ISSN: 0973-7510
E-ISSN: 2581-690X
Methicillin-resistant Staphylococcus aureus (MRSA) mastitis poses a significant threat to dairy herds worldwide, given its resistance to methicillin and other β-lactam antibiotics, which often leads to treatment failure. Consequently, there is an urgent need for safe and effective alternative therapeutic approaches. Recent investigations have highlighted the potential of baicalein, a natural flavonoid known for its potent anti-inflammatory and antibacterial properties, especially its synergistic effects with β-lactam antibiotics against MRSA. However, the limited solubility and bioavailability of baicalein hinder its biomedical utility. The present study assessed the therapeutic efficacy of encapsulated baicalein in chitosan, forming a tricomplex with a β-lactam antibiotic, using a murine model of MRSA-induced mastitis. The experimental design comprised seven groups, each consisting of six mice. We evaluated the ability of various treatment regimens to mitigate histopathological alterations and bacterial burden induced by MRSA infection, aiming to elucidate underlying mechanisms. Our results revealed that tricomplex treatment significantly reduced bacterial load in mammary tissue and preserved tissue integrity, resulting in decreased inflammatory responses post-MRSA inoculation. In addition, tricomplex treatment markedly reduced mean leukocyte and neutrophil counts in blood and suppressed the matrix metalloproteinase-9 (MMP-9) concentration and C-reactive protein (CRP) response. Notably, the synergistic interaction between baicalein and amoxicillin was particularly pronounced. Our findings suggest that chitosan-encapsulated baicalein combined with a β-lactam antibiotic holds promise as a therapeutic option for MRSA-induced mastitis. Further investigations, particularly in target animal species, are warranted to comprehensively evaluate its clinical feasibility.
Baicalein, Antibacterial activity, Mastitis, Antibiotic resistance, Encapsulation, β-lactam antibiotic
Methicillin-resistant Staphylococcus aureus (MRSA) stands as a formidable pathogen in both human and veterinary medicine, with its impact extending to the realm of dairy farming.1 Mastitis, characterized by inflammation of the mammary gland, poses a significant challenge to dairy cow health and milk production worldwide.2 Alarmingly, the incidence of MRSA-induced mastitis is on the rise among dairy cows, presenting a pressing concern for both animal welfare and food safety.3 Traditionally, antibiotics have served as the cornerstone of mastitis treatment, yet their efficacy is increasingly compromised by the emergence of antibiotic-resistant strains of S. aureus, including MRSA.2,3
The S. aureus mastitis model is frequently employed in research settings and holds particular relevance in the study of ruminant mastitis due to the striking parallels between murine and bovine mammary gland physiology and immunology.4,5 Leveraging this model provides invaluable insights into the pathogenesis of MRSA mastitis, facilitating the development and evaluation of novel therapeutic interventions.5 In S. aureus infection of mammary gland, the downstream signaling molecules such as nuclear factor-kappa B (NF-kB) is upregulated by activating the toll-like receptors, leading to expression of number of inflammatory cytokines (TNF-a, IL-1b and IL-6).6 NF-kB and inflammatory cytokines regulate the expression of matrix metalloproteinases (MMPs) that play a key role in recruitment of leukocytes and modulating inflammatory mediators in mammary gland.7 The MRSA expresses higher proportion of Panton-Valentine leukocidin gene that induces marked inflammatory response, resulting in acute phase response.8 The elevated level of inflammatory markers, C-reactive protein (CRP) and matrix metalloproteinase activities in milk and serum has been reported in mastitis.9
Flavonoids, the natural products from plant secondary metabolites, are known for its broad spectrum of health promoting effects owing to their anti-inflammatory, anti-bacterial, antioxidant and anticarcinogenic properties.10 Baicalein is a prominent flavonoid compound derived from Scutellaria baicalensis Georgi, a traditional Chinese medicinal herb, has garnered significant attention for its therapeutic potential in combating bacterial infections.11 Studies have demonstrated its efficacy in inhibiting biofilm formation, bacterial quorum sensing, and virulence gene expression, thereby exerting potent antibacterial effects.12 Moreover, baicalein exhibits anti-inflammatory properties by suppressing the MAPK pathway and reducing the expression of pro-inflammatory cytokines such as MMPs, highlighting its multifaceted pharmacological profile.13 Despite its promising therapeutic attributes, baicalein faces challenges stemming from its inherent poor solubility and low bioavailability, which hamper its clinical utility.14 However, research has shown that baicalein can synergistically enhance the antimicrobial efficacy of β-lactam antibiotics against MRSA, offering a potential solution to its limited efficacy when used alone.15 To address the solubility and bioavailability issues associated with baicalein, novel approaches such as nanoparticle-based drug delivery systems have emerged as promising strategies.16
Chitosan is a naturally-occurring linear polysaccharide that is an ideal nanoparticle for antimicrobial drug delivery apart form its own antibacterial property.17 Our study used chitosan to encapsulate baicalein and a β-lactam antibiotic to evaluate its therapeutic efficacy in MRSA-induced mouse mastitis. We hypothesized that treatment of chitosan encapsulated baicalein and β-lactam will reduce the inflammation, pathological changes and bacterial load of mammary tissue and improve the therapeutic efficacy as compared to baicalein or β-lactam alone in mouse mastitis. The present study investigated whether chitosan encapsulated baicalein and β-lactam antibiotic can be used to attenuate MRSA induced mastitis by accelerating the resolution of mammary tissue pathology and reducing bacterial load in mammary gland.
Bacterial strain
MRSA (S. aureus R1/BOV – Accession No. – KX181857) was isolated earlier from mastitis affected cows by our laboratory and preserved at -80°C as 30% glycerol stock.
Experimental animals
Forty-two adult healthy Swiss albino female and fourteen male mice of 6-8 weeks (weight > 25 grams) were obtained from laboratory animal resource section of the institute. The mice were housed in animal housing facility, Division of Medicine, ICAR-IVRI, Izatnagar as per CPCSEA guidelines. The protocol of the experiment No: F.1-53/2012-13/JD(R) was approved by the Institutional Animal Ethics Committee.
Induction of mastitis in murine model
The MRSA isolate from glycerol stock was sub cultured continuously to get the pure culture and then serial dilution of MRSA suspension was prepared and dose of bacteria was calculated by surface viable count.18
Experimental protocol
The preparation and characterization of baicalein green biomolecule were done as per the established protocol.19 Before initiation of experiment, the mice were allowed to acclimatize for 7 days. Then female and male (3:1) mice were kept for breeding. After 7 days of parturition, the pups were weaned from mice. On 8th day, 50 µL of MRSA culture (105 CFU/mL) was inoculated in each test of 36 female mice to induce mastitis whereas, 6 female mice were kept as health control that received no bacterial inoculation.
In total 42 mice were randomly allocated into seven groups and six mice of each group was allotted one of the following treatment protocol: (Group A) 100 µL sterile phosphate buffer saline (PBS, pH 7.2 ± 0.2) by oral route (healthy control group); (Group B) 100 µL sterile PBS by oral route (PC; positive control group); (Group C) 500 µL of CH suspension by oral route (2.0 mg/mL; CH group); (Group D) 200 µL of baicalein suspension by oral route (5.0 mg/mL; BC group); (Group E) 200 µL of CH encapsulated baicalein and amoxicillin by oral route (Tricomplex group); (Group F) 10 mg/kg body weight of amoxicillin by intramuscular route (AMX group); and (Group G) 8.0 mg/kg body weight clindamycin by intramuscular route (CLIN group). All the treatments were administered consecutively for seven days.
Analysis of blood samples
Blood was collected from each mouse by puncturing the retrobulbar venous plexus using microcapillary tubes and transferred into the tubes with and without anticoagulant. Total leukocyte count (TLC) and differential leucocyte count (DLC) in unclotted blood were measured manually.20 The concentrations of matrix metalloproteinase-9 (MMP-9) in serum were measured at 0, 24, 48 and 72 hours by using Immunotag™ MMP-9 ELISA kit (G Bioscience, Cat. # ITI4032, Geno Technology Inc., USA) according to the manufacturer’s instructions. The concentration of MMP-9 was expressed as nanogram/milliliter of serum. The C-reactive protein (CRP) in the serum samples was measured at 0, 48, and 72 hours by latex agglutination method (Lifescreen™–CRP, Kamineni Life Sciences Pvt. Ltd, India) following the manufacturer’s instructions. The reaction was graded as 0 to +4 based on the degree of agglutination.
Gross examination and estimation of bacterial load in mammary glands
The mammary glands were grossly examined for any lesions before and after the challenge.21 Gross examination of mammary glands was performed according to the standard clinical score card considering parameters like swelling, discoloration and exudation of mammary glands.9 For bacterial load, the mammary glands (R4) were removed and homogenized using pre-cooled sterile grinders with the addition of sterile PBS at a ratio of 1:5 (w/v). The mammary gland homogenates (10-fold serial dilution) were plated on mannitol salt agar plates, incubated at 37°C. Then S. aureus colonies were counted.22 Total bacterial count of the mammary gland was studied after sacrificing the mice.
Histological examination of mammary glands
The mammary tissues were collected at 10 days after initiation of treatment and fixed in 10% formaldehyde solution for paraffin embedding. After staining with hematoxylin and eosin (H and E), the histopathological alterations were examined using a light microscope (Olympus, Japan).23
Statistical analysis
Data were reported as the mean ± standard error of the mean (SEM). The obtained data were analyzed by statistical package for the social sciences (SPSS) version 20. Statistical significance was evaluated using one-way factorial analysis of variance (ANOVA) along with Tukey’s multiple-comparisons test. Values of p < 0.05 were considered to be statistically significant.
Total leukocyte count and differential leucocyte count
Haematological findings are included in Tables 1-4. The mean TLC and neutrophil (N) % were significantly (p < 0.05) increased at 48, 72, and 240 hours and lymphocyte (L) % were significantly (p < 0.05) decreased at 48 to 240 hours as compared to 0 hour in positive control group (Table 1). A persistently increased level of mean TLC from 24 to 240 hours and N % from 48 to 240 hours and significantly decreased level of mean L % was noted from 24 to 240 hours in both CH and BC-treated groups. In tricomplex–treated group, the mean TLC initially increased (p < 0.05) till 48 hours and thereafter it decreased (p < 0.05) to pretreatment value at 72 and 240 hours. The L % significantly dropped at 48, 72, and 240 hours whereas, the N% remained unaltered from 0 to 240 hours. The mean TLC and N % were significantly elevated and L % was significantly decreased at 72 to 240 hours as compared to 0 hour in both AMX and CLIN-treated groups. However, statistically, no significant changes in the mean monocyte (M) %, eosinophils (E) % and basophil (B) % were observed across the groups from 0 to 240 hours. When mean TLC, N % and L % were compared between positive control and treatment groups, it was observed that the mean TLC at 72 and 240 hours and N% at 48, 72, and 240 hours were significantly lower in tricomplex group and L% was significantly higher at 24 and 72 hours in tricomplex and at 48 and 240 hours in CLIN-treatment groups than other treatment groups (Table 1-4).
Table (1):
Total leukocytic count (Mean ± SD) in mice of different groups at 0, 24, 48, 72, and 240 hours post-challenge
Group (n=6) |
0 hour |
24 hours |
48 hours |
72 hours |
240 hours |
|---|---|---|---|---|---|
Group A |
4533 ± 167b |
4592 ± 191.56b |
4538 ± 158.39 b |
4573 ± 210b |
4735 ± 214b |
Group B |
4353 ± 146b |
5545 ± 425b |
7075 ± 1202.34a |
10200 ± 1215a |
10305 ± 1367b |
Group C |
5467 ± 148ab |
9517 ± 216.67a |
9183 ± 285ab |
8983 ± 247ab |
8287 ± 258ab |
Group D |
5765 ± 160ab |
8866 ± 365ab |
8265 ± 365ab |
8147 ± 419ab |
7998 ± 416ab |
Group E |
5760 ± 93ab |
10483 ± 217a |
9467 ± 424ab |
7182 ± 483ab |
6253 ± 462ab |
Group F |
4787 ± 242a |
6825 ± 936b |
7130 ± 717a |
7239 ± 827ab |
8127 ± 1040ab |
Group G |
4689 ± 83a |
6766 ± 1012b |
7216 ± 678a |
8067 ± 540ab |
6000 ± 611ab |
*Values with different superscripts are significant at level (p < 0.05), in rows (a, b, c)
Table (2):
Relative percentage of lymphocytes (Mean ± SE) in mice of different groups at 0, 24, 48, 72 and 240 hours post challenge
Group (n=6) |
0 hour |
24 hours |
48 hours |
72 hours |
240 hours |
|---|---|---|---|---|---|
Group A |
78 ± 0.56a |
76 ± 1.08b |
74 ± 0.87b |
71 ± 0.49b |
74 ± 0.42ab |
Group B |
76 ± 0.79b |
52 ± 2.75ab |
51 ± 2.23ab |
46 ± 1.53c |
36 ± 1.53c |
Group C |
78 ± 0.71a |
71 ± 1.15b |
68 ± 2.76ab |
65 ± 2.36ab |
61 ± 3.59a |
Group D |
75 ± 1.01b |
69 ± 0.33b |
68 ± 0.76ab |
65 ± 0.71ab |
64 ± 1.26a |
Group E |
77 ± 0.61ab |
73 ± 1.34b |
71 ± 1.41b |
68 ± 0.98b |
70 ± 2.01ab |
Group F |
76 ± 0.84b |
68 ± 0.54b |
67 ± 0.6b |
64 ± 1.28ab |
61 ± 1.89a |
Group G |
77 ± 0.95ab |
73 ± 1.65b |
72 ± 1.35b |
67 ± 1.96b |
69 ± 0.79ab |
*Values with different superscripts are significant at level (p < 0.05), in rows (a, b, c)
Table (3):
Relative percentage of neutrophils (Mean ± SE) in mice of different at 0, 24, 48, 72 and 240 hours post challenge
Group (n=6) |
0 hour |
24 hours |
48 hours |
72 hours |
240 hours |
|---|---|---|---|---|---|
Group A |
22.83 ± 1.14 |
24.83 ± 1.33* |
28.00 ± 1.83* |
27.00 ± 2.74*** |
30.00 ± 5.11* |
Group B |
24.00 ± 1.39 |
25.83 ± 2.14* |
43.17 ± 3.37*** |
48.17 ± 3.22* |
58.50 ± 3.19*** |
Group C |
24.67 ± 0.61 |
28.00 ± 1.75* |
36.67 ± 0.61*** |
41.17 ± 2.81* |
54.83 ± 2.07** |
Group D |
23.50 ± 1.61 |
24.33 ± 2.04* |
31.50 ± 5.43*** |
33.17 ± 5.47** |
41.50 ± 2.72** |
Group E |
23.83 ± 0.91 |
26.83 ± 1.28* |
25.83 ± 1.45** |
26.83 ± 1.90*** |
24.67 ± 1.09* |
Group F |
22.00 ± 0.86 |
29.67 ± 0.99* |
37.00 ± 2.27*** |
30.33 ± 2.82** |
40.00 ± 1.51** |
Group G |
24.33 ± 0.76 |
29.17 ± 1.45* |
36.67 ± 1.50*** |
31.67 ± 2.70** |
23.50 ± 2.42* |
*Values with different superscripts are significant at level (p < 0.05)
Table (4):
Relative percentage of eosinophil (Mean ± SE) in mice of different at 0, 24, 48, 72 and 240 hours post challenge
Group (n=6) |
0 hour |
24 hours |
48 hours |
72 hours |
240 hours |
|---|---|---|---|---|---|
Group A |
0.83 ± 0.31 |
1.00 ± 0.37 |
1.00 ± 0.26 |
0.67 ± 0.42 |
1.00 ± 0.37 |
Group B |
1.33 ± 0.49 |
1.50 ± 0.72 |
2.50 ± 0.34 |
2.83 ± 0.70 |
3.00 ± 0.37 |
Group C |
2.17 ± 0.54 |
2.33 ± 0.42 |
2.00 ± 0.45 |
2.50 ± 0.50 |
2.33 ± 0.61 |
Group D |
3.00 ± 0.58 |
2.17 ± 0.60 |
2.00 ± 0.00 |
1.33 ± 0.33 |
2.17 ± 0.40 |
Group E |
1.67 ± 0.67 |
1.50 ± 0.56 |
0.83 ± 0.40 |
1.00 ± 0.37 |
2.00 ± 0.52 |
Group F |
2.00 ± 0.45 |
2.67 ± 0.56 |
1.33 ± 0.21 |
1.50 ± 0.34 |
1.67 ± 0.49 |
Group G |
2.50 ± 0.34 |
2.50±0.56 |
2.83 ± 0.95 |
2.67 ± 0.76 |
1.00 ± 0.00 |
C-reactive protein
The agglutination reaction of CRP was negative for all the groups at 0 hour. The CRP response was significantly increased at 24 and 72 hours from 0 hour in positive control, CH, BC and AMX-treated groups. However, the CRP response was initially increased at 24 hours and thereafter the response was markedly decreased at 72 hours in both tricomplex and CLIN-treated mice. When mean scores of CRP were compared between positive control and treatment groups, it was observed that the mean CRP score was significantly lower in 24 hours in CLIN and at 72 hours in tricomplex and CLIN-treatment groups as compared to positive control and other treatment group.
Matrix metalloproteinase-9
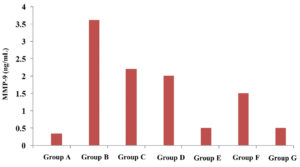
The average concentration of MMP-9 was persistently elevated (p<0.05) till 72 hours in positive control, CH, BC and AMX-treated groups. In tricomplex and CLIN-treated groups, the mean MMP-9 concentration was initially increased at 24 hours but the value gradually decreased at 72 hours. When mean MMP-9 concentrations were compared between positive control and treatment groups, it was observed that the mean MMP-9 concentration was significantly lower at 24 hours in CLIN and at 72 hours in tricomplex and CLIN-treatment groups as compared to positive control and other treatment groups (Figure 1).
Figure 1. Concentration of matrix metalloproteinase-9 (MMP-9) in serum in healthy, treated and untreated infected groups of mice
Gross examination of mammary glands
The clinical score card of animals in different groups are given in Table 5. Mice in all the groups were active and alert with normal appearance of mammary gland prior to bacteria inoculation. At 24 hours, the mice became dull and depressed with appreciable swelling and reddish discoloration in the mammary glands of positive control, CH, BC and AMX-treated groups whereas, mild inflammatory response was observed in group tricomplex and CLIN-treated groups (Figure 2). After 48 hours of bacterial inoculation, profound dullness and depression were observed in positive control group while their mammary glands showed appreciable swelling, enlargement and reddish discoloration with extravasations of pus and blood-stained exudates. There was decrease in inflammatory response in the mammary glands of CH and BC-treated mice, however, decrement in inflammatory response was much pronounced in tricomplex-treated mice. Swelling and hardness of mammary gland of BC-treated mice were seen.
Table (5):
The clinical score card of animals in different groups
Group (n=6) |
0 hour |
24 hours |
48 hours |
96 hours |
168 hours |
|---|---|---|---|---|---|
Group A |
21.5a |
4b |
3.5b |
4.5b |
5.5b |
Group B |
21.5a |
13ab |
21.3ab |
33.9a |
37a |
Group C |
21.5a |
32.08a |
29.25a |
21.25ab |
25.75ab |
Group D |
21.5a |
27a |
23.41a |
17.75ab |
18.83ab |
Group E |
21.5a |
22.5ab |
13.91ab |
13.33ab |
7.5b |
Group F |
21.5a |
29a |
32.25a |
35a |
34.75a |
Group G |
21.5a |
24.42ab |
21.83a |
12.75ab |
5.16ab |
*Values with different superscripts are significant at level (p<0.05), in rows (a, b, c)
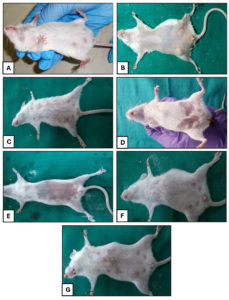
Figure 2. (A) Gross appearance of a normal healthy mammary gland in group A. (B) Mammary gland having swelling and redness in group B. (C) Mammary gland showing swelling and inflammation in group C. (D) Mild swelling of the mammary gland in group D. (E) Mammary gland of group E appearing normal. (F) Swollen mammary gland in group F. (G) Mammary gland of group G
Subsequently, at 96 hours, the mice were found severely depressed with discolored, heavily swollen mammary glands of positive control group. However, there was reduced discoloration and swelling and did not reveal gross pathological change in mammary glands of tricomplex and CLIN-treated mice. A decrement in inflammatory response was noted in BC-treated mice. At 168 hrs, the mice were found severely depressed with discolored (bluish), distorted and heavily swollen mammary glands engorged with purulent secretion in positive control group. In tricomplex-treated group, the mice were almost normal with no gross changes in mammary glands.
Bacterial load and histological examination in mammary tissue
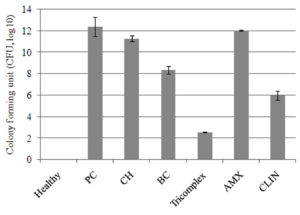
The mean log10 CFU of bacteria in mammary tissue was significantly (p < 0.05) decreased at 144 hours in tricomplex and CLIN-treated mice followed by BC, CS and AMX-treated mice as compared to positive control group (Figure 3). Differences were observed after 10 days post inoculation with 105 CFU of MRSA per gland in all the groups. The tissue sections of mammary gland of healthy mice revealed normal healthy lactating alveoli with tree like structures of the mammary lobules. Lactiferous ducts and acini in the lobules were well demarcated. Acini and ducts of mammary glands are lined by cuboidal epithelium. Presence of pinkish secretion was noticed. No bacterial colonies were observed in the secretion (Figure 4G1).
Figure 3. The mean log10 CFU of bacteria in mammary tissue of different treatment groups (Groups A-G) at 144 hours
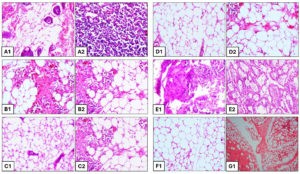
In positive control group, the mammary sections depicted abscess admixed with bacterial colonies necrotic alveoli, necrosis of alveoli with thickened interstitium, proliferation of mononuclear cells and classical botryomycosis lesions characterized by pyogranulomatous foci with fibrocellular reaction in the mammary parenchyma and dense fibrocellular reaction in the mammary parenchyma with inflammatory exudates in the lactiferous duct (Figure 4A1 and A2). The slides of mammary tissue depicted almost like healthy mammary acini with presence of pathological lesions, damaged mammary parenchyma, damaged secretory alveoli and associated duct showing any inflammatory lesions in CH-treated group (Figure 4B1 and B2). In BC-treated mice, the H and E stained histopathological slides depicted necrotic alveolar lumen filled with numerous bacterial colonies, alveolar lumen dilated with necropurulent exudates, thickened mammary interstitium showing marked congestion with heavy infiltration of mononuclear cells (Figure 4C1 and C2). Interestingly, the histopathological examination revealed no inflammatory reaction with almost normal mammary parenchyma, normal secretory alveoli and associated duct with little presence of showing any inflammatory cells surrounding the acini in Tricomplex-treated group (Figure 4D1 and D2).
In AMX-treated group, the slides depicted marked increase in neutrophil infiltration, extensive diffused necrotic changes and the normal architecture of the mammary alveolar was disrupted. The thickened inter-acinar septum, multiple focal abscesses typical of S. aureus was predominated and cellular exudates were evident. The acini were necrosed with the thickened parenchyma, presence of inflammatory exudates in the lactiferous duct with necrosed mammary epithelium and dense fibrocellular reaction in the mammary parenchyma (Figure 4E1 and E2). The histopathological sections depicted intact acini and ducts of mammary glands lined by cuboidal epithelium. Presence of pinkish secretion was noticed. No bacterial colonies were observed in the secretion. No inflammatory lesions were present in CLIN-treated group (Figure 4F1).
Figure 4. H and E stained section of mouse mammary gland (group B) showing (A1) classical botryomycosis lesions characterized by pyogranulomatous foci with fibrocellular reaction in the mammary parenchyma and (A2) Inflammatory exudates in the lactiferous duct. Group C shows the presence of (B1) inflammatory cells and proliferated fibroblasts along with (B2) heavy infiltration of mononuclear cells and thickened interstitium. Group D shows the presence of (C1) marked congestion and thickened interstitium along with (C2) damaged acini, purulent exudates in the alveoli and infiltration of a large number of neutrophils and mononuclear cells. Group E shows (D1) intact acini with well-maintained sheet of alveolar epithelial cells and (D2) Few inflammatory cells and complete acinar wall. Group F shows the presence of (E1) inflammatory exudate with necrotic abscess and thickened interstitium along with (E2) dense presence of inflammatory cells in the alveoli and damaged alveolar walls. Group G shows (F1) normal mammary intact alveoli, no inflammatory cells and exudates. Group A shows (G1) healthy lactating mammary glands with tree like structures, lactiferous ducts and acini in the lobules, The acini and ducts of mammary glands are lined by cuboidal epithelium
Methicillin and other β-lactam antibiotics, once mainstays in mastitis therapy, now face formidable resistance from MRSA strains, rendering conventional treatment regimens often ineffective.24 This escalating threat has prompted a surge of interest in the exploration of novel therapeutic modalities to combat MRSA mastitis. Among these approaches is the quest for antimicrobial agents capable of circumventing bacterial resistance mechanisms or reducing the necessity for high-dose and prolonged antibiotic therapy, thereby mitigating the risk of antimicrobial resistance.25 In this pursuit, drug combinations have emerged as a promising strategy to overcome bacterial resistance and enhance treatment outcomes in MRSA mastitis. By combining multiple agents with complementary mechanisms of action, synergistic effects can be achieved, effectively targeting MRSA while minimizing the likelihood of resistance development.26 Furthermore, drug combinations offer the flexibility to tailor treatment regimens to individual cases, optimizing efficacy while minimizing adverse effects.
By encapsulating baicalein within nanoparticles, its intracellular delivery can be enhanced, thereby augmenting its therapeutic efficacy and circumventing issues related to poor solubility and bioavailability.27 Furthermore, the development of bioactive and antimicrobial coatings presents another avenue for overcoming the limitations of baicalein.16,27 Such coatings could serve to enhance the stability and efficacy of baicalein, while also providing antimicrobial protection against bacterial colonization and biofilm formation, thereby improving its overall performance as a therapeutic agent.16,27
Despite the fact that baicalein is a potentially useful pharmaceutical compound, its commercialization is impeded by limitations such as low water solubility, bioavailability, and pharmacokinetic properties.28 Previous research has shown that self-assembled nanoparticles can improve the oral bioavailability of baicalein.29 Chitosan, a natural polysaccharide, has attracted the interest of researchers due to its high biocompatibility and biodegradability.30 In the current study, encapsulated baicalein and a β-lactam antibiotic was assessed for its ameliorative potential in MRSA-induced mastitis in mouse model.
The tricomplex group decreased the bacterial load in mammary tissue and lessened pathological damage in histopathology. Baicalein and β-lactam have a synergistic antibacterial effect in vitro, resulting in MRSA sensitivity at relatively low β-lactam concentrations.15 The β-lactam-baicalein synergism against S. aureus is due to contributions from two different types of activities: preventing β-lactamase hydrolysis of susceptible penicillin, which restores penicillin sensitivity, and inhibiting interactions between β-lactams and penicillin-binding proteins.13,15 In our study, the encapsulation of baicalin and amoxicillin within chitosan may have facilitated enhanced internalization and prolonged intracellular persistence of baicalin, particularly when administered orally.31,32 Recent research has indicated that encapsulating baicalin in lactobionic acid-modified chitosan further enhances the local bioavailability of therapeutic agents following oral administration.33 Another study showed that intracellular killing capacity of S. aureus increases by six-fold by chitosan loaded with antibiotic than the antibiotic alone in the infected site by increasing the efficiency of drug uptake by the macrophage and the epithelial cells.34
The ameliorative effect of chitosan encapsulated baicalein with β-lactam on MRSA-induced mouse mastitis likely stems from their synergistic antibacterial effects. These results underscore the potential of chitosan-encapsulated baicalein in combination with β-lactam antibiotics as a promising therapeutic intervention for mastitis. Nonetheless, further investigations involving target animal species are necessary to validate its clinical utility before widespread application.
In conclusion, the encapsulation of baicalein and a β-lactam antibiotic using chitosan demonstrated significant efficacy in reducing bacterial load and mitigating pathological damage in a mouse model of MRSA-induced mastitis. The observed synergistic antibacterial effect of baicalein and β-lactam in vitro, combined with the potential for enhanced bioavailability and intracellular persistence of baicalein through chitosan encapsulation, supports the therapeutic potential of this approach. Our findings indicate that chitosan-encapsulated baicalein with β-lactam could be a promising candidate for the treatment of mastitis caused by MRSA. Further research, particularly in target animal species, is warranted to fully evaluate its clinical applicability.
ACKNOWLEDGMENTS
The authors would like to thank the Director, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, India, for providing the necessary research facilities to carry out this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institute Animal Ethics Committee (IAEC), Indian Veterinary Research Institute (ICAR-IVRI), Izatnagar, Bareilly, Uttar Pradesh, India, vide order no.: F.1-53/2012-13/JD(R).
- Khanal S, Boonyayatra S, Awaiwanont N. Prevalence of methicillin-resistant Staphylococcus aureus in dairy farms: A systematic review and meta-analysis. Front Vet Sci. 2022;9:947154.
Crossref - Sharun K, Dhama K, Tiwari R, et al. Advances in therapeutic and managemental approaches of bovine mastitis: a comprehensive review. Vet Q. 2021;41(1):107-136.
Crossref - Cheng WN, Han SG. Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments – A review. Asian-Australas J Anim Sci. 2020;33(11):1699-1713.
Crossref - Camperio C, Armas F, Biasibetti E, et al. A mouse mastitis model to study the effects of the intramammary infusion of a food-grade Lactococcus lactis strain. PloS One. 2017;12(9):e0184218.
Crossref - Notebaert S, Meyer E. Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet Q. 2006;28(1):2-13.
Crossref - Zhao L, Jin L, Yang B. Saikosaponin A alleviates Staphylococcus aureus-induced mastitis in mice by inhibiting ferroptosis via SIRT1/Nrf2 pathway. J Cell Mol Med. 2023;27(22):3443-3450.
Crossref - Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, et al. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int J Mol Sci. 2020;21(24):9739.
Crossref - Holzinger D, Gieldon L, Mysore V, et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol. 2012;92(5):1069-1081.
Crossref - Dolma T, Mukherjee R, Pati BK, De UK. Acute Phase Response and Neutrophils: Lymphocyte Ratio in Response to Astaxanthin in Staphylococcal Mice Mastitis Model. J Vet Med. 2014;2014:147652.
Crossref - Roy A, Khan A, Ahmad I, et al. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res Int. 2022;2022:5445291.
Crossref - Bajek-Bil A, Chmiel M, Wloch A, Stompor-Goracy M. Baicalin-Current Trends in Detection Methods and Health-Promoting Properties. Pharm Basel Switz. 2023;16(4):570.
Crossref - Luo J, Dong B, Wang K, et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PloS One. 2017;12(4):e0176883.
Crossref - Wen Y, Wang Y, Zhao C, Zhao B, Wang J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int J Mol Sci. 2023;24(11):9317.
Crossref - Chen H, Gao Y, Wu J, et al. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014;354(1):5-11.
Crossref - Liu IX, Durham DG, Richards RME. Baicalin synergy with beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other beta-lactam-resistant strains of S. aureus. J Pharm Pharmacol. 2000;52(3):361-366.
Crossref - Lv Y, Li W, Liao W, et al. Nano-Drug Delivery Systems Based on Natural Products. Int J Nanomedicine. 2024;19:541-569.
Crossref - Jafernik K, Ladniak A, Blicharska E, et al. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems-A review. Mol Basel Switz. 2023;28(4):1963.
Crossref - Anderson JC, Chandler RL. Experimental Staphylococcal mastitis in the mouse. Histological, ultrastructural and bacteriological changes caused by a virulent strain of Staphylococcus aureus. J Comp Pathol. 1975;85(4):499-510.
Crossref - Soni S, Mukherjee R, De UK, et al. Preparation and characterization of chitosan encapsulated beta-lactam antibiotic and baicalein nanoparticles. Pharma Innov J. 2022;11(5):2198-2201.
- Jain NC. Schalm’s Veterinary Hematology.(Lea & Febiger 1986). https://www.cabdirect.org/cabdirect/abstract/19872289576. Accessed March 13, 2024.
- Gogoi-Tiwari J, Williams V, Waryah CB, et al. Mammary Gland Pathology Subsequent to Acute Infection with Strong versus Weak Biofilm Forming Staphylococcus aureus Bovine Mastitis Isolates: A Pilot Study Using Non-Invasive Mouse Mastitis Model. PloS One. 2017;12(1):e0170668.
Crossref - Pereyra EAL, Sacco SC, Dure A, et al. Immune response of Staphylococcus aureus strains in a mouse mastitis model is linked to adaptive capacity and genotypic profiles. Vet Microbiol. 2017;204:64-76.
Crossref - Gogoi-Tiwari J, Dorji D, Tiwari HK, Shirolkar G, Aleri JW, Mukkur T. Phenotypic PIA-Dependent Biofilm Production by Clinical Non-Typeable Staphylococcus aureus Is Not Associated with the Intensity of Inflammation in Mammary Gland: A Pilot Study Using Mouse Mastitis Model. Anim Open Access J MDPI. 2021;11(11):3047.
Crossref - Tesfaye K, Gizaw Z, Haile AF. Prevalence of Mastitis and Phenotypic Characterization of Methicillin-Resistant Staphylococcus aureus in Lactating Dairy Cows of Selected Dairy Farms in and Around Adama Town, Central Ethiopia. Environ Health Insights. 2021;15:11786302211021297.
Crossref - Annunziato G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int J Mol Sci. 2019;20(23):5844.
Crossref - Masimen MAA, Harun NA, Maulidiani M, Ismail WIW. Overcoming Methicillin-Resistance Staphylococcus aureus (MRSA) Using Antimicrobial Peptides-Silver Nanoparticles. Antibiot Basel Switz. 2022;11(7):951.
Crossref - Huang L, Huang XH, Yang X, et al. Novel nano-drug delivery system for natural products and their application. Pharmacol Res. 2024;201:107100.
Crossref - de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol Res. 2015;100:296-308.
Crossref - Wang W, Xi M, Duan X, Wang Y, Kong F. Delivery of baicalein and paclitaxel using self-assembled nanoparticles: synergistic antitumor effect in vitro and in vivo. Int J Nanomedicine. 2015;10(1):3737-3750.
Crossref - Detsi A, Kavetsou E, Kostopoulou I, et al. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics. 2020;12(7):669.
Crossref - Fang CL, Wang Y, Tsai KHY, Chang HI. Liposome-Encapsulated Baicalein Suppressed Lipogenesis and Extracellular Matrix Formation in Hs68 Human Dermal Fibroblasts. Front Pharmacol. 2018;9:155.
Crossref - Li X, Luo W, Ng TW, et al. Nanoparticle-encapsulated baicalein markedly modulates pro-inflammatory response in gingival epithelial cells. Nanoscale. 2017;9(35):12897-12907.
Crossref - Ahmed IS, Rashed HM, Fayez H, Farouk F, Shamma RN. Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver. Pharmaceutics. 2020;12(2):107.
Crossref - Maya S, Indulekha S, Sukhithasri V, et al. Efficacy of tetracycline encapsulated O-carboxymethyl chitosan nanoparticles against intracellular infections of Staphylococcus aureus. Int J Biol Macromol. 2012;51(4):392-399.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.