ISSN: 0973-7510
E-ISSN: 2581-690X
The World Health Organization (WHO) considers carbapenem-resistant organisms (CROs) to be critical-level pathogens. Regular screening for high-risk CRO colonization is essential, especially in the ICU. Direct detection of carbapenem-resistant genes is possible using the FDA-approved Xpert Carba-R assay. This study evaluated its reliability compared with the culture technique at a tertiary hospital in Indonesia. A high number of CRO colonization was found using the culture technique and the Xpert Carba-R assay with about 31 and 26 positive results out of 100 total samples, respectively. Both methods detected blaNDM in 11 samples, and the Xpert Carba-R assay detected one sample co-presenting with blaVIM that was not detected by PCR. The Xpert Carba-R assay did not detect the gene in 73 samples following negative results with the culture technique. Fifteen samples were detected gene by the Xpert Carba-R assay though there was no gene by the culture method, showing that the Xpert Carba-R assay demonstrated a high degree of sensitivity in identifying carbapenem-resistance genes. Carbapenem-resistance genes common in Indonesia other than those examined by Xpert Carba-R assay in this study (i.e., blaOXA-23 and blaOXA-24) or non-enzymatic mechanisms may also produce resistance in many colonies without the examined genes. Finally, the Xpert Carba-R assay produced faster findings than the culture technique.
Carbapenem-resistance, Xpert Carba-R Assay, Bacterial Colonization, ICU, Infectious Disease, Indonesia
In recent years, a worldwide outbreak of ESBL-producing bacteria led to an increase in the inappropriate use of carbapenem antibiotics and the emergence of carbapenem-resistant organisms (CRO) producing carbapenemase enzymes.1 Bacteria that produce carbapenemase enzymes are resistant to carbapenem and also to broad-spectrum beta-lactam drugs, leaving limited antibiotic therapy options available for them.2 Carbapenemases are generally divided into 3 molecular classes: penicillinases (class A carbapenemases) such as Klebsiella pneumoniae carbapenemase (KPC); metallo-beta-lactamases (class B carbapenemases) such as Imipenemase (IMP), Verona Integron-encoded Metallo-b-lactamase (VIM), and New-Delhi metallo b-lactamase (NDM); and oxacillinase (class D carbapenemases) such as Oxacillinase-48 (OXA-48).2
The increasing prevalence of CRO infection in several countries has become very troubling for patient care, especially in high care or intensive care units (ICU), because it is difficult to treat, extends the length of hospitalization, increases hospitalization costs, and involves high mortality rates.3 CRO infection is associated with CRO colonization as a risk factor, especially in critical patients. Thus it is important to carry out active and regular screening, especially in high-risk patients infected with CRO bacteria in the ICU, to prevent CRO infection.4-8
The World Health Organization (WHO) recommends continuous surveillance of patients with active or suspected CRO infections and individuals with elevated risk factors, including those undergoing multiple antibiotic medications, hemodialysis patients, transplant patients, and patients confined to intensive care.9 Culture surveillance for CRO-colonized asymptomatic patients should also be conducted based on epidemiology and local risk factor analysis.9
The limitations of culture-based surveillance include the requirement for molecular and/or phenotypic means of confirmation, the requirement for 24 to 48 hours for culture growth, issues with specificity and sensitivity based on growth media composition, and the specific enzymes of carbapenemase.10 Recently, molecular testing method, such as the Xpert Carba-R assay made by Cepheid (USA) have been developed to lessen these restrictions. This assay was an FDA-approved automated in vitro test using a multiplex real-time PCR technique to identify the blaNDM, blaOXA-48, blaKPC, blaIMP and blaVIM, genes related to its resistance.10-12 Data on the application of this method in clinical settings is still sparse, especially in Indonesia. In a tertiary hospital in Indonesia, This study evaluated the reliability of the Xpert Carba-R assay in comparison to the culture approach for screening CRO colonization in intensive care patients.
Patients had rectal swab samples obtained using a double sterile swab and then inserted into Stuart’s transport media. Samples that were not processed immediately were stored at 2-8°C with a maximum storage time of 7 days. After the double swab was gently rolled to minimize sampling bias probability, as directed by the manufacturer, one rectal swab was processed onto the test cartridge. The second rectal swab was cultured on MacConkey agar media supplemented with meropenem 2 µg/ml. Bacterial colonies that grew were suspected to be carbapenem-resistant. Identification of bacterial species used the VITEK®2 Compact system with GN card. The molecular approach verified the presence of carbapenem-resistant genes. Next, using primers to identify blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP-1 genes, DNA from CRO isolates was extracted for conventional PCR based on a previous protocol (Table 1).
Table (1):
Primer sequences and product length13,14
| Primer | Primer Sequence (5’-3’) | Product length |
|---|---|---|
| blaKPC | F: TGTTGCTGAAGGAGTTGGGC | 340 |
| R: ACGACGGCATAGTCATTTGC | ||
| blaNDM-1 | F: CTGAGCACCGCATTAGCC | 754 |
| R: GGGCCGTATGAGTGATTGC | ||
| blaIMP-1 | F: CTACCGCAGCAGAGTCTTTG | 587 |
| R: AACCAGTTTTGCCTTACCAT | ||
| blaOXA-48-like | R: GAGCACTTCTTTTGTGATGGC | 744 |
| R: CCCCTCTGCGCTCTACATAC | ||
| blaVIM | F: TGGGCCATTCAGCCAGAT C | 510 |
| R: ATGGTGTTTGGTCGCATATC |
F: forward primer; R: reverse primer
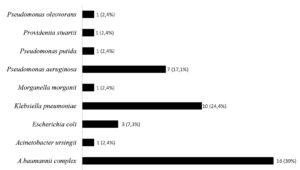
This study was conducted between January to June 2023. A total of 100 rectal swab samples meeting the inclusion criteria were consecutively obtained from hospitalized patients in the critical care unit who were older than eighteen. The Xpert Carba-R assay detected 26 rectal swab samples as positive, with 26 blaNDM genes and one blaVIM gene detected. The culture method detected 31 of 100 samples as positive for CRO colonization. Of the 31 patient samples with CRO colonization, 23 samples were isolated with one CRO species, six samples with two CRO species, and 2 samples with three CRO species. A total of 41 CRO isolates were collected (Figure).
Eleven rectal swab samples that agreed with the results of both the Xpert Carba-R assay and the conventional PCR from isolated colonies on selective media containing meropenem detected the blaNDM gene (Table 2). Carbapenem-resistant bacterial isolates from the concordance samples consisted of two Acinetobacter baumannii complex isolates, seven Klebsiella pneumoniae isolates, one Providencia stuartii isolate, and one Acinetobacter ursingii isolate. One sample obtained two isolates of carbapenem-resistant bacteria and detected blaNDM from rectal swab samples, concordant with PCR results from a Klebsiella pneumoniae isolate with the blaNDM gene. The same rectal swab sample was also detected for the blaVIM gene although PCR results for the Acinetobacter baumannii complex and Klebsiella pneumoniae isolates grown from the culture technique did not detect the blaVIM gene.
Table (2):
Xpert Carba-R assay gene identification in comparison to the culture approach
| Culture Method (gene detection by PCR) | |||||||
|---|---|---|---|---|---|---|---|
| Samples with isolate(s) growth | Samples without isolate growth | Total | |||||
| blaNDM (+) | blaNDM (-) | ||||||
| Xpert Carba-R assay | blaNDM (+) | 11 | 6 | 9 | 26 | ||
| blaNDM (-) | 1 | 13 | 60 | 74 | |||
| Total | 12 | 19 | 69 | 100 | |||
The blaNDM gene was identified via the Xpert Carba-R assay in 6 samples with colony growth where the same gene could not be detected by PCR from its growth isolates, including nine different isolates of the Acinetobacter baumannii complex, three Pseudomonas aeruginosa, two Klebsiella pneumoniae, and one Pseudomonas putida isolate. However, one sample with Pseudomonas aeruginosa colony growth was detected for the blaNDM gene by PCR but not by Xpert Carba-R assay from its swab sample. Nine samples tested positive for blaNDM by the Xpert Carba-R assay that yielded no colony growth on selective meropenem-containing MacConkey agar media. The gene was not found using the Xpert Carba-R assay in a total of 60 samples without colony growth. In addition, there were 13 samples with colony growth in which no resistance gene was found by either the PCR examination or the Xpert Carba-R assay (Table 1).
True positive carbapenem-resistance genes, especially blaNDM, were detected by the Xpert Carba-R assay examination using rectal swab samples and found to be in accordance with conventional PCR examination on isolates grown from carbapenem-resistant selective media in 11 samples. One sample co-presenting the blaVIM gene was not detected by the culture method. True negative results for carbapenem-resistance genes based on both methods were found in 73 samples.
We also identified 16 discordant results between these two methods. Of the 16 samples with discordant results, carbapenem-resistance genes were identified using the Xpert Carba-R assay method in 15 samples from the direct specimens, but not detected based on the culture method, where 9 samples showed no colony growth and 6 samples grew colonies of bacteria. One sample showed growth of two types of colonies of Acinetobacter baumannii complex and Pseudomonas oleovorans, 2 samples showed growth of Acinetobacter baumannii complex, 1 sample showed growth of Klebsiella pneumoniae, 1 sample had Pseudomonas aeruginosa growth, and 1 sample had Pseudomonas putida growth. One sample detected the blaNDM carbapenem-resistance gene in Pseudomonas aeruginosa colony growth through the culture method but the direct detection of the gene from the sample did not yield the same result in the Xpert Carba-R assay method.
In the 9 samples with no colony growth and the 6 samples with colony growth negative for carbapenem-resistant genes based on conventional PCR, the blaNDM detected by the Xpert Carba-R assay was probably carried by too small number of bacteria to be revealed by the culture method.15 The Carba Xpert-R assay was more sensitive for detecting carbapenem-resistance genes than conventional culture methods. Resistant bacterial colonies that did not reveal the targeted genes could result from non-enzymatic resistance mechanisms (i.e., non-carbapenemase producers), such as porin alterations that often occur in Acinetobacter baumannii complex and Pseudomonas species.16-18 Carbapenem-resistance genes other than the genes examined with the Xpert Carba-R assay (i.e. blaOXA-23 and blaOXA-24 genes), especially in Acinetobacter baumannii complex species, also may produce resistant colonies with no detected gene in this setting.14,19 One sample produced a false negative for the blaNDM gene from Pseudomonas aeruginosa that was not detected based on an Xpert Carba-R assay from the direct specimen. This may be due to a small number of bacteria, making it difficult to amplify the target gene to reach the limit of detection of the test.11
In terms of time efficiency, there were wide differences in turn-around time. The Xpert Carba-R assay only took around an hour, including a 48-minute running period, whereas the standard culture method needed 48-72 hours. On the diagnostic side, faster results help in making treatment decisions. This study used rectal swabs to screen CRO carriers so contact precautions could be taken and transmission to other patients could be avoided.
There were limitations to this study. There is no standard procedure for screening cultivation of carbapenem-resistant organisms, so organisms with decreased susceptibility to carbapenem drugs other than meropenem (i.e., ertapenem and imipenem) would be unculturable with meropenem-containing selective media. Thus, growth-isolate-based procedures for carbapenem-resistant organisms are more complex than the molecular-based procedure with the direct specimen. Bacterial colonies could not grow even containing a carbapenem-resistance gene. Moreover, in this study, it was challenging to establish a false positive conclusion in the sample with gene(s) detected by molecular methods from the direct specimens using the Xpert Carba-R assay without applying another molecular method also from the same direct specimens.
This study was the first in Indonesia to evaluate the FDA-approved Xpert Carba-R assay, which may be used as a screening tool for carbapenem-resistant organisms, in the ICU. The results revealed that the assay is more sensitive than the culture method on selective meropenem-containing media and has a faster processing time, making it useful in infection prevention and control strategies.
ACKNOWLEDGMENTS
The authors would like to thank Cepheid for providing Xpert Carba-R assay cartridges and the Institute of Tropical Disease-Unair for laboratory support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MAM, RS, BPS, KK, and EBK conceptualized the study. MAM, AMW, SROS, WS, BPS, PSA, KK, and EBK collected resources. MAM, SROS, and WS performed administration work. MAM, RS, KK, and EBK funding acquisition. AMW, BPS, KK, and EBK supervised the study. MAM, RS, BPS, KK, and EBK applied methodology. MAM, FSW, RS, BPS, KK, TS, and EBK performed analysis. MAM, and FSW performed visualization. MAM, FSW, RS, WS, AMW, SROS, BPS, PSA, KK, TS, and EBK performed data validation. MAM, FSW, RS, KK, and EBK wrote the original draft. MAM, FSW, RS, AMW, SROS, BPS, PSA, KK, TS, and EBK wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was supported by the Japan Initiative for the Japan Agency for Medical Research and Development (AMED) (Grant No. JP23wm0125009 and JP23fk0108664 for T.S.).
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
The study was approved by the Health Ethics Committee of Dr. Soetomo General Academic Hospital, Surabaya, Indonesia (approval number: 0558/KEPK/I/2023).
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Noel A, Huang TD, Berhin C, et al. Comparative evaluation of four phenotypic tests for detection of carbapenemase-producing gram-negative bacteria. J Clin Microbiol. 2017;55(2):510-518.
Crossref - Codjoe FS, Donkor ES. Carbapenem Resistance: A Review. Med Sci. 2017;6(1):1.
Crossref - Kaye KS, Pogue JM. Infections Caused by Resistant Gram-Negative Bacteria: Epidemiology and Management. Pharmacotherapy. 2015;35(10):949-962.
Crossref - Yan L, Sun J, Xu X, Huang S. Epidemiology and risk factors of rectal colonization of carbapenemase-producing Enterobacteriaceae among high-risk patients from ICU and HSCT wards in a university hospital. Antimicrob Resist Infect Control. 2020;9(1):155.
Crossref - Qin X, Wu S, Hao M, et al. The colonization of carbapenem-resistant Klebsiella pneumoniae: epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J Infect Dis. 2020;221(Suppl 2):S206-S214.
Crossref - Zhang D, Cui K, Wang T, et al. Risk factors for carbapenem-resistant Pseudomonas aeruginosa infection or colonization in a Chinese teaching hospital. J Infect Dev Cntries. 2018;12(08):642-648.
Crossref - Odih EE, Irek EO, Obadare TO, et al. Rectal Colonization and Nosocomial Transmission of Carbapenem-Resistant Acinetobacter baumannii in an Intensive Care Unit, Southwest Nigeria. Front Med (Lausanne). 2022;9:846051.
Crossref - Kontopoulou K, Iosifidis E, Antoniadou E, et al. The clinical significance of carbapenem-resistant Klebsiella pneumoniae rectal colonization in critically ill patients: from colonization to bloodstream infection. J Med Microbiol. 2019;68(3):326-335.
Crossref - World Health Organization. Implementation manual to prevent and control the spread of carbapenem-resistant organisms at the national and health care facility level: interim practical manual supporting implementation of the Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. World Health Organization; 2019. https://iris.who.int/handle/10665/312226
- Tato M, Ruiz-Garbajosa P, Traczewski M, et al. Multisite evaluation of cepheid xpert carba-r assay for detection of carbapenemase-producing organisms in rectal swabs. J Clin Microbiol. 2016;54(7):1814-1819.
Crossref - Moore NM, Canton R, Carretto E, et al. Rapid identification of five classes of carbapenem resistance genes directly from rectal swabs by use of the xpert carba-R assay. J Clin Microbiol. 2017;55(7):2268-2275.
Crossref - Traczewski MM, Carretto E, Canton R, Moore NM. Multicenter Evaluation of the Xpert Carba-R Assay for Detection of Carbapenemase Genes in Gram-Negative Isolates. J Clin Microbiol. 2018;56(8):e00272.
Crossref - Mlynarcik P, Roderova M, Kolar M. Primer Evaluation for PCR and its Application for Detection of Carbapenemases in Enterobacteriaceae. Jundishapur J Microbiol. 2016;9(1):e29314.
Crossref - Anggraini D, Santosaningsih D, Saharman YR, et al. Distribution of Carbapenemase Genes among Carbapenem-Non-Susceptible Acinetobacter baumanii Blood Isolates in Indonesia: A Multicenter Study. Antibiotics. 2022;11(3):366.
Crossref - Kim DK, Kim HS, Pinto N, et al. Xpert CARBA-R assay for the detection of carbapenemase-producing organisms in intensive care unit patients of a Korean tertiary care hospital. Ann Lab Med. 2016;36(2):162-165.
Crossref - Quale J, Bratu S, Gupta J, Landman D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2006;50(5):1633-1641.
Crossref - Verma P, Tiwari M, Tiwari V. Efflux pumps in multidrug-resistant Acinetobacter baumannii: Current status and challenges in the discovery of efflux pumps inhibitors. Microb Pathog. 2021;152:104766.
Crossref - Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin Microbiol Infect. 2006;12(9):826-836.
Crossref - Saharman YR, Karuniawati A, Sedono R, et al. Endemic carbapenem-nonsusceptible Acinetobacter baumannii-calcoaceticus complex in intensive care units of the national referral hospital in Jakarta, Indonesia. Antimicrob Resist Infect Control. 2018;7(1):5.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.