ISSN: 0973-7510
E-ISSN: 2581-690X
Pseudomonas aeruginosa is a Gram-negative, rod-shaped bacterium which can cause opportunistic infections in fishes. Ethanolic leaf extract of Moringa oleifera was used in the treatment of Pseudomonas aeruginosa infected Clarias gariepinus for a period of 10 days. Pathogen-free Clarias gariepinus fingerlings (n=120) were randomly distributed into four different groups (A-D). Group A, Infected Clarias gariepinus without treatment; group B, Infected Clarias gariepinus treated with ciprofloxacin 250 mg/mL); group C, Infected Clarias gariepinus treated with 500 mg/L Moringa oleifera extract; and group D, Infected Clarias gariepinus treated with 1500 mg/L Moringa oleifera extract. A 0.1 mL aliquot of 7.2×105 CFU/mL of Pseudomonas aeruginosa culture was intra-peritoneally injected into the body of the fingerlings to induce infection. The gill of fish was excised, homogenized and centrifuged to ascertain oxidative stress, while histological examination followed thereafter. The minimum inhibitory concentration of the ethanol extract was 125 mg/mL while the minimum bactericidal concentration was at 500 mg/mL. Gross morphology examination showed hemorrhage in the gill and mouth, and swollen abdomen, after 72 h of infection. The weight of Pseudomonas aeruginosa infected Clarias gariepinus before and after treatment showed no significant differences (P > 0.05) among the groups. There was significant (p<0.05) reduction in oxidative stress parameters examined. Histopathological changes noticed were minor hemorrhage epitheliocystis and damaged gill lamella. Total bacterial count showed a reduction in the Pseudomonas load in groups C and D over the period of study thus, indicating strong potentials of Moringa oleifera in the control of fish Pseudomonas infection.
Moringa oleifera, Antibiotic Resistance, Pseudomonas aeruginosa, Clariasgariepinus, Histopathology
Drug resistant infections are one of the major public health threats covered by the sustainable development goals. Studies into drug resistance by microorganisms have shown that resistance is not centered on humans alone but also on animals, especially food animals. Antimicrobial resistance (AMR) studies in agriculture covers drug resistance surveillance in animal husbandry, as well as in aquaculture. Aquaculture is one of the greatest contributors to the blue economy of Nigeria. Due to increased aquaculture practice and its consequent financial profit, fish farmers are encouraged to embrace sustainable methods for improvement of the culture system. Fish farming is a major aquaculture practice that impacts the blue economy of the world. Species of Clarias gariepinus, Oreochromis niloticus, amongst others have widely been patronized for consumption.1 This increased demand for fish protein sources gave rise to proliferation/boom in pond fish business especially, across West Africa and Nigeria precisely. C. gariepinus consumption spans across its use in delicacies like barbecues and soups. Thus, explaining the economic impact of fish farming. Fish farming, as a type of aquaculture, has been faced with a major challenge – infection by pathogenic microorganisms. Fish species have been faced with many diseases caused by protozoa, virus, fungi and bacteria.1,2 A common practice in fish farming that induces antimicrobial resistance issues is the use of antimicrobials for therapeutic and growth promoting purposes.
Pseudomonas aeruginosa is a normal flora of fish pond water, which however, causes opportunistic infections (hemorrhagic septicemia, gill necrosis, abdominal distension, friable liver, splenomegaly) in fishes when the pond becomes overcrowded.3 Algamal et al.3 P. areuginosa, a
gram-negative non-spore forming catalase and oxidase positive rod possessed both virulent genes (oprL and toxA) and multi-drug resistance genes (bla Tem, blaCTX-M and tetA) against fish species. Drug resistance, poisoning (due to overdose) and mild impairment of fish metabolism2 were associated with chemotherapeutics bottle necks, while adoption of botanicals has moderated the existing challenges. Administration of medicinal plants to fishes is mainly done by three established methods: oral, immersion or bathing and injection. Previous studies,4,5,6 have shown that medicinal plants can be used as promising antibiotic alternative for fish species, and they mainly act by increasing immune capacity of the fishes to resist infection. Plants such as: Ocimum sanctum,7 Azadiraetha indica,8 Sargassum dupilactum9 inter alia, have been reported. Moringa oleifera is a widely known medicinal plant with a tangible spectrum of activity against some microorganisms, mainly bacteria and fungi.10 M. oleifera has been reported to play meaningful roles in fish farming, ranging from growth promotion,11,12 to fish farm waste water treatment.13 Incorporation of M. oleifera in fish farming appears to be a more sustainable approach in aquaculture practice. Thus, the present study aims to assess the efficacy of Moringa oleifera leaf extract in the control of Pseudomonas aeruginosa infection in fish (Clarias gariepinus).
Experimental fish
Pathogen-free fingerlings of Clarias gariepinus (Catfish)(n=120), with a mean weight of 4.2±0.1 g, were obtained from Freedom Fishery, Nsukka, Enugu State, Nigeria. They were transported to the Department of Zoology and Environmental Biology, wet Lab, University of Nigeria, Nsukka (UNN), for this study. Prior to acclimatization, fishes were disinfected with 0.1% KMnO4 in 5 L of water in a quarantine tank to remove any possible external or internal (Ikele et al.). The fish were randomly distributed into four groups (A-D) in triplicates, group A (negative control), group B (standard drug), group C (50 mg/L ELEMO), and group D (1500 mg/L ELEMO). They were acclimatized for two weeks in a well-aerated chlorine-free tap water pond and were fed daily with commercial feed, Altech copens, Helmond, Netherland.
Animal Ethics
The study was approved by the Department of Zoology and Environmental Biology Postgraduate Committee, University of Nigeria, Nsukka. The fish were handled in accordance with the approved regulatory guidelines of the University of Nigeria, Nsukka Senate Committee on Medical and Research Ethics (UNN-ACC, Protocol No. 0921/2021).
Collection and processing of plant material
Fresh leaves of Moringa oleifera were plucked from the trees of Nsukka Environment and identified in the Herbarium unit (Voucher Number: UNH/021) of the Department of Plant Science and Biotechnology, UNN. The leaves were dried under room temperature, ground into powder, and sieved using 0.351 µm pore cheese cloth. The powdered herbs were concentrated in absolute ethanol for 48 h. The solution was filtered using Whatman filter Paper Number 1, and the filtrate was concentrated using rotary evaporation 0.5-2 L Teflon Scaling Mini Rotary Vacuum Evaporator (Model NoR-1001VN, China) at low temperature (30-40°C). The extract was weighed and refrigerated until further use.
Procurement of ciprofloxacin
Ciprofloxacin tablet (500 mg, May and Becker Nig. Limited) was procured from the Model Pharmacy, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka.
Isolation, characterization and molecular identification of microorganisms
Pseudomonas isolates were obtained from a water sample stored in a reservoir, using one-in-ten-fold serial dilutions in sterile water, and spread-plating on cetrimide agar (Titan Biotech, India), supplemented with 1% glycerol. Culture plates were incubated at 28°C for 24 h aerobically, and resultant mixed cultures from the water sample were separated to pure cultures by streaking according to modified methods of Ikele et al.14 Pseudomonas aeruginosa isolates were presumptively identified using routine biochemical tests. Antibiogram of isolates were profiled using commercial antibiotic discs (Optun, Nigeria), and the multi-drug resistant isolate was adopted for use. Confirmation of the multiple drug resistant isolate was done using 16S rDNA extraction and sequencing, at Inquaba biotech, South Africa, using the zymo biomics kit (USA), Illumina sequencer, and the NCBI website.
In vitro Antibacterial Assessment of Moringa oleifera ethanolic extract against P. aeruginosa
The tube dilution assay was employed to first determine the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) or the ethanolic plant extract. Two-fold dilution of 500 mg of the plant ethanol extract was made serially in sterile water, to get 250 mg/mL, 125 mg/mL, 62.5 mg/mL, 31.25 mg/mL and 15.625 mg/mL. Thereafter, 1 mL aliquot of each diluted extract was transferred into test tubes containing 1 mL peptone water with 0.1 mL 24h Pseudomonas culture. The setup was incubated for 24h at room temperature and turbidity was checked for in each tube. The agar well diffusion method was employed to determine the zones of inhibition of the MIC with Ciprofloxacin as the standard. A 100 µL aliquot of each concentration of plant extracted was placed separately in a well cut in sterile cetrimide Agar (with 1% glycerol) that was already seeded with Pseudomonas sp. The plates were incubated at 28°C for 24h and the diameters of zone of inhibition were measured using a meter rule.15
Infection and Gross Examination of Pseudomonas aeruginosa on Clarias gariepinus
A 0.1 mL aliquot of 7.2×105 CFU/mL of the Pseudomonas aeruginosa culture was intraperitoneally injected into the body of the Clarias gariepinus fingerlings to induce infection. Symptoms such as lethargy, redness of the gill and mouth, and swollen stomach were observed within 72h. Pseudomonas counts on infected tissues was determined by culturing 0.1 mL serially diluted (1 in ten-fold dilution) tissue homogenates on cetrimide agar (with 1% glycerol) at 28°C for 24 hours, using the pour-plate technique.
Weight determination
The weight of the fish pre-infection and post-infection during treatment was measured using weighing balance (model ME4002, Mettler Toledo, China) at nearest 0.01 g.
Evaluation of oxidative stress parameters
A 50 µl of clove oil dissolved in 1000 mL of water was used to anesthetize the fish. The blood was obtained from the vena caudal region of fish from each replicate, using a sterilized medical syringe rinsed with 2.7% EDTA. The serum was obtained pre and post treatment (0-10 days) intervals without anticoagulant and separated from blood by keeping the tubes in a slant position for 2 hours, centrifuged at 3,500 rpm for 10 minutes. The obtained serum was stored at -20°C until further assay.
Tissue lipid peroxidation was estimated through the determination of thiobarbituric acid reactive substance (TBARS) which are indices of membrane lipid peroxidation and expressed as nanomoles of TBARS formed/mg protein.16 Tissue catalase activity (CAT) assay based on the breakdown of H2O2 was also performed as described by Claiborne,17 and expressed as unit/mg protein in the gill and muscle tissues.
The activity of superoxide dismutase activity (SOD) was also determined by the method of Misra and Fridovich,18 and assay was based on the auto-oxidation of adrenalin due to the presence of superoxide anion and the wavelength was read at 420 nm using 752(N) UV-Vis spectrophotometer and the value expressed as a µmol of H2O decomposed/min/mg of protein.
The glutathione peroxidase (GPx) was determined following the protocol of Kumar et al.19 expressed as u/mg protein.
Liver enzymes assay
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphate (ALP), acid phosphatase (ACP) and total protein valueswere determined according to the methods described by Reitman and Frankel,20 and also following the Randox Kit procedures.
Examination of Pseudomonas load of fingerlings
Gill homogenates were obtained periodically and diluted in sterile water using one in ten-fold serial dilution. A 0.1 mL aliquot of the dilution factor tubes 10-2 to 10-4 were cultured aerobically on cetrimide agar supplemented with 0.5% glycerol at 28°C for 24 h, and microbial counts were then taken.
Histological examination
The histological analyses of the tissues were done according to the method described by Ikele et al.21 The fish gill and liver were fixed in vials with 10% buffered paraformaldehyde solution, dehydrated in increasing ethanol concentration (70 to 95%), cleared in xylene and paraffin embedded. Histological sections of 3 µm thickness were performed using LEICA RM 2125 RTS Rotary Microtome. Sections were mounted on glass microscope slides, stained with Mayer’s haematoxylin-eosin, mounted with Canada balsam and examined under Olympus CH Binocular Microscope Photomicrographs were obtained using Motic Images Plus 2.0.
Data Analysis
The Statistical Package for Social Solution (SPSS) version 21 was used. Mean values were analyzed for significant differences (P< 0.05) using the analysis of variance (ANOVA), followed by the post-Hoc test of Duncan multiple range test.
Isolation, identification and antimicrobial profile of Pseudomonas Isolate
The Pseudomonas isolate which showed the most resistance to antibiotics (six out of ten tested antibiotics) was identified with molecular typing as Pseudomonas aeruginosa strain KHRB-P1 (Table 1). M. oleifera had a minimum inhibitory concentration of 125 mg/mL against the multi-drug resistance isolate and a minimum bactericidal concentration of 500 mg/mL as shown in Table 2.
Table (1):
Antibiogram of Pseudomonas aeruginosa strain KHRB-P1
Antibiotics |
Results |
Diameter Zone of Inhibition (mm) |
|---|---|---|
Tarivid |
+ |
0 |
Reflacine |
– |
18 |
Ciprofloxacin |
– |
32 |
Augmentin |
– |
24 |
Gentamycin |
+ |
0 |
Streptomycin |
+ |
0 |
Ceporex |
+ |
0 |
Nalidixic acid |
– |
25 |
Septrin |
+ |
0 |
Ampicillin |
+ |
0 |
+ = Resistant to Antibiotic; – = Not resistant to Antibiotic
Table (2):
Antibacterial activity of Moringa oleifera ethanolic leaf extract against Pseudomonas aeruginosa strain KHRB-P1
Concen. (mg/mL) |
Results |
Diameter Zones of Inhibition(mm) |
|---|---|---|
500 |
+ |
15 |
250 |
+ |
14 |
125 |
+ |
13.6 |
62.5 |
– |
0 |
31.25 |
– |
0 |
15.625 |
– |
0 |
Ciprofloxacin |
+ |
12 |
+ = zones of Inhibition present; – = Zones of Inhibition absent
Effect of M. oleifera ethanolic leaf extract administration on body weight of infected Clarias gariepinus
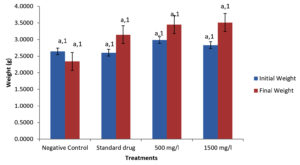
There was a significant (P < 0.05) difference in the weight of Pseudomonas aeruginosa infected Clarias gariepinus pre and post treatment as shown in Figure 1.
Figure 1. Weight of Pseudomonas aeruginosa infected Clarias gariepinus pre and post treatment with Moringa oleifera ethanolic leaf extract
Effect of M. oleifera ethanolic leaf extract administration on oxidative stress parameters of infected Clarias gariepinus
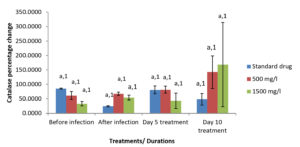
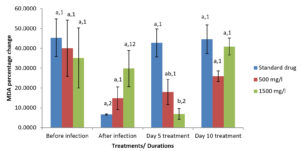
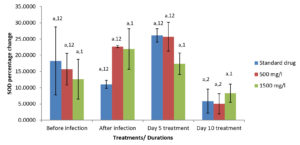
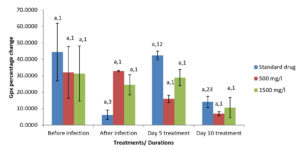
Moringa oleifera ethanolic leaf extract had significant effect (P < 0.05) on the catalase level in the gills of Clarias gariepinus infected with Pseudomonas aeruginosa in the control group and various treatment levels. There were also significant differences on the effect of Moringa olifera ethanolic leaf extract on the malondialdehyde (MDA) level in the gills of Pseudomonas aeruginosa infected Clarias gariepinus across various treatments at P < 0.05. The superoxide dismutase (SOD) levels in the gills of Pseudomonas aeruginosa infected Clarias gariepinus varied significantly (P < 0.05) across various treatments. There was however, no significant effect of Moringa oleifera ethanolic leaf extract of the Gpx level in the gills of Pseudomonas aeruginosa infected Clarias gariepinus (Figure 2-5).
Figure 2. Percentage changes in catalase level of Pseudomonas aeruginosa infected Clarias gariepinus gills pre and post treatment with Moringa oleifera ethanolic leaf extract
Figure 3. Percentage changes in MDA level of Pseudomonas aeruginosa infected Clarias gariepinus gills pre and post treatment with Moringa oleifera ethanolic leaf extract
Figure 4. Percentage changes in SOD level of Pseudomonas aeruginosa infected Clarias gariepinus gills pre and post treatment with Moringa oleifera ethanolic leaf extract
Figure 5. Percentage changes in Glutathione peroxidase (GPx) level of Pseudomonas aeruginosa infected Clarias gariepinus gills pre and post treatment with Moringa oleifera ethanolic leaf extract
Effect of M. oleifera ethanolic leaf extract administration on liver enzyme parameters of infected Clarias gariepinus
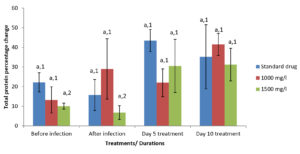
Moringa oleifera ethanolic leaf extract did not show significant effect (P > 0.05) on the AST level (Table 3); however, significant (p < 0.05) differences existed in their ALT, ALP, ACP and total protein values (Tables 4-6 and Figure 6).
Table (3):
Effect of Moringa olifera ethanolic leaf extract of the AST level in the liver of Pseudomonas aeruginosa infected Clarias gariepinus
| Treatments | Treatment duration | |||
|---|---|---|---|---|
| Pre infection | Post infection | Day 5 treatment | Day 10 treatment | |
| Negative Control | 17.33±1.33a,1 | 34.33±6.36a,1 | 30.00±3.51a,1 | 28.33±2.03a,1 |
| Standard drug | 16.67±0.88a,1 | 30.00±1.15a,1 | 18.67±0.88a,1 | 20.67±2.33a,1 |
| 1000 mg/l | 19.67±0.88a,1 | 29.67±3.93a,1 | 17.67±1.45a,1 | 16.33±0.88a,1 |
| 1500 mg/l | 18.00±1.53a,1 | 31.00±3.46a,1 | 26.67±2.73a,1 | 17.67±1.20a,1 |
Data are presented with means±SE, Significant means were separated with different alphabets along the columns for concentrations and numbers along each rows for duration differences using Least Significant Difference (LSD) for each parameter
Table (4):
Effect of Moringa olifera ethanolic leaf extract of the Alanine Transaminase (ALT) level in the liver of Pseudomonas aeruginosa infected Clarias gariepinus
| Treatments | Treatment duration | |||
|---|---|---|---|---|
| Pre infection | Post infection | Day 5 treatment | Day 10 treatment | |
| Negative Control | 20.00±3.21a,2 | 16.67±2.40c,2 | 29.00±6.56a,1 | 34.33±2.60a,1 |
| Standard drug | 17.67±1.45a,1 | 21.33±1.20b,1 | 18.67±3.67b,1 | 16.33±2.85b,1 |
| 1000 mg/l | 20.67±5.17a,2 | 33.33±3.84a,1 | 20.00±2.52b,2 | 17.33±2.19b,2 |
| 1500 mg/l | 23.00±1.15a,1 | 22.00±2.31b,1 | 17.33±1.20b,1 | 21.67±3.93b,1 |
Data are presented with means±SE, Significant means were separated with different alphabets along the columns for concentrations and numbers along each rows for duration differences using Least Significant Difference (LSD) for each parameter
Table (5):
Effect of Moringa olifera ethanolic leaf extract of the acid phosphatase (ACP) level in the liver of Pseudomonas aeruginosa infected Clarias gariepinus
| Treatments | Treatment duration | |||
|---|---|---|---|---|
| Pre infection | Post infection | Day 5 treatment | Day 10 treatment | |
| Negative Control | 34.33±4.98a,2 | 32.33±0.88b,2 | 42.67±1.76a,1 | 32.33±0.88a,2 |
| Standard drug | 36.33±3.38a,2 | 41.00±2.00a,1 | 30.67±0.88b,3 | 29.00±1.53ab,3 |
| 1000 mg/l | 27.67±2.85b,2 | 33.33±0.67b,1 | 29.67±0.33b,12 | 28.00±1.00b,2 |
| 1500 mg/l | 34.00±1.53a,2 | 39.67±1.45a,1 | 29.33±1.45b,3 | 27.67±1.67b,3 |
Data are presented with means±SE, Significant treatment combination means were separated with different alphabets along the columns for concentrations and numbers along each rows for duration differences using Least Significant Difference (LSD).
Table (6):
Effect of Moringa olifera ethanolic leaf extract of the alkaline phosphate (ALP) level in the liver of Pseudomonas aeruginosa infected Clarias gariepinus
| Treatments | Treatment duration | |||
|---|---|---|---|---|
| Before infection | After infection | Day 5 treatment | Day 10 treatment | |
| Negative Control | 41.33±4.98ab,3 | 46.00±2.08c,23 | 63.33±2.19a,1 | 50.33±3.18a,2 |
| Standard drug | 49.67±4.91a,1 | 49.00±9.54c,12 | 40.67±3.38b,2 | 41.00±3.61b,2 |
| 500 mg/l | 40.33±3.28b,2 | 65.67±5.24a,1 | 45.00±3.21b,2 | 45.33±0.33ab,2 |
| 1500 mg/l | 44.00±3.61ab,2 | 57.67±3.67b,1 | 46.33±4.10b,2 | 45.00±3.79ab,2 |
Data are presented with means±SE. Significant treatment combination means were separated with different alphabets along the columns for concentrations and numbers along each rows for duration differences using Least Significant Difference (LSD)
Figure 6. Percentage change in total protein level of Pseudomonas aeruginosa infected Clarias gariepinus liver before and after treatment with Moringa oleifera ethanolic leaf extract
Examination of Pseudomonas load of fingerlings
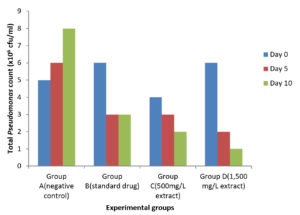
Reduced microbial counts were observed in the treatment groups from the 5th to the 10th day of treatment with both ciprofloxacin and M. oleifera extract. However, there was notable increase in Pseudomonas count in the negative control, over the ten-day monitoring period (Figure 7).
Figure 7. Total Pseudomonas count in Clarias gariepinus after 10 days post treatment with ethanolic leaf extract of Moringa oleifera and standard drug (ciprofloxacin)
Effect of M. oleifera administration of liver and gill tissues of test fingerlings
After 5th day and 10th day treatment with varied concentrations of Moringa oleifera extract, sinusoid enlargement, congestion of central vein and proliferation of cells were observed in liver tissues of both the treated groups and untreated groups throughout the treatment period, as shown in Figure 8.
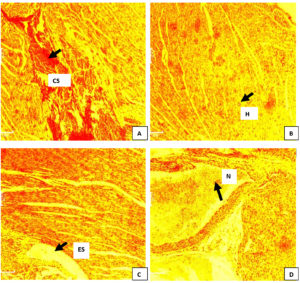
Figure 8. Photomicrograph of liver of C. gariepinus infected with Pseudomonas aeroginosa and treated with different concentrations of ethanol leaf extract of M. oleifera. Group A (infected not treated): severe congestion of sinusoid (black arrow) was observed. Group B (standard drug): Enlarged hepatocyte (black arrow) observed was observed. Group C (500 mg/L): enlargement of sinusoid (black arrow) observed Group D (1500 mg/L). Necrosis (black arrow) of hepatocytes was observed. H&E. mag. 100X
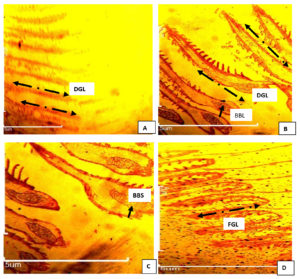
The photomicrograph of gill of the negative control (Group A) showed damaged gill lamella. Likewise, the secondary lamellae of the gill of Pseudomonas infected Clarias gariepinus treated with ciprofloxacin (group B) showed epitheliocystis, characterized with multiple basophilic ball shape attached to the capillary wall of the gill. At 1500 mg/L of extract, fusion of secondary lamellae and proliferation of interlamellar epithelial cells was observed after 5-days treatment. At the end of the 10-days treatment, negative control (group A) showed severely damaged gill lamella; while fusion of secondary lamellae and minor hemorrhage were observed in 500 mg/L leaf extract and 1500 mg/L extract, respectively as shown in Figure 9.
Figure 9. Photomicrograph of gill of C. gariepinus infected with Pseudomonas aeroginosa and treated with ethanol leaf extract of Moringa oleifera. Group A: infected not treated showed severe damaged gill lamella (DGL) (black arrow). Group B (cipro) fusion of secondary lamellae with multiple basophilic ball shape(BBS) (black arrow) attached to the capillary wall associated with epitheliocystis. Group C (500 mg/L) fusion of secondary lamellae with multiple basophilic ball shape attached to the capillary wall associated with epitheliocystis. Group D (1500 mg/L) fusion of secondary lamellae (FGL) by proliferation of interlamellar epithelial cells.Mag. X100.H&E
Histopathology
Changes in the liver of Pseudomonas spp infected Clarias gariepinus showed hypertrophy of hepatocytes, central vein congestion and sinusoid congestion was observed in the liver before treatment as shown in Figure 8 A, B, C and D. Similarly after 5 day and 10 day treatment with varied concentrations of Moringa oleifera extract, sinusoid enlargement, congestion of central vein and proliferation of cells were still observed in both the treated groups and untreated groups throughout the treatment period.
The photomicrograph of gill of infected untreated showed damaged gill lamella (Figure 9). The secondary lamellae of the gill of Pseudomonas-infected Clarias gariepinus treated with ciprofloxacin (group B) showed epitheliocystis characterized by multiple basophilic ball shape attached to the capillary wall of the gill. At 1500 mg/L of extract, fusion of secondary lamellae and proliferation of interlamellar epithelial cells was observed after 5-days treatment. At the end of the 10-days treatment, infected not treated (group A) showed severe damaged gill lamella while fusion of secondary lamellae (Figure 9 A and B), fusion secondary lamellae (Figure 9C) and minor hemorrhage (Figure 9D) were observed in 500 mg/L leaf extract and 1500 mg/L extract, respectively.
Pseudomonas species are known to cause opportunistic infections to fishes in the event the fish health status is under compromise.1,3 This present study evaluated the antibiogram of different Pseudomonas species obtained from pond feed-water. It was observed that the obtained isolates expressed resistance to some of the tested antibiotics. Moringa oleifera which is a well-documented medicinal plant was employed for the control of Pseudomonas fish bacteriosis, as an alternative to antibiotic use. It’s in vitro activity against P. aeruginosa corresponds with the report of Rabilu et al.10 Adoption of M. oleifera in aquaculture for growth promotion and infection control has been reported by Emam et al.11 and Tabassum et al.22 They posited that the nutritional values of this medicinal plant make it a choice destination for phytotherapy incorporation in aquaculture.
The present study reported appreciable weight gain in C. gariepinus fingerlings administered with ethanolic extract of the plant. This finding is in consonance with the reports of Tiimub et al.12 and Tabassum et al.22 who reported the weight gain inducing activity of M. oleifera leaves and seeds, when incorporated into the diets of Cirrhinus mrigala fingerlings. The nutritional capacity of M. oleifera leaves has been reported by Igwilo et al.,23 Emam et al.11 and Tabassum et al.22 Emam et al.11 reported the growth promoting effect of M. oleifera on Nile tilapia.
Impact of the antioxidant roles of the ethanolic leaf extract on the fingerlings, showed the modulation of catalase level, superoxide dismutase and malondialdehyde levels of the test C. gariepinus fingerlings. It appears that the administration of M. oleifera during the treatment of the infected fingerlings showed a marked increase in the listed oxidative stress enzyme markers, which provides a degree of insight on the pathogenicity level of the multi-drug resistant isolate being controlled. One could infer from the data that the pathogenicity of the organism may have induced the production of reactive oxygen species, hydroxyl and hydrogen peroxide moieties into the body system of the fishes,24,25 which consequently got mopped up by the possibly known free radicals- quercetin and chlorogenic acid contained in M. oleifera. The outcome of this process being seen in the increase in catalase, superoxide dismutase and malondialdehyde values of the test fishes. Fishes dosed with 1500 mg/mL extract seemed to have higher anti-oxidant scavenging activity at different periods of administration. Blood liver enzyme profiling of the test fishes showed significant (P< 0.05) decreases in ALT, ALP and ACP values in groups dosed with the leaf extract; in comparison to the negative control. These findings correspond with results reported on ALT and AST activities by Emam et al.11
Antibacterial activity of the leaf extract in vivo was monitored by determining the periodic Pseudomonas counts. There was observed significant (P< 0.05) decrease in P. aeruginosa counts with the administration of the extract. This study probably is one of the few studies that have attempted to study the in-vivo antibacterial activity of this plant extract with C. gariepinus fingerlings as the host. This host-pathogen study using a multi-drug resistant (MDR) strain of P. aeruginosa goes to show that M. oleifera could be one of the potent non-chemotherapeutic means of controlling the emergence of MDR-P. aeruginosa fish infection reported by Algammal et al.3
The histopathological study of the gills showed that gills are the primary initial target of toxicity, and the cytological changes in gills morphology in fish usually occur as a result of contaminant exposure Ikele et al.21 The fusion of secondary lamellae, minor hemorrhage and epitheliocystis observed in the infected-treated group showed respiratory impairment caused by P. aeruginosa. Erosion of the gill epithelium would not only decrease the surface area available for oxygen diffusion but would increase the oxygen distance between water and blood which in turn could cause tissue hypoxia.1,19 The observed swelling of hepatocytes observed in the Pseudomonas-infected treated fish caused congestion and enlargement of sinusoids. The histopathological changes in the current study coincided with the reports of Omeet and Atheer,2 and Magdy et al.,1 on African catfish Clarias gariepinus. Necrotic presentations in the liver tissues indicated cell injury and failed blood supply due to the interaction between the bacteria and host immunological defense system.2
This study has been able to show that Moringa oleifera is a potent biological control agent against Pseudomonas infection in Clarias gariepinus. The extract showed to possess potent antibacterial and antioxidant properties, which were both beneficial in the disease modulation. Since the world has shifted interest to adopting natural antimicrobials and natural antioxidants for fisheries and general animal husbandry, M. oleifera has shown to be a potential alternative from the findings in this study.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The study was approved by the Department of Zoology and Environmental Biology Postgraduate Committee, University of Nigeria and Nsukka Senate Committee on Medical and Research Ethics (UNN-ACC, Protocol No. 0921/2021).
- Magdy L, El-Hady M, Ahmed H, Elmeadawy S, Kenwy A. A Contribution on Pseudomonas aeruginosa Infection in Africa Catfish (Clariasgariepinus).Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(5):575-588.
- Omeet FMH, Atheer HA. Histopathological changes in the liver and spleen of common carp Cyprinuscarpio I. challenged with Pseudomonas aeruginosa (Schroeter, 1872) fed with dietary Chitosan and ciprofloxacin. Basrah Journal of Agricultural Science 2019; 32(2): 193- 07.
Crossref - Algamal AM, Mabrok M, Sivaramasamy E, Youssef FM, Atwa MH, El-Kholy AW, Hetta HF, Hozzein WN. Emerging NDR-Pseudomonas aeruginosa in Fish Commonly Harbor oprLand toxA Virulence Genes and blaTEM, blaCTX-M, and tetAAntibotic Resistance Genes.Scientific Reports 2020;10: 15961.
Crossref - Gupta N, Kar SR, Chakraborty A. A Review on Medicinal Plants and Immune Status of Fish.Egyptian Journal of Aquatic Biology and Fisheries 2021;25(2): 897-912.
Crossref - Adel M, Abedian AA, Zorriehzahra J, Nematolahi A, Esteban MA. Effects of dietary peppermint (Menthapiperita) on growth performance, chemical body composition and hematological and immune parameters of fry Caspian white fish (Rutilusfrisiikutum). Fish and Shellfish Immunology 2015; 45: 841-847.
Crossref - Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B. and Sasal, P. (2014). Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 2014; 433: 50- 61.
Crossref - Logambal SM, Venkatalakshmi S, Michael RD.Immunostimulatory effect of leaf extract of Ocimum sanctum Linn. inOreochromismossambicus (Peters). Hydrobiologia 2000; 430: 113–120.
Crossref - Thanigaivel S, Vijayakumar S, Gopinath S, Mukherjee A, Chandrasekaran N, Thomas J. In vivo and in vitro antimicrobial activity of Azadirachtaindica (Lin) against Citrobacterfreundii isolated from naturally infected Tilapia (Oreochromismossambicus). Aquaculture 2015; 437: 252-255.
Crossref - Immanuel G, Sivagnanavelmurugan M, Balasubramanian V, Palavesam A. Effect of hot water extracts of brown seaweeds Sargassum spp. on growth and resistance to white spot syndrome virus in shrimp Penaeusmonodonpostlarvae. Aquaculture Research 2010; 41: 545-553.
Crossref - Raubilu IA, Isah U, Ahmad MB. Antimicrobial Activity of Moringaoleifera: A Short Review. Bayero Journal of Pure and Applied Sciences 2019;12 (1): 128-132.
Crossref - Emam MA, Shourbela RM, El-Hawany WN, Abo-kora SY, Gad FA, El-Latif AMA, Dawood MAO.Effects of Moringaoleifera Aqueous Extract on the Growth Performance, Blood Characteristics, and Histological Features of Gills and Livers in Nile Tilapia.Aquaculture and Fisheries 2021.
Crossref - Tiimub BK, Mpanga IK, Tiimub GL, Tiimub RW, Baani I, Tiimub EL. Effect of Moringaoleifera Feed Supplements on All-Male Tilapia Growth Performance at Tano-Dumasi Pilot Aquaculture Center. EAS Journal of Biotechnology and Genetics 2020; 2(5): 67-83.
Crossref - Tong CY, Yusuf FH, Derek CJC. Fish Farm Waste Water Treatment using Moringaoleifera Seed Powder as Natural Coagulant.Earth and Environmental Science2021; 945: 012070.
Crossref - Ikele MO, Ezeonu IM, Umeh CN. Prebiotic Roles of Ocimumgratissimum Extract in the Control of Colibacillosis in Broilers. Journal of Animal Health and Production 2020;8(4): 206-211.
Crossref - Kabir OA, Olukayode O, Chidi EO, Christopher CI, Kehinde AF. Screening of Crude Extracts of Six Medicinal Plants used in South-West Nigeria Unorthodox Medicine for Anti-methicillin Resistant Staphylococcus aureusActivity.BMC Complement Alternative Medicine 2005;5:1-7.
Crossref - Lushchak VI, Bagnyukova TV, Husak VV, Luzhna LI, Lushchak V, Storey KB. Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. International Journal of Biochemistry and Cell Biology2005; 37(8): 1670 – 1680.
Crossref - Claiborne AL. Catalase activity. In : CRC Handbook of Methods for Oxygen Radical Research . CRC Press, Florida 1986.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry 1972;247(10): 3170-3175.
Crossref - Kumar S, Raman RP, Kumar K, Pandey PK, Kumar N, Mallesh B, Kumar A. Effect of azadirachtin on haematological and biochemical parameters of Argulus infested goldfish Carassiusauratus (Linn. 1758). Fish Physiology and Biochemistry2013;39(4): 733–747.
Crossref - Reitman S, Frankel S. Colorimetric Methods for the Determination of Serum Transaminases. American Journal of Clinical Pathology1957; 28: 56-61.
Crossref - Ikele BC, Obiezue RN, Okoye IC, Ikele M O. Histopathology And Activities Of Proteases , Aminases And Glutamate Dehydrogenase In Clariasgariepinus Fingerlings Exposed To Linear Alkyl Benzene Sulphonate ( LAS ). Advances in Life Science and Technology 2014:16: 2224-7181.
- Tabassum S, Hussain SM, Ali S, Arsalan MZ, Ahmad B, Asar M, Sharif A. Partial Replacement of Fish Meal with Moringaoleifera Leaf Meal in Practical Diets of Cirrhinusmrigala Fingerlings. Brazilian Journal of Biology. 2021; 83: e246333.
Crossref - Igwilo IO, Okonkwo JC, Ugochukwu GC, Ezekwesili CN, Nwenyi V. Comparative Studies on the Nutrient Composition and Anti-nutritional Factors in Different Parts of Moringaoleifera Plant Found in Awka, Nigeria. The Bioscientist 2017;5(1): 1-12.
- Mao C, Yuan J, Lv Y, Gao X, Yin Z, Kraus VB, Luo J, Chei C, Matchar DB, Zheng Y. Associations between Superoxide Dismutase, Malondialdehyde and All-cause Mortality in Older Adults: A Community-Based Cohort Study. BMC Genetics 2019; 19:104.
Crossref - Olaoye AB, Ologunde CA, Molehin OR, Nwankwo I. Comparative Antioxidant Analysis of Moringaoleifera Leaf Extracts from South Western States in Nigeria. Future Journal of Pharmaceutical Sciences 2021;7: 68.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.