ISSN: 0973-7510
E-ISSN: 2581-690X
Rapid nucleic acid assays have been approved by FDA for managing the COVID-19 pandemic, however its analytical efficiency has not been thoroughly validated. This study evaluates the detection and identification of COVID-19 virus using Abbott ID-Now to rapidly identify cases and intervention practices in comparison to nucleic acid detection. Nasopharyngeal Swabs collected from 611 participants were tested for Abbott ID-NOW and LabGun COVID-19 ExoFast RT-PCR Kit as per manufacturer’s protocol. The results from the ID NOW™ COVID-19 assay were evaluated by comparing results with the standard RT-PCR, which served as a standard reference. The infection burden of SARS-CoV-2 in the population of UAE was 11.62%. Compared to detection using real time-based platforms, the sensitivity, specificity, positive and negative predictive values of the ID-Now were 84.51%, 99.81%, 98.36% and 98.00% respectively for COVID-19. A stratified analysis was also carried out using cycle threshold (Ct) values categorizing as Ct>33 as with low viral loads while those with Ct<33 as high. This demonstrated statistically significant (P<0.0001) decrease in sensitivity in samples (97.87% in low Ct value samples versus 58.33% in high Ct value samples). Even though the sensitivity for Abbott ID NOW™ in this study was lower, the specificity, positive predictive values and negative predictive values were significant in low viral load samples. It is easy to use and interpret, giving early information to support clinical decision-making ID-NOW could be possibly used as a point-of-care test after evaluation in epidemic and endemic settings.
COVID-19, ID NOW, Diagnostic testing, SARS-CoV-2, Rapid tests
Globally, roughly 450 million confirmed cases, including 6 million deaths of COVID-19, has been reported to WHO1; however, owing to limited resource for diagnostic testing in underprivileged regions and inaccurate COVID-19 data reporting to the WHO, the current data likely represent only a fraction of actual infections and mortality from the COVID-19 pandemic.2
The COVID-19 pandemic response has shown that diagnostics are crucial. Several innovative diagnostic tests for COVID-19 have been developed. Three primary diagnostic test types are available for the diagnosis and treatment of the patient: serology tests that identify the host’s antibodies in response to the infection or vaccination; antigen tests that identify viral proteins; and nucleic acid detection tests that identify viral RNA.3 As per the World Health Organization, for identifying and confirming SARS-CoV-2, the gold standard method is real time RT-PCR.4 The demand for COVID-19 diagnostic testing is still very significant following the recent global outbreak. A robust and efficient testing infrastructure is critical in identifying people infected with COVID-19 for a health system to reduce transmission and limit the spread of COVID-19 disease. Acknowledging the fast-developing COVID-19 pandemic, the U.S. Food and Drug Administration (FDA) implemented emergency use authorization enabling the adoption of various molecular assays for in vitro diagnosis.5
Numerous rapid nucleic acid detection tests have received emergency authorization, such as ID NOW™ COVID-19 (Abbott), Xpert® Xpress SARS-CoV-2 (Cepheid), and SimplexaTM COVID-19 Direct (Diasorin), detect COVID-19 in 15-90 minutes.6 The Abbott ID-NOWTM
COVID assay uses isothermal nucleic acid amplification (Nicking enzyme Amplification reaction- NEAR) of the viral target RNA (RdRp) in comparison to the other assays with a manufacturer-stated limit of detection (LOD) of 125 genome equivalents/ml. Its turnaround time is between 5 and 13 minutes depending upon whether the sample is positive or negative, and it is designed to be utilized as a point-of-care test (POCT).7,8 Results from most moderate-to high-complexity laboratory tests, like RT-PCR, take many hours, often 8 to 24 hours as samples. The length of time it takes for a doctor or patient to get a test result depends on how frequently the test is performed at the lab; typically, tests are done in batches, which may prolong turnaround times. Also, trained technical personnel are required to man such laboratories.
The objective of this study was to access the test characteristics of Abbott ID-NOW™ to a Real Time RT-PCR-based assay for detection of SARS-CoV-2 for determine its efficacy for patient testing. We have assessed these parameters in patients with low and high virus loads determined by cycle threshold (Ct) values above and below 33.
A hospital-based retrospective cross-sectional study was conducted at the Molecular Biology Laboratory of our hospital between December 2021 and March 2022. This project was reviewed and approved by the Institutional Review Board (BH/REC/019/22) and the Abu Dhabi Health Research and Technology Ethics Committee – Department of Health (DOH/CVDC/2022/1460). All symptomatic and asymptomatic patients attending the COVID-19 Testing Clinic were included in this study. Two nasopharyngeal swabs were collected per patient: One dry swab was placed into a 15 ml centrifuge tube, while the other one was placed into a viral transport medium (HiViralTM Transport Medium, HiMedia Laboratories LLC). The samples were placed in biohazard plastic bags and transported immediately to the laboratory for testing. Dry swabs samples (n=611) were tested by Abbott ID NOW™ and reports were released within two hours from the time of collection, with a disclaimer “confirm all NEGATIVE RAPID PCR test with the routine real time PCR”. Swabs in viral transport medium were tested by real time platforms using the LabGun™ COVID-19 ExoFast RT-PCR Kit (LabGenomics, Korea) within 24 hrs.
Abbott ID NOW COVID-19 assay
The ID NOW™ Instrument is a rapid molecular in vitro diagnostic test that makes use of isothermal nucleic acid amplification technology and identifies the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) gene segment. Fluorescently labelled molecular markers are used to precisely identify each target of the amplified RNA and is made to detect SARS-CoV-2 viral nucleic acid qualitatively in direct anterior nasal, nasopharyngeal, or throat swabs from individuals who have COVID-19-like symptoms. According to the manufacturer’s guidelines, it takes roughly 13 minutes to complete. It has the FDA emergency use approval. The results of the test are populated on the instrument screen once the test is completed.
Comparator: RT-PCR assay kit
The LabGun™ Exofast COVID-19 RT-PCR Kit is a CE-IVD certified RT-PCR assay intended to identify SARS-CoV-2 virus RNA in respiratory specimens. The primers and probes set are specific to detect the N and RdRp genes of the SARS-CoV-2 virus. The comparator assay was validated in-house for routine diagnostic of SARS-CoV-2 detection in respiratory specimens. Roche MagNA Pure 96 DNA and Viral NA Small Volume Kit was used to isolate and purify nucleic acids from nasopharyngeal tissue. The isolated nucleic acid was amplified directly on the ABI QuantStudio™ 5 Dx Real-Time PCR System using this kit which targets the RdRp and N genes.
Statistical analysis
The results from the ID NOW™ COVID-19 assay were assessed to those from the standard RT-PCR, which served as a standard reference. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were analyzed, along with the corresponding Wilson 95% confidence intervals (CI). The results were shown as mean±SD., The Fisher’s exact test and chi-square test were performed for categorical variables. SPSS was employed for statistical analysis, with a statistically significant p-value of 0.05.
The performance characteristics were recalculated in consideration of previous literature on Abbott ID-Now’s performance in samples with a low viral load. Based on the prior literature that was accessible, the samples from the RT-PCR positive patient were categorized as low viral load (defined as Ct≥33) and high viral load (Ct<33).9
The study was conducted during a span of a year namely from December 2021 to March 2022. It has included 611 individuals, were the mean age of the patients was 35.67 (SD±16.03) years, majority were males (58.26%). Most of the patients were adults (86.60%) staying in and around the study site, baseline patient demographics data summarized in Table 1. Table 2 summarizes Abbott ID-NOW™ performance characteristics. The disease burden of SARS-CoV-2 infection was 11.62%.
Table (1):
Baseline distribution of patient demographics data
| Demographic data | Value | |
|---|---|---|
| Sex | Female | 255 (41.73%) |
| Male | 356 (58.26%) | |
| Mean age | 35.67 ± 16.03 | |
| SARS CoV-2 RT PCR Positive | 71 (11.62%) | |
| Total (N) | 611 | |
Table (2):
Test characteristics of Abbott ID-NOW™ for detection of SARS-CoV-2
| Performance of ABBOTT ID-NOW for detection of SARS-CoV-2 | ||||
|---|---|---|---|---|
| RT-PCR Results | Total | |||
| Positive | Negative | |||
| ABBOTT ID- NOW™ RDT | Positive | 60 | 1 | 61 |
| Negative | 11 | 539 | 550 | |
| Total | 71 | 540 | ||
| Statistic | Value | 95% Confidence interval | ||
| Sensitivity | 84.51% | 73.97% to 92.00% | ||
| Specificity | 99.81% | 98.97% to 100.00% | ||
| Positive Likelihood Ratio | 456.34 | 64.23 to 3242.01 | ||
| Negative Likelihood Ratio | 0.16 | 0.09 to 0.27 | ||
| Disease prevalence (*) | 11.62% | |||
| Positive Predictive Value (*) | 98.36% | 89.41% to 99.77% | ||
| Negative Predictive Value (*) | 98.00% | 96.61% to 98.83% | ||
| Accuracy (*) | 98.04% | 96.59% to 98.98% | ||
(*) These values are dependent on disease prevalence.
Table (3):
Test Characteristics Of Abbott ID-NOW™ for detection of SARS-CoV-2, stratified by cycle-threshold value <33
| Performance of ABBOTT ID-NOW for detection of SARS-CoV-2 | ||||
|---|---|---|---|---|
| RT-PCR Results | Total | |||
| Positive (CT VALUE <33) | Negative | |||
| ABBOTT ID- NOW™ RDT | Positive | 46 | 1 | 47 |
| Negative | 1 | 539 | 540 | |
| Total | 47 | 540 | ||
| Statistic | Value | 95% Confidence interval | ||
| Sensitivity | 97.87% | 88.71% to 99.95% | ||
| Specificity | 99.81% | 98.97% to 100.00% | ||
| Positive Likelihood Ratio | 528.51 | 74.58 to 3748.59 | ||
| Negative Likelihood Ratio | 0.02 | 0.00 to 0.15 | ||
| Disease prevalence (*) | 8.16% | |||
| Positive Predictive Value (*) | 97.91% | 86.88% to 99.70% | ||
| Negative Predictive Value (*) | 99.81% | 98.70% to 99.97% | ||
| Accuracy (*) | 99.66% | 98.77% to 99.96% | ||
(*) These values are dependent on disease prevalence.
Table (4):
Test characteristics of ABBOTT ID-NOW™ for detection of SARS-CoV-2, stratified by cycle-threshold value ≥33
| Performance of ABBOTT ID-NOW for detection of SARS-CoV-2 | ||||
|---|---|---|---|---|
| RT-PCR Results | Total | |||
| Positive (CT Value ≥33) | Negative | |||
| ABBOTT ID- NOW™ RDT | Positive | 14 | 1 | 16 |
| Negative | 10 | 539 | 549 | |
| Total | 24 | 540 | ||
| Statistic | Value | 95% confidence interval | ||
| Sensitivity | 58.33% | 36.64% to 77.89% | ||
| Specificity | 99.81% | 98.97% to 100.00% | ||
| Positive Likelihood Ratio | 315.00 | 43.18 to 2297.87 | ||
| Negative Likelihood Ratio | 0.42 | 0.26 to 0.67 | ||
| Disease prevalence (*) | 4.25% | |||
| Positive Predictive Value (*) | 93.33% | 65.71% to 99.03% | ||
| Negative Predictive Value (*) | 98.18% | 97.11% to 98.86% | ||
| Accuracy (*) | 98.05% | 96.54% to 99.02% | ||
(*) These values are dependent on disease prevalence.
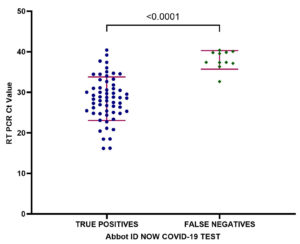
The test characteristics of the analysis based on the mean Ct values for the targets (RdRp and N genes specific to the SARS-CoV-2 virus) were summarized in Tables 3 and 4. A stratified analysis by Ct values showed a statistically significant (P<0.0001) drop in sensitivity in patients with low Ct values 97.87% (95% CI: 88.93% to 99.95%) versus 58.33% (95% CI: 36.64% to 77.89%) in patients with high Ct values. Figure illustrates the mean Ct values of all RT-PCR positive patients, the correlation between false negative ID-NOW™ tests and higher Ct values.
The world has seen the destructive power of COVID-19, one of the worst public health crises. The primary issues related to a sudden lack of resources include intensive care unit capacity, hospital admission capacity and individual protection equipment. It was crucial to have a reliable method for determining which patients need hospital isolation, especially those who required intense or less-intensive care. Additionally, RT-PCR testing requires a lengthy procedure time because each test swab must be delivered to the hub center for processing and analysis takes hours. For SARS-CoV-2 testing, accurate results and fast turnaround times are crucial not only for patient management but also for preventing the illness from spreading throughout community.10
In most patients, the symptoms of COVID-19 were mild and does not indicate any urgent medical intervention or hospitalization, essentially in vaccinated individuals.11 Early detection could avoid additional strain on an already stretched healthcare infrastructure. To identify the epidemiologic burden, the extensive use of clinical testing is important, but the availability of resources for testing is variable across the globe. The availability of sophisticated testing for viral disease heavily depends on the resources, which are very much limited to such drastic number of samples from the mild symptomatic outpatients.
In comparison to RT-PCR, our study showed that ID-NOW™ has a 99.81% specificity and 84.51% sensitivity for detecting SARS-CoV-2. All the RT-PCR positives were confirmed to be true positive patients by repeat testing using a second swab as is the policy laid down by the regulatory bodies on this country. The discrepancy between the two tests were seen in 12 samples; one of the ID-NOW™ positives were determined to be false positive with negative repeated testing on RT-PCR. Eleven samples were considered as false negatives by Abbott ID Now™. As recommended for both RT PCR as well as for rapid PCR tests the potential for false-negative results exists, due to either variation of sampling or viral load. Hence, a single negative test does not exclude viral infection in symptomatic patients. If the suspicion for COVID-19 remains, in terms of symptoms that are highly suggestive repeat testing is advised for prognostic and infection control.
The test performance characteristics indicated a statistically significant (P<0.0001) reduction in sensitivity at low viral loads (97.87% in low Ct value<33 patients versus 58.33% in high Ct value>33 patients). The small sample size of high Ct value patients (n = 24) is a limitation of the current study. The disparity between the assays could be due to probable variation in sampling and the fact that some of the samples were from repeat testing of COVID-19 positive patients on treatment who had higher Ct values indicating lower viral burden. Patients who shed low viral load appears to be less contagious and rapid detection might be the advantage of POCT with high sensitivity to detect contagious patients in the early patient care decisions.12
Also, we should keep in mind that the number of asymptomatic patients might have played a major role in the low positive results. The variation of results among the methods might be due to the Limit of Detection of RT-PCR being much lower than the isothermal PCR ID NOW assay, samples should be expected to be positive for longer period even after the peak viral infection load.13 Much relevant to this statement, our analysis showed that the false negativity of ID Now was found to be 90.9 % in the less viral load population (mean CT value>33). Another fact which may contribute to the false negative might be the sampling difference (dry swab and swab in viral transport media), yet many studies have stated that the RNA stability of SARS-CoV-2 in dry swab is stable upto 9 days.14-16 We have processed the samples of the patient group within 1 hour from the time of sample collection.
Our study suggests that Abbott ID-Now™ can be used as a POC test for COVID-19 in health facilities without expensive equipment and technical expertise when the results are desired immediately, especially in symptomatic/acute cases. This is especially useful in outbreak situations when large numbers of samples are expected for testing. It performed very well in comparison to the reference standard. From symptomatic patients with high viral loads to patients visiting the emergency department, ID NOW™ can be used as a rapid POC test for the diagnosis of COVID-19 and proceed with the isolation of patient at the earliest. However, there is a chance that ID NOW™ might miss infections in the symptomless infected population with low viral loads. Therefore, negative results should be co-related to clinical signs and symptoms of COVID-19 and repeat testing must be advised. It may be wise to restrict using Abbott ID for asymptomatic cases considering the variability in viral loads in this population.
In conclusion, the ID NOW™ test may be utilized as a point-of-care test since it is simple to perform and interpret. It can provide early information to support clinical decision-making. However, they must be reviewed before being used for routine or point-of-care diagnostics.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Shamyla Siddique and Dr. Khaled Musallam for their constant cooperation and support throughout the span of work. Authors also would like to convey thanks to Mr. Mayur Sabhani, Group Director – Laboratory Services, Mr. Adil Hussain, Lab operation manager, for providing all the facilities to carry out this project in the laboratory.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
OAJ and SO designed the study. SO supervised the study. JA performed data collection. OAJ carried out the laboratory testing. OAJ, MMPV, AB, VA, MS and SO performed data analysis. OAJ drafted the manuscript. AB, MS, JA and VA reviewed the manuscript. MMPV and SO edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Review Board (BH/REC/019/22) and the Abu Dhabi Health Research and Technology Ethics Committee – Department of Health (DOH/CVDC/2022/1460).
- World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed Dec 15, 2022.
- Mathieu E, Ritchie H, Rodיs-Guirao L, Appel C, Giattino C, Hasell J, et al. Coronavirus Pandemic (COVID-19). https://ourworldindata.org/coronavirus. Accessed Dec 15, 2022.
- Peeling RW, Heymann DL, Teo YY, Garcia PJ. Diagnostics for COVID-19: moving from pandemic response to control. Lancet. 2022;399(10326):757-768.
Crossref - Wu J, Liu J, Li S, et al. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med Infect Dis. 2020;37:101673.
Crossref - U.S. Food and Drug Administration, In Vitro Diagnostics EUAs. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas#individual-molecular. Accessed Dec 15, 2022.
- Zhen W, Smith E, Manji R, Schron D, Berry GJ. Clinical evaluation of three sample-to-answer platforms for detection of SARS-CoV-2. J Clin Microbiol. 2020;58(8):e00783-20.
Crossref - U.S. Food and Drug Administration, In Vitro Diagnostics EUAs. https://www.fda.gov/media/136525/download. Accessed Dec 20, 2022.
- Abbott Diagnostics Scarborough, Inc. ID NOW COVID-19 Product Insert. https://www.alere.com/en/home/product-details/id-now-covid-19.html. Accessed Dec 15, 2022.
- Ramachandran A, Noble J, Deucher A, Miller S, Tang PW, Wang RC. Performance of Abbott ID Now rapid nucleic amplification test for laboratory identification of COVID-19 in asymptomatic emergency department patients. J Am Coll Emerg Physicians Open. 2021;2(6):e12592.
Crossref - Ward S, Lindsley A, Courter J, Assa’ad A. Clinical testing for COVID-19. J Allergy Clin Immunol. 2020;146(1):23-34.
Crossref - Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474-1484.
Crossref - Hinson JS, Rothman RE, Carroll K, et al. Targeted rapid testing for SARS-CoV-2 in the emergency department is associated with large reductions in uninfected patient exposure time. J Hosp Infect. 2021;107:35-39.
Crossref - Tu YP, Iqbal J, O’Leary T. Sensitivity of ID NOW and RT-PCR for detection of SARS-CoV-2 in an ambulatory population. Elife. 2021;10:e65726.
Crossref - Alfaro-Nunez A, Crone S, Mortensen S, et al. SARS-CoV-2 RNA stability in dry swabs for longer storage and transport at different temperatures. Transbound Emerg Dis. 2022;69(2):189-194.
Crossref - Mosscrop L, Watber P, Elliot P, et al. Evaluation of the impact of pre-analytical conditions on sample stability for the detection of SARS-CoV-2 RNA. J Virol Methods. 2022;309:114607.
Crossref - Gordhan BG, Ealand CS, Kana BD. Survival and detection of SARS-CoV-2 variants on dry swabs post storage. Front Cell Infect Microbiol. 2022;12:1031775.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.