a gram of compost. Bacteria are responsible for compost degradation and heat generation by a variety of enzymes that chemically degrade a wide range e of organic materials. In the beginning mesophilic bacteria (0-40°C) predominate but when the compost temperature rises above 40°C, thermophilic bacteria like Bacillus dominate at this phase, followed by mesophilic bacteria and actinomycetes at the end of the process when the temperature decreased. Also, Actinomycetes, contribute significantly to composting by degrading complex organics such as cellulose, lignin, chitin, and proteins. Because of their enzymes, they can chemically decompose resistant debris. Some Actinomycete species emerge during the thermophilic phase, whereas others emerge during the cooler curing phase.7
The microaerophilic Gram-negative plant growth-promoting A. chroococcum is a mesophile, dark-brown water-soluble pigment producer (melanin) and fixes nitrogen under aerobic conditions in the presence of P-3, K+, S-2, Mg+2, and Ca+2.8,9 Azotobacter is commonly found in soil and is used as a bio-fertilizer to enhance plant growth and yield due to making nitrogen available to plants. The application of Azotobacter with Moringa compost has been shown to increase the nitrogen content of the soil and improve crop yield.10
Streptomyces is a genus of soil bacteria that are known for their ability to produce a wide range of bioactive compounds and lytic enzymes. The use of Streptomyces as a bio-fertilizer has been shown to enhance plant growth and yield. Applying Streptomyces with Moringa compost has been shown to improve soil fertility and increase crop yield.11
Using Moringa compost with Azotobacter or Streptomyces to enhance plant growth and yield is a sustainable and eco-friendly approach to improving soil fertility5 due to their ability to fix atmospheric nitrogen and produce bioactive compounds. Sagar et al.12 reported that Azotobacter and Moringa leaf extract significantly improved the growth and yield of tomato plants. The treated plants had higher plant height, number of branches, number of fruits, and fruit yield compared to the control group. Similarly, Kumar et al.11 detected an increase in the growth and yield of wheat plants after the application of Streptomyces and Moringa compost. The increase was clear in plant height, number of grains/spike, and grain yield compared to the control group. The use of this combination enhances plant growth and yield due to the potential of these microorganisms on soil fertility.
Phaseolus vulgaris is one of the most widely cultivated and economically important legume crops, that face drought, salinity, and nutrient deficiencies that adversely affect their growth and production. To address these challenges and promote sustainable agriculture; this study aimed to explore a promising innovative strategy that detects the effect of the combined use of plant-based compost and the inoculation of rhizosphere soil with the heterotrophic diazotroph Azotobacter alone or in combination with Streptomyces under greenhouse conditions.
Moringa peregrine compost preparation
The abundant and nutrient-rich Moringa healthy and fresh leaves were collected, dried under sunlight for 5 days, and powdered because smaller particles decompose faster which increases the composting efficiency. The powder was mixed with soil (3:1 w/w) to facilitate the decomposition process and the pile was watered and mixed well every two days to maintain moisture contents around 55% to maintain microbial activities.13 Regularly, turn the compost pile to aerate it, providing oxygen to the microorganisms responsible for decomposition and speeding up the composting process.13,14 The compost pile is left to mature for 3 months, depending on environmental conditions and the size of the pile, and the produced heat during this process kills the pathogens and weed seeds. Finally, the organic matter breaks down into rich, dark, and crumbly compost.
The effect of compost extract and the bacterial filtrates on seed germination
Phaseolus vulgaris (common bean) seeds were obtained from Al-Tawfeeq Agricultural Supplies, Jeddah, and surface sterilized using 10% NaOCl (3 min), washed with sterile distilled water, and air dried. Compost extract was prepared by shaking 50 g of compost in 50 ml sterile water and the mixture was filtered through filter paper. Surface sterile seeds were soaked in sterile compost extract, filter sterilized (Millipore filter, 0.45 mm) culture filtrate of Azotobacter, Streptomyces, their mixture (1:1, V/V) or distilled water for 24 hrs and 5 seeds were transferred to glass Petri dishes plates with sterile filter paper at the bottom, filled with water and all plants were incubated in the dark for 6 days until complete germination.15 Three replications of each treatment were carried out and germination percentage (%) and index were calculated.16
Germination Index= Sum of germinated seed for a certain period / Total days × Total seeds
Seedlings were transplanted in filled sterile pots in the outdoor greenhouse under optimum conditions of temperature, humidity, light, and day/night rhythm from April to May 2022.
Determination of moisture content, pH and EC, and the organic matter of compost
Compost sample (100 g) were oven-dried for 24 hrs at 105°C, weighted and moisture content was calculated by the variance in weight. Moreover, the pH, EC, and organic matter were measured according to Motsara and Roy.17 After acid digestion, nitrogen content (N), organic matter, P, K, and Fe ions was detected as described in Allen et al.18 and the C/N ratio in compost samples was estimated.19
Microbial analyses of compost and soil
Bacteria, actinomycetes, and fungi had a role in compost preparation and their types and counts changed every time. Compost or soil samples were collected, and serially diluted, and microbial counts were estimated on media of Nutrient agar and nitrogen-free agar for bacteria, Starch nitrate agar for actinomycetes, and PDA for fungi using plate count agar.20 All plates were incubated at 25°C and 45°C for 1-2 days for bacteria and 25°C for actinomycetes and fungi. Also, total counts of phosphate-solubilizing bacteria were detected using Pikovskaya’s medium. Compost suspension was spread on the previous agar medium and the plates were incubated at 30°C for 2 days. The colonies with clear zones around bacterial growth were counted and recorded as positive results for phosphate solubilization.21
Bacterial identification
The most dominant free-living bacterial isolate MB5 which grows well in nitrogen-free medium and actinomycete isolate MB11 which showed leathery and powdery colonies on Starch nitrate agar were selected, characterized, and identified using morphological and physiological methods. The selected isolates were gram staining and examined under light microscopes and biochemically characterized by starch hydrolysis, oxidase test, carbohydrate fermentation, and color of diffusible pigment22,23 in addition to molecular methods using partial sequencing of 16S rDNA. The universal eubacterial primers 16S rDNA 27F (AGAGTTTGATCMTGGCTCAG) and 1492R (TACGGYTACCTTGTTACGACTT) were used.24 The PCR reactions were performed and the amplification process was confirmed by ethidium bromide fluorescence in 1% agarose gel.
Plant-promoting bacterial growth and activities
The growth of the two isolated bacteria was measured by determining the optical density at 500 nm. The bacterial cells were collected by centrifugation at 5000 rpm for 10 min and cells were prepared in a sterile saline solution to inoculate soil while filtrate was filtered sterilized and used for seed soaking experiment and determining the percentage of seed germination. The two selected bacterial isolates were screened in vitro for their phosphate solubilizing activity using Pikovskaya’s medium,25 indole Acetic Acid (IAA) was detected after growth in a medium containing 2 g/L tryptophan as described by Ndeddy Aka and Babalola26 while HCN production was detected as described by Bakker and Schippers.27
Soil preparation and plant experiment
Air-dried sand soil (sieved acid-washed sand) was sterilized 2 times in two successive days, air dried, and mixed well with 20% Moringa compost. Sterile pots were filled with either 2 kg of sterile sand soil only (G1) or filled with 2 Kg of the previous soil mixture (G2-G5) and were inoculated with AZ (G3), ST (G4), and AZ+ST (G5). Each group contained 10 replicates and five seedlings per pot which were grown at 25°C and soil water potential stayed near field capacity.
Inoculation of soil by bacteria and plant growth studies
Azotobacter cells were grown on nitrogen-free agar (Agar 1.5%, Sucrose 0.5%, CaCO3 0.5%, MgSO4 0.02%, NaCl 0.02%, KH2PO4 0.02%, FeSO4 0.0005%) and isolate MB11 was grown on starch nitrate agar for 4 days at 100 rpm and 25°C. and cells were collected, re-suspended in saline solution (A500nm =0.5) and 2 ml of the suspension (2×106 CFU/ml) was used to inoculate nitrogen-free broth or starch nitrate broth in 250 ml Erlenmeyer flasks containing 50 ml of the medium. After 4 days of inoculation, cells were collected, and, re-suspended in saline solution (A500nm 0.45-0.59) and used to inoculate soil with Azotobacter (AZ) or Streptomyces (ST). About 20 ml of the bacterial suspension (2×106 CFU/ml) was used to inoculate each pot with 2 kg of soil. The filtrate was used for soaking the P. vulgaris (common bean) seeds.
Greenhouse experiment was carried out 5 seedlings were transferred to each plastic pot (25×25 cm2), filled with 2 kg of steam-sterilized sand soil mixed with 20% Moringa compost. The pots were divided into 5 groups (G), G1: control plants (normal soil without compost), G2: plants grown in normal soil, G3: the plants grown in soil and inoculated with Azotobacter (20 ml of cell suspension of 8×105 CFU/ml), G4: plants grown in soil with compost and inoculated with Streptomyces (20 ml of cell suspension of 8×105 CFU/ml), and G5: plants grown in soil with compost and inoculated with both bacteria (40 ml of a mixture of cell suspensions of Azotobacter and Streptomyces, 2×106 CFU/ml, V/V). After a week, irrigation was applied with 200 ml two times/week of half strength of Hoagland nutrient solution, composed of these materials in mM: KH2PO4, 1.0; KNO3, 5; Ca(NO3)2, 5, (NH4)Mo7O24, 0.0002, MgSO4, 2, Fe/ EDTA, 0.1, H3BO3, 0.005, MnCl2, 0.010, ZnSO4, 0.008, CuSO4,0.004.28
Plant growth and chemical analysis
After one month, plant growth was measured by detected root depth, shoot length, shoot and root fresh, and dry weights,29 dry weight was obtained after oven drying by at 70°C for 2 days. In addition, the fresh leaf content of chlorophylls and carotenoids were measured using UV-VIS Spectroscopy after extraction with 95% ethyl alcohol.30 Chlorophyll’s concentrations (μg/ml) were calculated as follows:
Chlorophyll a (Chla)= (13.36 A664) – (5.19 A649).
Chlorophyll b (Chlb)= (27.43 A649) – (8.12 A664),
Carotenoid (Car) = 1000 A470 – (2.13 Chla – 97.63 Chlb) /209.
Moreover, plant shoots of each treatment were collected, dried, and analyzed for protein, and sugar according to protocols methods described in Allen et al.18 The soluble carbohydrate of the shoot was estimated by the Anthrone method while the total content of soluble proteins was estimated according to the Lowry method.31 After acid digestion, shoot or phosphorus and nitrogen were detected using the methods of Wilde et al.32 while mineral contents of K+, and Ca++ were determined using Shimadzu Atomic Absorption Spectrophotometer (AA -7800 Series).
Statistical analysis
Data of all parameters were collected and analyzed by using the statistical software SPSS. The data were subjected to a one-way analysis of variance (ANOVA) to evaluate the difference in mean values (P < 0.05) of treatments.
Composting refers to the dynamic process of converting macromolecular organic substances in organic solid waste into carbon dioxide, water, NH3, and humus substances through microbial metabolism in a high-temperature.33 One of the main challenges for crop production in sandy soils is the limitations of available nutrients. These soils suffer from continuous and significant losses in nutrients, and this might negatively affect plant growth. To improve the physical and chemical characteristics of these soils, organic compost is recommended.34
Moringa leaves were collected mixed with soil and fermented for about 3 months with watering and shaking until a black color and good smell were obtained. Compost’s chemical and microbial properties, including pH, electrical conductivity (EC), temperature, moisture, organic matter, and mineral content were summarized in Table 1. The color was brown and the compost temperature was 30-35°C, pH 7.5, EC was 4.16 dS m-1 with 45% moisture content while mineral contents were summarized in Table 1. Compost contained the highest count of true bacteria (2.201×107 CFU/g) followed by thermophilic bacteria (0.113 x107 CFU/g), then actinomycetes (0.009 x107 CFU/g) and fungi (0.006 x107 spore/g). The lowest counts were recorded for P-solubilizing bacteria (2.111×104 CFU/g) and Free-living N-fixing bacteria (0.003 x104 CFU/g).
Table (1):
Compost chemical and microbial properties, including pH, electrical conductivity (EC), temperature, moisture, organic matter, mineral content and microbial counts
Parameter |
Results |
Parameter |
Results |
Microbe |
Counts (CFU or spore/g) |
|---|---|---|---|---|---|
Color |
Brown |
Total N % |
2.760 ± 0.80 |
Total bacterial counts |
2.201 x 107 |
EC (dS m-1) |
4.163 ± 0.34 |
Total P % |
0.467.30 ± 0.06 |
Thermophilic bacterial counts |
0.113 x 107 |
Temperature °C |
30-35 |
Total Ca % |
0.379 ± 0.51 |
Actinomycetes |
0.009 x 107 |
pH |
7.5 ± 0.14 |
Total Fe % |
0.121 ± 1.09 |
Fungi |
0.006 x 107 |
Moisture % |
45.1 |
Total K % |
0.271± 1.23 |
P solubilizing bacteria |
1.111 x 104 |
Organic matter % |
27.661 ± 1.5 |
C/N |
17.961 ± 0.30 |
Free-living N fixing bacteria |
0.003 x 104 |
Data are means of three replicates ± standard deviation.
The pH level is an essential parameter during composting.35 Microbial activities during the composting process are influenced by time. Typically, compost has a pH ranging from 6 to 9.5.36 The EC of compost is a crucial salinity indicator and indicates its suitability for use. EC typically rises during composting as a result of organic matter degradation, which produces inorganic compounds. The EC values of the mature composts fell within the previously documented range of 2.8-10.1 dS m-1 for composts derived from solid fraction wastes. One of the most important elements for monitoring the composting process is temperature. Composting is an exothermic process that is affected by the starting substrate, temperature and biodegradability of the microbe present.37 Under suitable moisture content, Moringa leaves give a good bioavailability to composting by soil microorganisms with high metabolic activities.35 It is reported that under high moisture content, bacteria and fungi convert wastes into humus.13 The ideal moisture content was 45%-60% is the most suitable and 53% gives the maximum results and the longest duration of the thermophilic period (15 days) which enhances nitrogen content.38 Moringa wastes were degraded by microbial activity to compost with a high decomposition rate which was noticed by the reduction in the pile volume and change in color. The detected organic matter was 27%, while the high-quality compost is in the range of 25-80%.39 Aeration and the produced high temperature are required to enhance compost formation.40 Carbon, nitrogen, and carbon/nitrogen ratio were important measured for the compost and the C/N ratio in compost may vary depending on the bioavailability of carbon and nitrogen.41 The high carbon content may be due to the high presence of lignin, cellulose, and hemicelluloses complex structures which are difficult to degrade.42 Moreover, the detected total nitrogen content (2.7%) is lower than that estimated which was 3.2%.43 It was documented that compost with a C/N ratio of 15–20 is satisfactory as a good nitrogen source while low C/N ratio are due to carbon release as CO2.44 Microorganisms need a C/N ratio of approximately 10, which is optimal for their metabolism. Microorganisms require carbon, nitrogen, phosphorus, phosphate, and potassium as primary nutrients for optimal microbial activities. Moringa wastes stayed only for three months to be composted but in contrast to our study, it may stay for a year or even more without complete decay and extending composting time is necessary to produce compost with high nitrogen content.
Compost is a rich source of P and other nutrients e.g., Ca, Fe, and N (Table 1) which were essential elements for numerous physiological functions of the plants.45 In this work, the most abundant bacteria that were noticed during compost preparation were selected and counted using different agar media. The counts of total mesophilic and thermophilic bacteria, Nitrogen fixing and phosphate solubilizing bacteria in addition to actinomycetes and fungi were recorded in the compost. Most microbes maintained their growth ability when the temperature was 25°C. Total counts of mesophilic ≥ phosphate solubilizing ≥ thermophilic bacteria ≥ actinomycetes ≥ fungi ≥Nitrogen-fixing bacteria. Various bacteria like the genera of Thermus, Bacillus, and Streptomyces were detected.46 The type of raw materials in the compost leads to different microbiota compositions during composting. Simultaneously, bacteria might have different responses to nutrients in the raw material, and the bacteria that can degrade these materials rapidly increase in abundance and counts.42
Table (2):
The tested morphological, physiological and biochemical characters of the isolate MB5 and MB11
Tested character |
Isolate MB5 |
Isolate MB11 |
Tested character |
Isolate MB5 |
Isolate MB11 |
|---|---|---|---|---|---|
Shape |
Cocci |
filamentous |
Temperature (°C) |
25-32 |
ND |
Gram stain |
Negative |
Positive |
Growth in nitrogen-free medium |
+ |
– |
Color |
Black |
Dark Gray |
pH range |
5-8 |
5-11 |
Soluble pigment |
Found |
Found |
Cyst formation |
Found |
Not Found |
Mycelia and spore chain |
ND |
Found |
Phosphate solubilization |
+ |
+ |
HCN production |
+ |
+ |
ACC |
+ |
+ |
H2S production |
+ |
+ |
Siderophore production |
+ |
+ |
IAA production |
+ |
+ |
Nitrate reduction |
+ |
+ |
Utilization of phenylalanine |
+ |
+ |
Gelatinase, catalase and oxidase |
+ |
+ |
Decomposition of xanthine, casein, chitin, pectin, urea |
– |
+ |
Resistance to
Kanamycin, Rifampin and Tetracycline |
+ |
+ |
Tolerance to NaCl (10%) |
+ |
+ |
Tolerance to NaCl (15%) |
– |
+ |
+: positive results, -: negative results
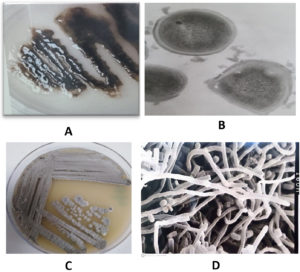
The most abundant isolates MB5 and MB11 were selected and identified using morphological and physiological methods (Table 2). The isolate MB5 belongs to free-living bacteria that grow well in the nitrogen-free medium as gelatinous colonies with dark black color and the cells were cocci, motile Gram-negative, cyst forming (Figure 1A and B), oxidase, and catalase positive. They grew well at 25-32°C and generated indole, citrate, catalase, and oxidases according to,47 and using several approaches, it was recognized as a species belonging to the genus Azotobacter.40,48 Using the previous morphological and biochemical characters in addition to molecular methods using partial sequencing of 16S rDNA, the isolate MB5 was identified as A. chroococcum MB5 (Figure 2). The cells are abundant in soil, increasing soil nitrogen concentration and agricultural sustainability.
Figure 1. The isolate MB5 on nitrogen-free medium after 5 days of growth at 25°C (A), Under transmission electron microscope (B), isolate MB11 on starch nitrate agar after 7 days of growth at 25°C (C), under scanning electron microscope (D)
The most dominant actinomycete isolate MB11 was obtained from compost and showed leathery and powdery grey color colonies on Starch nitrate agar. After examination under light and electron microscopes, the cells were Gram-positive with well-developed filamentous aerial and substrate mycelia carrying straight long spore chains with smooth surfaces and colonies produced pale yellow soluble pigments on agar medium (Figure 1C and 1D). Cells were gram-positive and molecular analysis showed that these isolates belong to genus Streptomyces and identified as S. griseus MB11 with a 94% similarity level (Figure 3). Similar results was recorded by the previous species.49
In this work, IAA was found in the culture filtrate of A. chroococcum MB5 and S. griseus MB11. It was confirmed that bacteria and actinomycetes produce IAA in growth media.50,51 Streptomyces rochei, S. livaceoviridis, and S. rimosus were a producer of IAA, which increase plant growth, and other bacteria were found as efficient producers of IAA, siderophore, and phosphate-dissolving organisms.52-54 Soil bacteria synthesize phytohormones that promote plant growth and alter the morphology and structure of roots.55 These bacteria are considered eco-friendly biofertilizers since they are inexpensive and provide a sustainable source of nutrients to plants, reducing reliance on chemical fertilizers. They also play an important role in boosting nutrient availability, which promotes plant growth.56 In this investigation, the filtrates of A. chroococcum MB5 or S. griseus MB11 or their mixture increased seed germination, which could be related to the presence of IAA and other secondary metabolites.
Soil pH, EC, and microbial counts of the detected bacteria and fungi in inoculated and inoculated soil after one month of plant growth were summarized in Table 3. After plant growth, soil pH was not affected by the inoculated microorganism except, in the case of using cells of AZ+ST, a significant increase was noticed (Table 3). Also, the addition of compost to the soil increases soil pH from 6.5 to 6.9. Moreover, soil EC was increased with soil inoculation compared to control (soil with 20% Moringa compost only). Total bacterial counts at 25°C were increased while the counts at 45°C were under detection limits but the counts of AZ or ST were increased in their inoculated soil. In contrast, there is non-significant increase in fungi counts compared to control.
Table (3):
Soil pH, EC and microbial counts of the detected bacteria and fungi in inoculated and inoculated soil after 2 months of plant growth
| Treatment | Soil pH | Soil EC | Counts in soil after plant growth x 104 CFU/g | ||||
|---|---|---|---|---|---|---|---|
| Bacterial Counts | Nitrogen fixing | Actino-mycetes | Fungi | ||||
| 25⁰C | 45⁰C | ||||||
| Soil alone# | 6.5 ± 0.45* | 0.16 ± 0.01* | ND | ND | ND | ND | ND |
| Soil+MC (control) | 6.9 ± 0.24 | 0.29 ± 0.05 | 81 | 0.009 | 0.005 | 0.23 | 0.11 |
| Soil+MC+AZ | 6.9 ± 0.56 | 0.33 ± 0.06 | 198* | ND | 0.196* | 0.28 | 0.17 |
| Soil+MC+ST | 6.9 ± 0.33 | 0.63 ±0.06* | 106* | ND | 0.003 | 0.88* | 0.14 |
| Soil+MC+AZ+ST | 7.0 ± 0.19* | 0.61 ± 0.11* | 191* | ND | 0.194* | 0.97* | 0.19 |
ND: under detection limits, *: significant differences at p > 0.05, #: acid washed sterile washed soil, MC: Moringa compost, AZ: Azotobacter, ST: Streptomyces.
Plant growth enhancement by soil bacteria and compost
The percentage of seed germination and Germination Index were increased by soaking in MC or AZ or ST extracts and the significant increase was clear in the case of using MC+AZ or MC+AZ+ST extracts (Table 4). The effect of Zn and Mn on seed quality and germination in common beans (P. vulgaris L.) was studied.57 The bacterial biofertilizer Azotobacter, and of gibberellic acid affect the germination of guava seeds and the single effect of each agent was superior in all studied characteristics and the control treatment recorded the lowest rate.58
Table (4):
Effect of Moringa compost and inoculation with bioagents on Fresh and dry weights, Leaf number, plant height, and root length of Phaseolus vulgaris grown under greenhouse conditions for one month
Property |
Soil |
Soil+MC (Control) |
MC+AZ |
MC+ST |
MC+AZ+ST |
|---|---|---|---|---|---|
% of seed germination |
66 |
69 |
79* |
74 |
79.3* |
Germination Index |
0.12 |
0.13 |
0.15 |
0.14 |
0.15 |
Leaf number |
7.9* |
8.9 ± 1.22a |
8.3 ± 1.58a* |
8.3 ± 1.50a* |
10.3 ± 2.11b* |
Fresh weight (g) |
9.0* |
10.1 ± 0.90 a |
12.12 ± 0.85a* |
16.62 ± 0.93b* |
19.10 ± 0.92c* |
Dry weight(g) |
2.3 |
2.9 ± 0.18 a |
2.99 ± 1.18 a |
3.40 ± 0.06 b |
4.9 ± 0.15c |
Plant height (cm) |
23.8 |
31.0 ± 4.4a |
33.30 ± 2.50c |
37.00 ± 0.99b |
39.11 ± 0.50d |
Root depth (cm) |
14.0 |
16.33 ± 1. 7d |
21.5 ± 2.01b |
28.66 ± 1.07a |
18.33 ± 1.97c |
Data are means values of 10 replicates ± standard deviations. *: statistically significant results compared to control at p < 0.05. The results with different letters in the same row were statistically significant (p < 0.05), MC: moringa compost, AZ: Azotobacter, ST: Streptomyces.
The bacterial inoculum can be applied to the soil containing organic fertilizers, such as compost or manure, or by spraying it onto the soil surface during the seedling stage and the efficacy of Azotobacter or Streptomyces as a biofertilizer depends on several factors, including the soil type, crop species, and environmental conditions.12,59,60 In this experiment, soil containing compost was inoculated with the bacterial inoculum before seedlings were transferred to the pots. After two months of growth, inoculation of soil with AZ, ST and a mixture of AZ+ST had a beneficial effect on plant growth and chemical analysis. Microbial inoculation or the presence of compost enhances the availability of nitrogen and other nutrients to plants, promotes nutrient cycling, suppresses plant pathogens, and enhances plant growth-promoting activities.
In the present study, plant height, root depth, and leaf number, as well as fresh and dry weight, were significantly affected after applying compost treatments or bioagents to the soil (Table 4). It was clearly shown that P. vulgaris shoot height, root depth, fresh weight, and dry weight were significantly increased in all treatments compared to the control (compost only). Moringa compost increased all tested plant parameters compared to plants grown in soil without compost (Figure 4). In agreement with the present study, previous reported that compost addition to sandy soil led to higher plants,61 while the application of different levels of termite mound compost material to sandy soil significantly improved plant height and leaf number.62 Adding compost and dry Azolla to the soil improved both the quality and growth of squash plants.63 Thus, enhancing soil quality facilitates nutrient absorption by the plant and promotes optimal root development and growth, increasing the total number of roots.34,64 The increase may be related to N fixation and production of IAA.
Figure 4. The growth of plants for 30 days after seedling in soil with 20% Moringa compost and inoculated with both Azotobacter (AZ) and Streptomyces (AZ) or uninoculated and compared to control plants grown in soil only
Many researchers demonstrated that the enhanced mineralization of nutrients and decomposition of organic matter by bacteria could be ascribed to improved plant growth and productivity.65,66 Furthermore, it is hypothesized that the compost with growth-promoting bacteria exerts a direct influence on plants through hormonal mechanisms (cytokinins and auxin-like activity) and an indirect influence via metabolic soil microorganisms in response to changes in soil nutrient uptake and physical properties.67
Chlorophyll and carotenoids are pigments widely distributed in the chloroplasts in plant leaves and play an important role in plant photosynthesis and there is a close correlation between the leaf chlorophyll content and nitrogen availability. As shown in Table 5, there were significant differences in chlorophyll contents of the leaves in all treatments. However, C+AZ+ST showed significant improvement in chlorophyll a and b, with 1.86±0.02 and 9.69±0.04 mg/g, respectively. Meanwhile, carotenoid content slightly increased in plants inoculated with C+AZ+ST. It was reported that chlorophyll contents were positively and significantly correlated to organic fertilizers. There is a correlation between nutrient uptake by plant tissues and leaf content of chlorophyll. Therefore, the continuous nutrient supply from the soil by compost addition or inoculation with plant growth-promoting bacteria increases the adsorption of essential elements needed for effective plant photosynthesis.
Table (5):
Chlorophyll a, b, and carotenoid and macro-nutrient and mineral contents of common bean grown in the presence of Moringa compost and bioagents
| treatments | Chlorophyll (mg/g FW) | Essential compounds (mg/g) | Mineral (mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chl a | Chl b | Car | Carbo-hydrates | Protein | P | N | Ca | K | |
| Soil alone | 0.66 ± 0.01b | 4.56 ± 0.17 b | 2.36 ± 0.05 a | 0.97 ±0.07c | 1.55±0.02a | 5.26b | 5.13d | 15.99a | |
| Soil+MC | 0.89 ± 0.01b | 6.27 ± 0.17 a | 2.31 ± 0.05a | 1.36 ± 0.03b | 2.44 ± 0.006b | 5.92a | 6.29d | 6.24a | 25.23d |
| Soil+MC+AZ | 0.89 ± 0.04b | 8.69 ± 0.04 c | 2.99 ± 0.08 b | 1.65 ±0.02a | 3.89 ± 0.07c | 7.3c | 8.57a | 8.23b | 26.44d |
| Soil+MC+ST | 1.08 ± 0.01c | 8.45 ± 0.07 c | 2.23 ± 0.03a | 1.79±0.02a | 4.38 ± 0.004d | 785b | 6.51d | 8.00b | 25.23d |
| Soil+MC+AZ+ST | 1.86 ± 0.02a | 9.69 ± 0.04 c | 2. 99 ± 0.10 b | 1.91 0.02a | 5,.99 ± 0.02d | 8.05d | 8.16a | 8.11b | 26.23d |
The results with different letters in the same column were statistically significant (p < 0.05), MC: Moringa compost, AZ: Azotobacter, ST: Streptomyces
Following inoculation with AZ, ST, or a combination of the two, soil microbiota boosted root and shoot growth, mineral, and protein content which may be due to nitrogen fixation, auxins, and the synthesis of unidentified chemicals. Similarly, plants inoculated with A. chroococcum, Azospirillum brasilense, and S. mutabilis showed a significant increase in growth, mineral contents like P, Mg, and N, and total soluble sugars due to IAA release and/or nitrogen fixation by soil microbiota.68,69
Due to the presence of nutrient elements, particularly potassium, which aids in photosynthesis, carbohydrate transport, regulation of stomatal opening, and respiration, the physiological properties of plants treated with final compost are enhanced.70 These results are consistent with prior research that demonstrated the effectiveness of chlorophyll as well as confirmed the final compost’s maturity and high quality.71 Furthermore, compost increased the levels of chlorophyll and carotenoids in comparison to control, as demonstrated.72
In soil, plant residues or compost are changed to value-added products by microorganisms and this increases plant growth and nutrient contents, namely protein, carbohydrates, and lipids. In addition, compost is a good bio-fertilizer that contains nutrients that have the potential to be utilized in agriculture.73 However, during composting, microorganisms degraded the nutrients to simple forms at the same time, N contents were increased in plants grown in inoculated soil with AZ compared to Control, ST, and AZ+ST, respectively. In this study, addition of compost to soil increased plant protein and carbohydrate and P, N, Ca, and K contents (Table 4) compared to control (soil only) and inoculation of soil with AZ, ST, or AZ+ST improved all the previous parameters compared to plants grown in soil with compost only, therefore, addition of Moringa compost and inoculation with benefit microorganisms can be utilized to enhance plant growth and productivity and may lead to distinct functional evolutions of microorganisms. In the present study, compost slightly increased the protein and carbohydrate content of the plant compared to control. This result is in close agreement with other results74 that reported carbohydrates and protein mainly were increased due to increased microbial activity during composting and plant growth. The inoculum of three strains of nitrogen-fixing bacteria, two concentrations of Moringa leaf extract, and their combinations significantly increased fennel plant growth, yield, and oil components in addition to plant height, branch number per plant, herb fresh weight, fruit weight, umbel number per plant, and fruit yield. Photosynthetic pigments, total phenols, and oil components were also improved.75 It was reported that bacteria might have been capable of absorbing nutrients to endure the composting process. Nitrogen-fixing bacteria Azotobacter and plant growth-promoting Streptomyces have many biological activities like nitrogen fixation and production of IAA, phosphate solubilization, and other eco-friendly activities that increase plant growth. When N was easily accessible, plants would predominantly produce compounds with high N content (e.g., proteins for growth). When N availability was scarce, metabolism shifted toward carbon-containing compounds like starch and cellulose, phenolics, and terpenoids. A balanced proportion of nitrogen in soil enhances protein production, hence increasing microbial activity resulting in a faster degradation rate of unsoluble compounds and good compost which increases plant growth. Moreover, K+ uptake is extremely selective and is intimately linked to plant metabolic activity. Potassium acts as an activator or cofactor for numerous enzymes involved in metabolizing carbohydrates and proteins. Meanwhile, potassium ions are necessary for the activity of over fifty enzymes and plants prefer it in soluble form.75 Soil N content affects K+ uptake consequently influencing the levels of carbohydrates and proteins. The nitrogen concentration in the compost was relatively low, thus, the presence of bioagents increased the nitrogen content of the soil, which impacted the carbohydrate and protein content of the plant.
The nutrient content in the soil significantly increased after adding compost which is rich in nutrients, P, N, K, and Ca contents. For plant development, phosphorus is always an essential nutrient and microbial P solubilization seems to be an effective process to release the precipitated P in soil. In the present work, the two selected isolates belong to P-solubilizing bacteria which has beneficial effects on plant yields. Similarly, P solubilizers Pseudomonas, Bacillus, and Paenibacillus were isolated from Jujube rhizosphere.9
In the results of this work, the total P content was shown to be higher in all treatments compared to compost as the control. The gradual increase in P content during the composting process occurred due to the increase in the P water solubility by bacteria during the decomposition of plant wastes. The Ca, P, K and N content in plant shoots increased in microbial-treated plants compared to the control. Organic fertilizers improve soil’s organic matter content and nutrient availability which balance plant nutrition and improve soil structure, organic matter, and microbial activity.
The importance of bioagents formula with compost of Moringa to enhance plant growth was noticed (Figure 4), whereas the free-living A. chroococcum and Streptomyces were used as bioagents, which are known to have many biological activities and the presence of plant-based compost like Moringa compost plays a vital role in improving soil structure and fertility and enhance plant growth. The results also emphasize the importance of sustainable agriculture and the use of eco-friendly dry Azolla increased the nutrient efficiency in soil compared to other fertilization methods.63 Microorganisms from the genera Streptomyces, Bacillus, Trichoderma, Pseudomonas, and nitrogen-fixing bacteria in addition to their enzymes have a role in biological process and plant infection resistance.76 The use of compost and some plant growth-promoting (PGR) bacteria for enhancing plant growth is more practical, easy to handle, less expensive, and low-cost due to their presence in the soil as agricultural wastes or soil microflora.77 The use of Moringa compost increases plant growth helps in recycling organic matter, contributes to sustainable agriculture, and enhances soil fertility.78 The incorporation of compost into the soil enhances water retention, aeration, and nutrient availability, thereby mitigating the adverse effects of stress conditions and promoting beneficial microbial activity, which aids in nutrient cycling and the suppression of soil-borne pathogens.79 Nitrogen-fixing Azotobacter introduces an additional source of nitrogen, reducing the plants’ reliance on external nitrogen inputs and potentially mitigating nutrient deficiencies,59 thus enhancing plant growth and development, especially when nitrogen availability is limited. There are synergistic effects of compost and A. vinelandii inoculation when used in combination and increase tomato growth due to many benefits, the first through enhanced stress tolerance to drought and salinity, improved soil structure, and increased nutrient availability resulting from compost application and the additional nitrogen supply from A. vinelandii.80 There is increased nutrient uptake and utilization by plants due to compost solubilization and nutrient release by the action of the soil microbes81 and there is disease suppression by promoting the healthy soil microbiome, which can help suppress soil-borne pathogens.80 Plant-based compost reduces environmental impacts and makes it a highly desirable option for sustainable and productive plants. With the evidence from various scientific studies supporting its effectiveness, the use of plant-based compost should be encouraged as a natural and eco-friendly approach to maximizing plant yield, improved soil structure also reduces the risk of soil erosion and compaction, which are critical factors for successful plant growth.81,82 Compost is a valuable source of essential nutrients for plant growth and contained a variety of macronutrients (nitrogen, phosphorus, and potassium) and micronutrients (such as calcium, magnesium, and iron) that are slowly released into the soil as the compost decomposes.83 This gradual nutrient release provides a consistent supply of essential elements to plants, promoting steady growth and healthy fruit production.
The Saudi Arabia Standard Organization has released guidelines for organic fertilizers and compost to promote sustainability, environmental protection, and public health. According to the guidelines, compost should be produced from organic materials that are free from harmful substances, weed seeds, and contaminants. Additionally, the compost must have a balanced nutrient composition of nitrogen, phosphorus, and potassium to qualify as an effective fertilizer.84,85 A quality control system should also be in place to ensure that the compost meets the required standards. Common metals that are tested for in compost include P, N, Ca, and P. A compost sample analysis results were compared to regulatory limits or guidelines to determine if the compost is safe for use in agriculture or other applications. Furthermore, Actinobacteria has shown a biosynthetic potential to produce large amounts of bioactive secondary metabolites with novel structures and remarkable biological activity in agriculture.86
Plant-based composts also contribute to developing a diverse and thriving soil microbial community. A healthy soil microbiome is particularly crucial for plant growth. Research has shown that the incorporation of composts and bioagents in the soil can lead to a reduction in the population of harmful pathogens, creating a more disease-resistant environment for plant growth.87 Utilizing compost for plants reduces the environmental footprint of agriculture and supports eco-friendly farming which aligns with sustainable agricultural practices, reduces the need for chemical fertilizers, helps divert organic waste from landfills, and reduces greenhouse gas emissions, which can have detrimental effects on the environment.88
The characterization and identification of two bacterial isolates, A. chroococcum MB5 and S. griseus MB11, and studying their ability to promote plant growth through the production of phytohormones, and nutrient solubilization is one of agriculture’s promising and sustainable approaches. Both isolates were found to produce indole acetic acid, which facilitates plant growth because they are considered eco-friendly biofertilizers that enhance nutrient availability and reduce dependence on chemical fertilizers. Inoculum of beneficial microorganisms with eco-friendly organic bio-fertilizer Moringa compost is an effective way to enhance plant growth, improve soil fertility, increase crop yield, and reduce the dependence on chemical fertilizers. This strategy improves soil structure, enriches the soil with essential nutrients, and promotes the growth of beneficial microorganisms. Moreover, the nitrogen-fixing ability of bacteria offers a sustainable and eco-friendly solution for addressing nutrient deficiencies, particularly in environments where stress factors are prevalent. Further research and field trials are necessary to optimize the application of this approach for various stress conditions, ensuring the continued advancement of sustainable plant cultivation.
ACKNOWLEDGMENTS
None.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia, on 20/May/2022.
- Dong OX, Ronald PC. Genetic Engineering for Disease Resistance in Plants: Recent Progress and Future Perspectives. Plant Physiol. 2019;180(1):26-38.
Crossref - Lucas JA. Plant pathology and plant pathogens, 4th Ed. ISBN: 978-1-118-89385-2, April 2020, Wiley-Blackwell, 432.
- Akrami M, Golzary H, Ahmadzadeh M. Evaluation of different combinations of Trichoderma species for controlling Fusarium rot of lentil. African J Biotech. 2011;10(14): 2653-2658.
Crossref - Brimner TA, Boland GJ. A review of the non-target effect of fungi used to biologically control plant diseases. Agriculture, Ecosystem and Environment. 2003;100(1):3- 16.
Crossref - Bari MUH, Alam MMS, Khan MS, Sundas F, Razaullah, S. Effect of Moringa Leaf Extract on Growth and Yield of Tomato. International Journal of Sustainability in Research, 2024;2(1): 37–58.
Crossref - Sari PN, Auliya M, Farihah U, Nasution NE. The effect of applying fertilizer of moringa leaf (Moringa oliefera) extract and rice washing water to the growth of pakcoy plant (Brassica rapa L. spp. Chinensis (L.). Journal of Physics: Conference Series 1563 (2020) 012021.
Crossref - Partanen P, Hultman J, Paulin L, Auvinen P, Romantschuk M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010;10:94.
Crossref - Wani SA, Chand S, Ali T. Potential Use of Azotobacter chroococcum in Crop Production: An Overview. Curr Agric Res J. 2013; 1(1): 35-38.
Crossref - Wang J, Li R, Zhang H, Wei G, Li Z. Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol. 2020;20(1):38.
Crossref - Bari MUH, Alam MMS, Khan MS, Sundas F, Razaullah S. Effect of Moringa Leaf Extract on Growth and Yield of Tomato. Inter. J. Sustain. Research, 2024;2(1):37-58.
Crossref - Emongor VE. Effects of Moringa (Moringa Oleifera) Leaf Extract on Growth, Yield and Yield Components of Snap Beans (Phaseolus Vulgaris). Current Journal of Applied Science and Technology. 2014;6(2):114-22.
Crossref - Sagar A, Sayyed RZ, Ramteke PW, et al. Synergistic Effect of Azotobacter nigricans and Nitrogen Phosphorus Potassium Fertilizer on Agronomic and Yieldtraits of Maize (Zea mays L.). Front. Plant Sci. 2022; 13:952212.
Crossref - Ofei-Quartey MN, Appiah-Effah E, Akodwaa-Boadi K, Ampaw B, Taylor TS, Millogo ZE. Enhancing the economic potential of organic waste by co-composting using ratio modelling toward a circular economy. Journal of Material Cycles and Waste Management. 2023;25(3):1560-80.
- Sarpong D, Kwarteng SO, Gyasi SF, et al. Biodegradation of heterogeneous mixture of organic fraction of municipal solid waste by black soldier fly larvae (Hermetia illucens) under the tropical climate conditions. Int J Innov Res Sci Eng Technol. 2018; 5(3):1–11. https://ijiset.com/vol5/v5s3/IJISET_V5_I03_29.pdf
- Alghamdi S. Enhancing Tomato Growth and NaCl Stress Using ACC Deaminase-Producing Streptomyces Isolate Alone or In Combination with Azotobacter vinelandii MM1. Egypt Acad J Biol Sci C. 2022;14(2):267-285.
Crossref - Mrkovacki N, Djalovic I, Jockovic D, Jarak M, Bijelic D. Efficiency of inoculation with Azotobacter chroococcum on agronomic characteristics and yield of maize and sugar beet. In Book of proceedings: Fifth International Scientific Agricultural Symposium” Agrosym. 2014:23-26.
- Motsara MR, Roy NR. Guide to Laboratory establishment for plant nutrient analysis. FAO Fertilizer and Plant Nutrient Bulletin Rome, 19th Edition. 2008:42-88.
- Allen SE, Grimshaw HM, Parkinson JA, Quarmby C. Chemical Analysis of Ecological Materials. Blackwell Scientific Publications, Oxford, London. 1974:565.
- Watteau F, Villemin G. Ultrastructural study of the biogeochemical cycle of silicon in the soil and litter of a temperate forest. Eur J Soil Sci. 2001;52(3):385-396.
Crossref - Ramesh MA. Inoculating curiosity in fungal biology for a new generation of students. Fungal Biology Reviews. 2016;30(1):15-23.
Crossref - Chen Q, Liu S. Identification and Characterization of the Phosphate-Solubilizing Bacterium Pantoea sp. S32 in Reclamation Soil in Shanxi, China. Front Microbiol. 2019;10:2171.
Crossref - Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol. 1966;16:313- 340.
Crossref - Chukwuneme CF, Babalola OO, Kutu FR, Ojuederie OB. Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J Plant Interact. 2020;15(1):93-105.
Crossref - Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory:
pluses, perils, and pitfalls. J Clin Microbiol. 2007;45(9):2761-2764.
Crossref - Qureshi MA, Ahmad ZA, Akhtar N, Iqbal A, Mujeeb F, Shaki MA. Role of phosphate solubilizing bacteria (PSB) in enhancing p availability and promoting cotton growth. J Anim Plant Sci. 2012;22(1):204-210.
- Aka RJN, Babalola OO. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int J Phytorem. 2016;18(2):200-209.
Crossref - Bakker A, Schippers B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth- stimulation. Soil Biol Biochem. 1987;19(4):451-457.
Crossref - Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular. 1950;347:1-32.
- Aly MM, El-Sabbagh S, El-Shouny W, Ebrahim MKH. Physiological response of Zea mays to NaCl stress with respect to Azotobacter chroococcum and Streptomyces niveus. Pak J Biol Sci. 2003;6(24):2073-2080.
Crossref - Lichtenthaler HK. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350-382.
Crossref - Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J biol Chem, 1951;193(1):265-275.
Crossref - Wilde SA, Voigt GK, JC Iyer. Soil and plant analysis for tree culture. Oxford and IBH Publ. Co., New Delhi, India (Distributed in North America by Dr. S. A. Wilde, Madison, Wisconsin) 209; 1972.
- Troy SM, Nolan T, Kwapinski W, Leahy JJ, Healy MG, Lawlor PG. Effect of Sawdust Addition on Composting of Separated Raw and Anaerobically Digested Pig manure. J Environ Manage. 2012;111:70-77.
Crossref - Gomah HH, Ahmed MMM, Abdalla RM, Farghly KA, Eissa MA. Utilization of some organic wastes as growing media for lettuce (Lactuca sativa L.) plants. J Plant Nutr. 2020;43(14):2092-2105.
Crossref - Wu K, Yuan S, Wang L, et al. Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol Fertil Soils, 2014;50, 961-971.
Crossref - Boen A, Hammerass B, Magnusson C, Aasen R. Fate of the potato cyst nematode Globodera rosto-chiensis during composting. Compost Science and Utilization, 2006;14:142-146(12).
Crossref - Kulikowska D. Kinetics of organic matter removal and humification progress during sewage sludge composting, Waste Management, 2016, 49,16,196-203.
Crossref - El-Tarabily KA. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase- producing streptomycete actinomycetes. Plant and Soil. 2008; 308(1):161-174.
Crossref - El-Tarabily KA, Sivasithamparamb K. Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem. 2006;38(7):1505-1520.
Crossref - Aly M, El-Sayed H, Sayed A, Jastaniah SD. Synergistic Effect between Azotobacter vinelandii and Streptomyces sp. Isolated From Saline Soil on Seed Germination and Growth of Wheat Plant. J Am Sci. 2012;8(5):667-676.
- Sumreen H, Asma A, Bilal A, et al. Actinobacteria: Potential Candidate as Plant Growth Promoters, Plant Stress Physiology, IntechOpen. 2020.
Crossref - Li M, He X, Tang J. Influence of moisture content on chicken manure stabilization during microbial agent-enhanced composting, Chemosphere. 264(2):128549
Crossref - Varma V S and Kalamdhad A S. Composting of Municipal Solid Waste (MSW) mixed with cattle manure, International Journal on Environmental Sciences, 2013;3:2068-2079 https://api.semanticscholar.org/CorpusID:36622777
- Santos-Beneit F, Ceniceros A, Nikolaou A, Salas JA, Gutierrez-Merino J. Identification of Antimicrobial Compounds in Two Streptomyces sp. Strains Isolated From Beehives. Front Microbiol. 2022;13:742168
Crossref - Trautmann NM, Richard T, Krasny M. Cornell Composting, Science and Engineering. In: The Science and Engineering of Composting Background Information. Cornell Waste Management Institute 1996, Cornell University Ithaca, NY 14853. https://ecommons.cornell.edu/server/api/core/bitstreams/ccce3cbc-ab98-4e29-adbe-093e141f1148/content
- Chen Z, Zhang S, Wen Q , Zheng, J. Effect of aeration rate on composting of penicillin mycelial dreg. Journal of Environmental Sciences, 2015; 37:172-178.
Crossref - Bueno P, Tapias R, López F, Díaz MJ. Optimizing composting parameters for nitrogen conservation in composting. Bioresource Technology, 2008;99(11): 5069-5077.
Crossref - Asquer C, Cappai G, Gioannis G D, Muntoni A, Piredda M, Spiga D, Biomass ash re-utilisation as an additive in the composting process of organic fraction of municipal solid waste. Waste Management, 2017;69:127-135.
Crossref - Abou-Hussien E, Nada W, Elgezery M. Influence of Sulphur Compost Application on Some Chemical Properties of Calcareous Soil and Consequent Responses of Hordeum Vulgare L. Plants. Egyptian Journal of Soil Science, 2020; 60(1):67-82.
Crossref - Manzoor, Ma L, Ni K, Ruan J. Influence of Organic and Inorganic Fertilizers on Tea Growth and Quality and Soil Properties of Tea Orchards’ Top Rhizosphere Soil. Plants (Basel). 2024;13(2):207.
Crossref - Williams SC, Hong Y, Danavall DCA, et al. Distinguishing between living and nonliving bacteria: Evaluation of the vital stain propidium iodide and its combined use with molecular probes in aquatic samples. Journal of Microbiological Methods, 1998;32(3):225-236.
Crossref - Santos LF, Olivares FL, Plant microbiome structure and benefits for sustainable agriculture. Current Plant Biology, 2021;26:100198.
Crossref - Antony-Babu S, Stach JEM, Goodfellow M. Genetic and phenotypic evidence for Streptomyces griseus ecovars isolated from a beach and dune sand system. Antonie van Leeuwenhoek. 2008;94(1):63-74.
Crossref - Ashry NM, Alaidaroos BA, Mohamed SA, Badr OAM, El-Saadony MT, Esmael A. Utilization of drought- tolerant bacterial strains isolated from harsh soils as a plant growth-promoting rhizobacteria (PGPR). Saudi J Biol Sci. 2022;29(3):1760-1769.
Crossref - Fadiji AE, Babalola OO. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities with Multifunctional Prospects. Front Bioeng Biotechnol. 2020;8:467.
Crossref - Pal S, Singh HB, Farooqui A, Rakshit A. Fungal biofertilizers in Indian agriculture: perception, demand and promotion. J Eco-friendly Agri. 2015;10(2):101-113.
- Yarnia M, Bouljak MS, Bolouri P, Tolay I. Zinc and manganese effect seed quality and germination in common bean (Phaseolus vulgaris L.). Turk J Agric For. 2011;1(1):154-168.
Crossref - Alkalabi CKJ, Bajlan SGH. The Effect of the Bacterial Biofertilizer Azotobacter and Gibberellic Acid Solution and Kinetin in Germination of Guava Seeds (Psidium guajava L.) IOP Conf Ser: Earth Environ Sci. 2023;1214:012020.
Crossref - Bashan Y, de-Bashan LE, Prabhu SR. Superior Polymeric Formulations and Emerging Innovative Products of Bacterial Inoculants for Sustainable Agriculture and the Environment. In: Singh, H, Sarma B, Keswani C. (eds) Agriculturally Important Microorganisms. Springer, Singapore.
Crossref - Berdy J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J Antibiot. 2012;65(8):385-395.
Crossref - Serwaa SY. Co-composting organic solid waste with Moringa oleifera leaves, sawdust and grass clippings. MSc Thesis, School of Hraduate Studies, Kwame Nkrumah University of Science and Technology. production. Agronomy. 2018;8(9):180.
- Garba M, Cornelis WM, Steppe K. Effect of termite mound material on the physical properties of sandy soil and on the growth characteristics of tomato (Solatium lycopersicum L.) in semi-arid Niger. Plant and Soil. 2011;338(1-2):451
- Youssef, M.A.; AL-Huqail, A.A.; Ali, E.F.; Majrashi, A. Organic Amendment and Mulching Enhanced the Growth and Fruit Quality of Squash Plants (Cucurbita Pepo L.) Grown on Silty Loam Soils. Horticulturae. 2021;7(9):269.
Crossref - Alharbi S, Majrashi A, Ghoneim AM, et al.A new method to recycle dairy waste for the nutrition of wheat plants. Agronomy. 2021;11(5):840.
Crossref - Ojo JA, Olowoake AA, Obembe A. Efficacy of organomineral fertilizer and un-amended compost on the growth and yield of watermelon (Citrullus lanatus Thumb) in Ilorin Southern Guinea Savanna zone of Nigeria. Int J Recycl Org. Waste Agricult. 2014;3:121-125.
Crossref - Rady MM, Semida WM, Hemida KA, Abdelhamid MT. The effect of compost on growth and yield of Phaseolus vulgaris plants grown under saline soil. International Journal of Recycling of Organic Waste in Agriculture. 2016;5:311-321.
Crossref - Semida WM, Abd El-Mageed T, Howladar SM. A novel organo-mineral fertilizer can alleviate negative effects of salinity stress for eggplant production on reclaimed saline calcareous soil. International Symposium on Growing Media and Soilless Cultivation. 2014;1034:493-499.
Crossref - Ahmed W, Shahroona B, Zahir ZA, Arshad M. Inoculation with ACC-deaminase containing rhizobacteria for improving growth and yield of wheat. Pak J Agric Sci. 2004;41(3-4):119-124.
- Cohen AC, Bottini R, Pontin M, et al. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol Plant. 201;153(1):79-90.
Crossref - Sarkar MD, Rahman MJ, Uddain J, et al. Estimation of Yield, Photosynthetic Rate, Biochemical, and Nutritional Content of Red Leaf Lettuce (Lactuca sativa L.) Grown in Organic Substrates. Plants. 2021; 10(6):1220.
Crossref - El Hayany B, En-Nejmy K, El Mezouari El Glaoui G, Hafidi M, El Fels L. Chlorophyll performances as an indicator of compost quality: Effectiveness of liquid humic substances and compost tea. International Journal of Recycling Organic Waste in Agriculture. 2023;12(4):683-698.
Crossref - El-Fatah AHR, Hosni AAM, Mahmoud H, Slim MH. Effect of compost fertilization on some growth parameters and yield of Calendula officinalis variety (Costa yellow). J Environ Sci. 2019;48(3):43-69.
Crossref - Franco RT, Buffiere P, Bayard R. Effects of storage conditions, total solids content and silage additives on fermentation profiles and methane preservation of cattle manure before anaerobic digestion. Waste and Biomass Valorization. 2018;9 (12): 2307-2317.
Crossref - Lu Q, Zhao Y, Gao X, et al. Effect of tricarboxylic acid cycle regulator on carbon retention and organic component transformation during food waste composting. Bioresour Technol. 2018;256:128-136.
Crossref - El-Serafy RS, El-Sheshtawy AA. Effect of nitrogen fixing bacteria and moringa leaf extract on fruit yield, estragole content and total phenols of organic fennel. Scientia Horticulturae. 2020;265.
Crossref - Huang CJ, Wang TK, Chung SC, Chen CY. Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28-9. J Biochem Mol Biol. 2005;38(1):82-88.
Crossref - Khan MR, Shahana M, Fayaz AM, Nabilah K. A new bioprocess to produce low cost powder formulations of biocontrol bacteria and fungi to control fusarial wilt and root-knot nematode of pulses. Biological Control. 2011;59(2):130-140.
Crossref - Mvumi C, Tagwira F, Chiteka AZ. Effect of Moringa Extract on Growth and Yield of Maize and Common Beans. Greener Journal of Agricultural Sciences. 2013; 3(1): 055-062.
Crossref - Hasnain M, Chen J, Ahmed N, et al. The Effects of Fertilizer Type and Application Time on Soil Properties, Plant Traits, Yield and Quality of Tomato. Sustainability, 2020;12(21):9065.
Crossref - Chen S, Zhao H, Zou C, et al. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Frontiers in Microbiology, 2017;8:2516.
Crossref - Bashan Y, de-Bashan LE, Prabhu SR, Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998-2013). Plant and Soil. 2014;378(1-2):1-33.
Crossref - Choudhary RC, Bairwa HL, Kumar U, et al. Influence of organic manures on soil nutrient content, microbial population, yield and quality parameters of pomegranate (Punica granatum L.) cv. Bhagwa. PLoS One. 2022;17(8):e0266675.
Crossref - Rehman Su, De Castro F, Aprile A, Benedetti M, Fanizzi FP. Vermicompost enhancing plant growth and combating abiotic and biotic stress. Agronomy. 2023; 13(4):1134.
Crossref - Abd El-Hack ME, Alagawany M, Elrys AS, et al. Effect of Forage Moringa oleiferaL. (moringa) on Animal Health and Nutrition and Its Beneficial Applications in Soil, Plants and Water Purification. Agriculture. 2018;8(9):145
Crossref - Mashamaite CV, Ngcobo BL, Manyevere A, Bertling I, Fawole OA. Assessing the Usefulness of Moringa oleifera Leaf Extract as a Biostimulant to Supplement Synthetic Fertilizers: A Review. Plants (Basel). 2022;11(17):2214.
Crossref - Khallaf H, Amasha R, Alaidaroos B, Aly M, Jastaniah S. Isolation and molecular characterization of some plant growth-promoting actinomycetes from cultivated soil for management of some pathogenic microbes. AGBIR. 2023;39(1):412-420.
- Francioli D, Schulz E, Lentendu G, Wubet T, Buscot F, Reitz T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. J. Frontiers in Microbiology, 2016;7.
Crossref - Enebe MC, Babalola OO. Effects of inorganic and organic treatments on the microbial community of maize rhizosphere by a shotgun metagenomics approach. Ann Microbiol., 2020;70:49.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.