ISSN: 0973-7510
E-ISSN: 2581-690X
Reverse transcription-quantitative PCR (RT-qPCR)-based assays are extensively being utilized to detect coronavirus disease 2019 (COVID-19). However, due to a lack of RT-qPCR testing capability, these tests cannot be carried out in community clinics. The intention of our study was to evaluate the specificity and sensitivity of Rapid Antigen Detection (RAT) tests versus those of RT-qPCR using nasopharyngeal and oropharyngeal specimens. Respiratory swab specimens were collected from the COVID-19 patients admitted at Dr. Bhimrao Ambedkar Memorial Hospital, Raipur, CG, India, during March to April 2022. RAT and RT-qPCR were performed using standard methods as per guidebook instructions, and subjects were chosen using a convenience sample technique. 100 swabs from patients, who had earlier verified positive and 100 from who had earlier verified negative for SARS-CoV-2 via RT-qPCR, were taken for study. Study was approved by the institutional ethical committee before data collection and initiation of the study. We evaluated for the sensitivity and specificity of the STANDARD Q COVID-19 Ag test kit (SD Biosensor). On testing, an over-all sensitivity and specificity of the kit was recorded as 74% and 100%, respectively in comparison to the RT-qPCR kit. Further, the assay’s sensitivity was shown to be 100%, 94.87%, 77.27%, and 55.56%, respectively, for samples with cycle thresholds (Ct) of 15-25, 25-30, 30-35, and >35. We draw the conclusion that the RT-qPCR assay has superior sensitivity and specificity to the antigen assay. However, in all situations where RT-qPCR testing is difficult, the antigen assay could serve as a rapid and simple option for separating SARS-CoV-2 contagious from non-contagious patients.
Rapid Antigen Detection, RAT, COVID-19, RT-qPCR, Ag-detection, SARS-CoV-2
Ongoing pandemic, COVID-19, is a result of SARS-CoV-2 and has symptoms like pneumonia, and more intense death. COVID-19 exposed as a singular human pathogen from Wuhan, China and emerged at the give up of 2019.1 As in keeping with WHO data of twenty third, December 2022, there were 651,918,402 showed instances of COVID-19, consisting of 6,656,601 deaths and as of twenty first, December 2022, a total of 13,073,712,554 vaccine doses have been administered (https://covid19.Who.Int/) to save you the unfold of disease.2 Once individual infected with SARS-CoV-2, right away patient isolation and documentation are crucial to stopover the spread of the contamination caused by the virus. Specific and properly-timed identity of beyond and acute infections with SARS-CoV-2 is an essential need of hour.3 RT-qPCR check is the most effective method for diagnosing COVID-19 using nasopharyngeal, and oropharyngeal swabs. Preferred RT-qPCR procedures are sensitive and unique but they may be laborious and luxurious.4 RT-qPCR assessments necessitate properly-prepared settings and hospitals and they’re restrained to neighborhood clinics in addition to authorities hospitals where the suspect desires to take a look at on an pressing basis. Therefore, patients’ specimens want to be transported to the well-equipped laboratory which has RT-qPCR setup.5 Packing, transportation, and turnaround time (ToT) of the RT-qPCR check delay the the test findings and heightened the fear of those who may have COVID-19.6-10 Rapid Antigen Test (RATs) for COVID-19, which do not need any devoted device or a variety of cash, have been legitimate for clinical utilization by means of WHO, everywhere in the international to help with this COVID-19 contamination condition and may be accomplished in short temper.11-12 RATs are believed to be less touchy and specific than the RT-qPCR, additionally tests end result achieves in a lesser amount of time. However, Nucleic acid amplification strategies are available with a time of much less than 1 hour such as CB-NAAT, Turenat but again those setups needed exclusive type of machine and facilities. Currently, SARS-CoV-2 unique antigen assays have come to be a short and clean replacement for nucleic acid amplification assays. With this thought, in current study we evaluated specificity and sensitivity of SARS-CoV-2 RAT kit with that of RT-qPCR kit.
Respiratory nasopharyngeal and oropharyngeal swabs were collected from patients admitted to Dr. Bhimrao Ambedkar Memorial Hospital, Raipur, CG, India, to verify whether SARS-CoV-2 viral nucleic acid has been detected or not, with a gold star the Thermo Fisher Scientific’s CoviPathTM COVID-19 RT-qPCR Kit (Applied BiosystemTM) was used. A total 100 positive and 100 negative RT-qPCR samples were taken and same were used for the further RAT evaluation. To gather samples from the participants, convenience sampling was used. After taking the approval from Institutional Ethical Committee (IEC), Pt. JNM Medical College, Raipur, CG, India, study was started. All samples were collected from March 2022 to April 2022 after collection samples were transported to state’s Virology Research and Diagnostic Laboratory (VRDL), Pt. JNM Medical College, Raipur, CG, India, for examination. Sensitivity and specificity of SARS-CoV-2 Rapid Antigen Test (SD-Biosensor Ag-test kit) was compared with the Thermo Fisher Scientific’s CoviPathTM COVID-19 RT-PCR Kit (Applied BiosystemTM). A total of 100 positives and 100 negative samples were taken to test the efficacy of the RAT (SD Biosensor Ag-test kit). This antigen kit is a fast immunochromatographic assay for identifying IgM/IgG antibodies to SARS-CoV-2 in samples of human serum, plasma, or whole blood. In accordance with the manufacturer’s instructions, specimen were examined using the lateral flow assay SARS-CoV-2 Rapid Antigen Test, without a temporary interruption of the freeze-thaw cycle, 300 μL of swab transport medium and manufacturer-supplied extraction buffer were mixed during the procedure. The mixture was thoroughly mixed and slightly twisted before being applied in three drops (10 µL) to the lateral flow device. The test outcome was announced following a 15-30 minute after incubation period at room temperature. Swab samples showing the control line alone were considered SARS-CoV-2 antigen negative, while samples displaying the SARS-CoV-2 antigen was deemed to be present in both the control line and the test line. The SARS-CoV-2 RAT, was used to examine nasopharyngeal swabs from patients who had tested positive for SARS-CoV-2 RNA using the gold star SARS-CoV-2 RT-PCR Kit which covered an extensive range of cycle thresholds (ct). In case of COVID RT-qPCR kits targeted confirmatory genes were ORF1ab and N genes. Cq values cut-off ≤37 for both were considered as positive. In each run for the Positive Control (PC) to pass, the N gene and ORF1ab must be detected, as measured by the Cq cutoff values and for the Negative Control (NC) to pass, the N gene, ORF1ab, and RNase P must not be detected, as measured by the Cq cut-off values. RT-qPCR was carried out using a redesign software on a Biorad CFX96 Real Time PCR equipment; UNG incubation 2 min at 25°C (1 cycle), reverse transcription 10 min at 53°C (1 cycle), activation 2 min at 95°C (1 cycle), denaturation and annealing 3 sec and 30 sec at 95°C and 60°C (40 cycles), respectively.
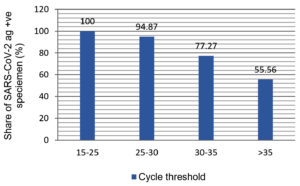
All nasopharyngeal and oropharyngeal swabs were collected from patients using sticking a sterile swab inside the nostril and oropharynx of the patient, by gradually rotating and extending the surface of the posterior nasopharynx and oropharynx, swab was pushed until resistance is encountered at the turbinate level. After collection all samples were submitted to VRDL, Pt. JNM Medical College, Raipur, CG, India, for testing and confirmation of SARS-CoV-2 viral RNA by RAT as well as RT-qPCR. 100 SARS-CoV-2 positive and 100 SARS-CoV-2 negative nasopharyngeal swabs, previously confirm by RT-qPCR, were analyzed using the SARS-CoV-2 STANDARD Q COVID-19 Ag test kit. RATs are immunochromatographic assays, as is well known; consequently, the binding kinetics of the monoclonal antibodies utilized in each RAT kit determines their sensitivity, proportion of the specimen in the analyte, composition of the lysis buffer, and the technique used to display the results all have an impact on the sensitivity. Results were visualized and assessed by the individual’s eye at 15-30 min, after adding the specimen. Types of specimens recommended for STANDARD Q COVID-19 Ag Test is swab from human nasopharynx but for RATs and RT-qPCR both naso and oropharyngeal types of specimens were taken. In our findings out of 100 specimens tested SARS-CoV-2 viral RNA negative by RT-qPCR, for those samples SARS-CoV-2 RAT kit also showed 100% similarity and all were tested as negative (Table). This calculates to a specificity of Rapid Antigen Kit is of 100 % (Table). Of the 100 samples which found to be positive for SARS-CoV-2 RNA via RT-qPCR, only 74 individuals had a positive result for SARS-CoV-2 Rapid Antigen Test kit. This results in an overall sensitivity of RATs kit is of 74%. However, a comprehensive evaluation of the assay is unachievable because the overall sensitivity is entirely dependent on the distribution of cycle thresholds (Ct) values among the population of specimens. Thus, we have also calculated the sensitivity-based Ct-dependent manner for each specimen (Figure). The SARS-CoV-2 Rapid Antigen Test had a 100% sensitivity for samples with a high viral load (Ct 15-25). The computed sensitivity for samples with a medium (Ct 25-30) virus load was 94.87%. Sensitivity was estimated to be 77.27% and 55.56%, respectively, based on the viral load (Ct 30-35 and >35). The manufacturer’s guidelines manual states that the SARS-CoV-2 RAT has a sensitivity and specificity range of 88.7% and 95-98%, respectively.
Figure. Sensitivity of STANDARD Q COVID-19 Ag test kit (SD Biosensor) with respect to the viral load of clinical specimen
Table:
Number of SARS-CoV-2 Antigen positive and negative in a population of 100 SARS-CoV-2 positive patients and 100 SARS-CoV-2 negative patients
SARS-CoV-2 Antigen Positive |
SARS-CoV-2 Antigen Negative |
||
|---|---|---|---|
SARS-CoV-2 RT-PCR Positive |
74 |
26 |
74 (sensitivity) |
SARS-CoV-2 RT-PCR Negative |
0 |
100 |
100 (specificity) |
In the current investigation, we assessed the sensitivity and specificity of the STANDARD Q COVID-19 Ag test kit and compared it to that of the CoviPath TM COVID-19 RT-PCR Kit (applied BiosystemTM) from Thermo Fisher Scientific. Our findings showed that an overall specificity and sensitivity of antigen test kit found to be 100% and 74%, respectively in tested specimens. According to manufacturer’s report, using positive and negative samples for SARS-CoV-2, The site-specific RT-qPCR test was contrasted with the STANDARD Q COVID-19 Ag Test in terms of sensitivity and specificity. The combined sensitivity in was recorded as 76.6% (62.8-86.4%) and the pooled specificity was recorded as 99.3% (98.6-99.6%) in clinical specimen, data recorded form Germany whereas the testing center in Brazil recorded a sensitivity of 88.7% (81.3 93.4%) and a pooled specificity of 97.6% (95.2-98.8%), respectively.13 In our case we recorded 100% specificity, however, this is rare, but few reports are available which showed 100% specificity of the antigen test kits and matching with our findings.14 Shrestha et al. also reported that sensitivity and specificity of antigen test kit was calculated as 85% and 100%, respectively, with overall accuracy of 93.80% in 113 clinical specimens. similarly, Michael et al, reported 100% specificity regardless of the cycle threshold (Ct) value, for all nasopharyngeal samples which were submitted for the antigen test.15 However, if antigen the test’s sensitivity and specificity found to be higher but still it is important to analyze the test results cautiously depending on the prevalence of COVID-19 infection in the local community as well as epidemiological circumstances of the test subject. If any uncertainty RT-qPCR should be performed and confirm for clinical co-relation.16 The viral load in the specimen, the duration of the disease’s development, the quality of sample collection, and the test subject’s performance are few examples of the factors that affect the tests’ sensitivity and specificity, and the detailed description of the test kits’ components.17 In our findings sensitivities reduces as Ct value reduces with the viral load in specimen. RAT sensitivity was reducing from 100%, 94.87%, 77.27%, 55.56% respectively, for samples with increases CT (low viral load) of 15-25, 25-30, 30-35, and >35 respectively. Similar to prior findings, the Rapid Antigen test showed good sensitivity (84.9%) for samples with high viral loads but was substantially less sensitive (15.4%) for samples with low viral loads. The visual readout of this test may have decreased sensitivity. Additional visual band analysis (Rapid Antigen Kit, Respi-Strip CORIS) was recently assessed in two European trials.18 Though, detection rates increased for samples with high viral loads (with Ct 25), achieving sensitivities of 73.9% to 82.2%. Overall sensitivity extended from 50% to 57.6% with various Ct values. Additionally, in some cases from which the virus was isolated via RT-qPCR, RAT was failed to detect viral antigens with the higher Ct values samples more than 35. Hence, current RAT will miss some COVID-19 patients who are shedding infectious SARS-CoV-2 in the society.19-23 Rapid Antigen Test might have an inadequate appropriateness for the detection of the SARS-CoV-2 RNA and infection status of patients. Patients in the early or late stages of the infection, which are often accompanied by a low viral load, would not have their COVID-19 infection identified with this assay; Therefore, COVID-19 infection won’t be detected in people in the early or late stages of the infection, which are often characterized by a low viral load.20 Additionally, research has shown that specimens with Ct values >30 typically do not permit the virus to be cultured, indicating limited infectivity. Despite having low viral loads, these people may be considered non-contagious.18-20,22 The approach used to collect samples from infected people has a significant impact on the relevance of the RAT assay’s result outcome. The viral load in the patient’s respiratory tract may not always match the viral load in a collection. Only with an effective sample collection technique result can be meaningful. Otherwise, the patient’s viral load can be exaggerated.21 The standard method for detecting COVID-19 infection is RT-qPCR, but because it takes so long to complete, it still has difficulty reaching rural and isolated areas of the nation. It is now important to use a quick device to enable testing outside of laboratories.23-24 We needed a quicker and more trustworthy alternative, and the antigen test’s price is one of its benefits.
All notwithstanding, we arrived at the conclusion that the RT-qPCR assay has superior sensitivity and specificity to that Rapid Antigen Test, and we would like to stress that the gold standard RT-qPCR has a wide range of applications, from clinical to basic biology. To stop the transmission of infection, however, the antigen assay may be a simple, quick, and inexpensive method to separate SARS-CoV-2 infectious patients from less infectious or non-infectious patients at the community level in situations where RT-qPCR is not readily available or practicable. To put it another way, Rapid antigen testing can be regarded as a screening method and it can be suggested that you could have an infection, but an RT-qPCR test is required to validate the exact findings.
Study limitations
A small sample size was employed in the study, which was conducted over just six months. So, to confirm the results of this investigation, a larger sample size study over a longer time period is recommended. Because samples were only collected from the hospital’s OPD, the findings of this study cannot be generalized to other contexts within the state.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
NSi, NSh and TN conceptualized and visualized the study. JJ, OK, NSI, NSH and TN applied methodology. NSi, NSh, TN, NS and AS performed experiments. JJ and OK performed data curation. AS and NS performed data analysis. AN drafted the manuscript. NSi wrote the manuscript. AN, JJ and OK reviewed the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This work was supported by the NUHM (National Urban Health Mission; No/Micro/2022/ 1256, Raipur date: 20.06.2022), India.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Pt. JNM Medical College, Raipur, India, with approval Number No./MC/Ethics/2020/11 Raipur, Dated 07/01/2021.
- Na Z, Dingyu Z, Wenling W, et al. A Novel Coronavirus from Patients with Pneumonia in China. N Eng J Med. 2019;382(8):727-733.
Crossref - WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. https://covid19.who.int/
- Anna M, Mario H, Jurgen J, et al. Marcus Panning, Ad hoc laboratory-based surveillance of SARS-CoV-2 by real-time RT-PCR using minipools. J Clin Virol. 2020;127:104381.
Crossref - Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588(7836):E6.
Crossref - Nagura-Ikeda M, Imai M, Tabata K, et al. Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-qPCR), Direct RT-qPCR, Reverse Transcription-Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test to Diagnose COVID-19. J Clin Microbiol. 2020;58(9):438-440.
Crossref - Mak GC, Cheng PK, Lau SS, et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129:104500.
Crossref - Lambert S, Cuffel A, Le P, et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129:104500.
- Porte L, Legarraga P, Vollrath V, et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. 2020;99:328-333.
Crossref - Scohy A, Anantharajah A, Bodeus M, et al. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020;129:1044-55.
Crossref - Mertens P, De Vos N, Martiny D, et al. Development and Potential Usefulness of the COVID-19 Ag Respi-Strip Diagnostic Assay in a Pandemic Context. Front Med. 2020;7:1-11.
Crossref - Blairon L, Wilmet A, Beukinga I, Tré-Hardy M. Implementation of rapid SARS-CoV-2 antigenic testing in a laboratory without access to molecular methods: Experiences of a general hospital. J Clin Virol. 2020;129:104472.
Crossref - Updated CLIA SARS-CoV-2 Molecular and Antigen Point of Care Test Enforcement Discretion. Available from Guidance for Antigen Testing for SARS-CoV-2 for Healthcare Providers Testing Individuals in. available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/point-of-care-testing.html
- Clinical management of severe acute respiratory infection when novelcoronavirus(nCoV) Infection is suspected. Interim guidance2020. Available from: https://www.who.int/publications/i/item/10665-332299.
- Shrestha B, Neupane AK, Pant S, et al. Sensitivity and Specificity of Lateral Flow Antigen Test Kits for COVID-19 in Asymptomatic Population of Quarantine Centre of Province 3 Kathmandu. University Journal of Medicine and Medical Specialities. 2021;18(2):36-39.
Crossref - Wolfl-Duchek M, Bergmann F, Jorda A, et al. Sensitivity and Specificity of SARS-CoV-2 Rapid Antigen Detection Tests Using Oral, Anterior Nasal, and Nasopharyngeal Swabs: a Diagnostic Accuracy Study. Microbiol Spectr. 2020;10(1):e202921.
Crossref - Anna E, Mario H, Wenzel JJ, et al. Ad hoc laboratory-based surveillance of SARS-CoV-2 by real-time RT-PCR using minipools of RNA prepared from routine respiratory samples. J Clin Virol. 2021;127:104381.
Crossref - Yamayoshi S, Sakai-Tagawa Y, Koga M, et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses. 2020;12(12):1420.
Crossref - Weitzel T, Legarraga P, Iruretagoyena M. et al. Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples.BioRxiv;2020.
Crossref - Routsias JG, Mavrouli M, Tsoplou P, Dioikitopoulou K, Tsakris A. Diagnostic performance of rapid antigen tests (RATs) for SARS-CoV-2 and their efficacy in monitoring the infectiousness of COVID-19 patients. Sci Rep. 2021;11(1):22863.
Crossref - Lee MJ. Quantifying SARS-CoV-2 viral load: current status and future prospects. Expert Rev Mol Diagn. 2021;21(10):1017-1023.
Crossref - Kendall EA, Arinaminpathy N, Sacks JA, et al. Antigen-based Rapid Diagnostic Testing or Alternatives for Diagnosis of Symptomatic COVID- 19: A Simulation-based Net Benefit Analysis. Epidemiology. 2021;32(6):811-819.
Crossref - Dachert KF, Lupoli CB, Oztan GN, et al. Ten rapid antigen tests for SARS-CoV-2 widely differ in their ability to detect Omicron-BA.4 and -BA.5. Med Microbiol Immunol. 2023;212(5):323-337.
Crossref - Meumann EM, Robson JM. Testing for COVID-19: A 2023 update. Australian Prescriber. 2023;46(1):13-17.
Crossref - Shafie MH, Antony DM, Shaberi HS, Zafarina Z. Screening and confirmation tests for SARS-CoV-2: benefits and drawbacks. Beni-Suef Univ J Basic Appl Sci. 2023;12(1):6.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.