ISSN: 0973-7510
E-ISSN: 2581-690X
Latilactobacillus curvatus has a strong carbohydrate fermentative ability and antibacterial ability. It is considered as a promising probiotic by its excellent fermentation attributes and health advantages. Pickled shrimp derived from the fermentation process is highly appreciated by its unique texture, taste and flavor. However, this product is easily decomposed by spoilage bacteria especially Staphylococcus. This research evaluated the inoculation of L. curvatus (0.1-0.5 %) and different fermentation temperatures (28-30 oC) on the reduction of Staphylococcus aureus, pH and overall acceptance of the pickled shrimp after 6 weeks of fermentation. Results showed that the fermentation process should be conducted at 29 oC with 0.3 % Latilactobacillus curvatus (at initial density 9 log cfu/ml) to reduce pH to 3.70, completely against Staphylococcus aureus, obtain the highest sensory score (8.91).
Fermentation, Latilactobacillus curvatus, overall acceptance, pH, pickled shrimp, Staphylococcus aureus
The FAO/WHO definition of a probiotic—“live microorganisms which when administered in adequate amounts confer a health benefit on the host”—was reinforced as relevant and sufficiently accommodating for current and anticipated applications1. Health Canada has accepted Bifidobacterium and Lactobacillus in food at a level of 9 log CFU/g2. European Union countries suggest the utilization of specific species for nutrition and health advantages3. The Italian Ministry of Health has regulated the application of probiotic bacteria in the food industry under several terms, including a minimum number of viable cells (9 log CFU) administered per day, a full genetic characterization of the probiotic strain and a demonstratable history of safe use in the Italian market4. Overall benefit of probiotic was a supporting on healthy digestive tract against infectious diarrhoea, antibiotic-associated diarrhoea, gut transit, abdominal pain and bloating, ulcerative colitis and necrotizing enterocolitis5-9. The major benefits of probiotic were improvement of healthy immune system, reproductive tract, oral cavity, lungs, skin and gut–brain axis10-11. Probiotics inhibited prospective pathogens or released helpful metabolites/ enzymes to improve intestinal or extraintestinal immune effects12-13. Latilactobacillus curvatus shows milky white, translucent, and smooth colonies. It’s a candidate probiotic included in the list of recommended biological agents for certification by the European Food Safety Authority. It had perfect fermentation characteristics and health advantages14. It is unique by its bacteriocinogenic property against pathogenic and spoilage bacteria especially Listeria monocytogenes and Staphylococcus aureus, Bacillus cereus, and Enterococcus faecium15-18. Bacteriocin extracted from Latilactobacillus curvatus can be utilized to coat on polyethylene film as positive product coating19-20. Organic acids were also derived from Latilactobacillus curvatus. These organic acids were responsible for pH reduction and fatty acid hydrolization to impart desirable flavor and aroma during sausage production21-22. Latilactobacillus curvatus is considered as beneficial probiotic to relieve obesity and hyperlipidemia by regulating the colon micro-system through attending with nutritional components or converting antimicrobial amino acids, retarding adipocyte differentiation and lowering fat accumulation via discharging of cholesterol and coprostanol via cholesterol metabolism23-24. Latilactobacillus curvatus is commonly isolated from fermented vegetable, beef, fish25-28. It’s able to metabolize different carbohydrates like sucrose, glucose, trehalose, lactose, galactose, cellobiose and esculine29-31. Moreover, Latilactobacillus curvatus is also able to catabolize ascorbic acid, alcohol32. Latilactobacillus curvatus has ability to create tissue cantons via inter-binding to establish in the colon route contributing to probiotic role by interfering with harmful microorganisms to support the owner and retard the foodborne bacteria33-34. Hydrophobicity is a decisive variable affecting cell adhesion. Latilactobacillus curvatus has peptidoglycan layer of the cell partition to overcome high concentration of lysozyme in saliva, low pH in stomach and bile in the upper intestine by altering the profile and absorbent of the cell lining or forming outer polysaccharides35-41.
White leg shrimp (Litopenaeus vannamei) is one important seafood in the world. It has great nutritional values because it contains an excellent source of proteins, minerals, polyunsaturated fatty acid content, but low fat, less cholesterol42-43. White shrimp can be fermented into value-added product like pickle. Pickled shrimp is highly appreciated by its specific texture, taste and flavor44. Pickled shrimp has much more amino acid, glycogen and mineral compared to pickled vegetable45-47. Staphylococcus was identified as the main spoilage bacteria in decomposition of pickled shrimp48-49. Purpose of our research was to investigate the impact of several ratios of L. curvatus as starter culture and different fermentation temperature on the elimination of pathogenic bacteria (Staphylococcus aureus), pH and overall acceptance of the pickled shrimp after 6 weeks of fermentation.
Material
White shrimps (Litopenaeus vannamei) were purchased from local market in Bac Lieu, Vietnam. They were temporarily preserved in flake ice ready for experiments. Besides white shrimp, this research also used other ingredients such as sodium chloride, calcium chloride, saccharose, ethanol, galanga, plastic jar. Latilactobacillus curvatus and Staphylococcus aureus were supplied from Rainbow Trading Co. Ltd, Vietnam.
Researching method
White shrimps were rinsed with clean water before mixing with 13.5% of NaCl, 1.5% of CaCl2, 6.5% of sucrose, 5% of ethanol, 10% sliced galangal. Staphylococcus aureus was inoculated into shrimp mixture at 0.05 % with the initial density 9 log cfu/g. Latilactobacillus curvatus was activated before experiments. Stock density of Latilactobacillus curvatus was enumerated at 9 log cfu/ml. Latilactobacillus curvatus was inoculated into shrimp mixture at different ratios (0.1-0.5 %). The fermentation temperature was conducted in range (28-30 oC) for 6 weeks. Samples were taken to evaluate Latilactobacillus curvatus colony survival, pH and overall acceptance. This sampling lasted until the 6th week. Latilactobacillus curvatus enumeration was performed by method described by Van Reckem et al50. 5 gram of pickled shrimp was injected into a mixing pouch with diluents in ratio 1:10 and 0.1% peptone. This mixture was homogenized by stomacher for 1.5 minutes and adequate dissolving in buffer solution were prepared, cover on MRS film. MRS film was kept at 29±1 ◦C for 72 h. Latilactobacillus curvatus density (cfu/g) was enumerated by colony counter. Staphylococcus aureus was enumerated by Petrifilm plate. pH of samples was evaluated by pH meter. Overall acceptance (sensory evaluation) was examined by a panel of specialists basing on 9-point Hedonic scale ranging from 1 = Dislike extremely and 9 = Like extremely. The hedonic scale admitted that specialists’ interests exist on a constant and that their feedbacks could be classified into like and dislike.
Statistical analysis
All testings were set in 3 replications. The data were expressed as mean±standard deviation. Statistical parsing was based on the Statgraphics Centurion software version XVI.
Fermentation was a useful preservative method to prolong the seafood stability for long-term consumption51. It was prepared by mixing raw material with salt, keeping at room condition52. Table 1 showed the survival of Staphylococcus aureus (log cfu/g) in the pickled shrimp affected by inoculation ratio (0.1-0.5 %) of Latilactobacillus curvatus (9 log cfu/ml) and fermentation temperature (28-30 oC) after 6 weeks of fermentation. It’s obviously noticed that 0.3 % Latilactobacillus curvatus (at initial density 9 log cfu/ml) could effectively suppress Staphylococcus aureus growth (the highest 1.15±0.01 log cfu/ml at 28 oC to the lowest 0.00±0.00 log cfu/ml at 29 oC). 29 oC was identified as appropriate temperature for Latilactobacillus curvatus to proliferate against pathogenic and spoilage bacteria (the highest survival of Staphylococcus aureus 1.04±0.00 log cfu/ml at 0.1 % inoculum to the lowest survival of Staphylococcus aureus 0.00±0.00 log cfu/ml at 0.3 % inoculum). It has a distinct capacity to emit antibacteria substances with powerful antimicrobial activity against pathogen and spoilage bacteria in meat preservation. As a kicked microbial for fermented sausage, L. curvatus improves desirable flavor for the final product53. It can decrease the load of L. monocytogenes, a main pathogen in fermented sausages54. Moreover, it also greatly retard the proliferation of the harmful bacteria like Enterobacteriaceae, Pseudomonas fragi, Pseudomonas putida, Brochothrix thermosphacta55.
Table (1):
Survival of Staphylococcus aureus (log cfu/g) in the pickled shrimp affected by inoculation ratio (%) of Latilactobacillus curvatus (9 log cfu/ml) and fermentation temperature (oC) after 6 weeks of fermentation.
| Fermentation temperature (oC) | Inoculation ratio (%) of Latilactobacillus curvatus (9 log cfu/ml) | ||||
|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| 28.0 | 4.21±0.02a | 2.85±0.00ab | 1.15±0.01b | 0.66±0.00bc | 0.25±0.02c |
| 28.5 | 2.65±0.01a | 1.32±0.03ab | 0.63±0.02b | 0.21±0.01c | 0.12±0.03c |
| 29.0 | 1.04±0.00a | 0.57±0.02b | 0.00±0.00c | 0±0.00c | 0±0.00c |
| 29.5 | 1.93±0.03a | 1.26±0.01ab | 0.29±0.03b | 0.12±0.02bc | 0.07±0.01c |
| 30.0 | 3.41±0.02a | 1.94±0.00ab | 0.89±0.01b | 0.53±0.03bc | 0.20±0.02c |
Note: the numbers were presented as the mean of 3 samples; the same symbol was considered insignificant difference (α = 5%).
Table (2):
pH of the pickled shrimp affected by inoculation ratio (%) of Latilactobacillus curvatus (9 log cfu/ml) and fermentation temperature (°C) after 6 weeks of fermentation.
| Fermentation temperature (oC) | Inoculation ratio (%) of Latilactobacillus curvatus (9 log cfu/ml) | ||||
|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| 28.0 | 4.67±0.00a | 4.46±0.02ab | 4.31±0.00b | 4.22±0.03bc | 4.13±0.01c |
| 28.5 | 4.11±0.03a | 4.02±0.01ab | 3.91±0.00b | 3.83±0.02bc | 3.74±0.00c |
| 29.0 | 3.96±0.01a | 3.81±0.00b | 3.70±0.02c | 3.59±0.00c | 3.50±0.03c |
| 29.5 | 4.05±0.02a | 3.97±0.03ab | 3.84±0.01b | 3.71±0.01bc | 6.62±0.02c |
| 30.0 | 4.33±0.00a | 4.15±0.01ab | 4.08±0.00b | 3.99±0.02bc | 3.85±0.01c |
Note: the numbers were presented as the mean of 3 samples; the same symbol was considered insignificant difference (α = 5%).
Table 2 presented pH reduction in the pickled shrimp affected by inoculation ratio (0.1-0.5 %) of Latilactobacillus curvatus (9 log cfu/ml) and fermentation temperature (28-30 oC) after 6 weeks of fermentation. There was a down trend of pH by increasing inoculation ratio of Latilactobacillus curvatus and fermentation temperature. The highest fermentation efficiency recorded at 29 oC, pH of the fermentation batch decreased from 3.96±0.01 to 3.50±0.03. Latilactobacillus curvatus had a strong ability to ferment different carbohydrates to form organic acids29-31. Organic acids were produced from Latilactobacillus curvatus to be used in pH reduction and fatty acid hydrolization to enhance desirable flavor and aroma21-22.
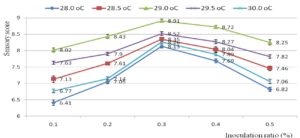
Fig. 1. Overall acceptance of the pickled shrimp affected by inoculation ratio (%) of Latilactobacillus curvatus (9 log cfu/ml) and fermentation temperature (°C) after 6 weeks of fermentation.
Fig. 1 revealed the overall acceptance of the pickled shrimp affected by inoculation ratio (0.1-0.5 %) of Latilactobacillus curvatus (9 log cfu/ml) and fermentation temperature (28-30 oC) after 6 weeks of fermentation. The fermentation process should be conducted at 29 oC with 0.3 % Latilactobacillus curvatus (at initial density 9 log cfu/ml) to achieve the highest sensory score (8.91±0.04). Meanwhile the lowest overall acceptance (6.41±0.05) was noticed at 28 oC with 0.1 % inoculum. L. curvatus can metabolize nitrosamines and fatty acids via different dedicated enzymatic systems56-58. L. curvatus could decompose sarcoplasmic protein to release peptides and amino acids59. In aging, these peptides and amino acids straight forward improve sensory attributes of final products60. Short-chain and medium-chain free fatty acids also released from hydrolyzing esters by L. curvatus. These fatty acids contribute to improvement of sensory characteristics of the sausage. According to Nguyen et al., white shrimp should be fermented at 28oC for 28 days to get a pleasant taste44. A supplementation of garlic into pickled white shrimp was reported61. In pickling, a great amount of unique amino acids was released. Pickled product was safe and stable over 6 months at ambient condition62 (Chandrashekhar, 1979). Pasteurized marinated shrimp in green curry paste was safe for 15 days at 0-3°C63.
Latilactobacillus curvatus is a promising starter culture recommended for meat processing industry. It can utilize carbohydrate for fermentation. It exhibits bioprotective properties by releasing bacteriocin against harmful microorganism. This bacteria has numerous adoptions in seafood preservation and in human wellness improvement. This research has successfully demonstrated the influence of different ratio of L. curvatus as starter culture and various fermentation temperature on the reduction of pathogenic bacteria (Staphylococcus aureus), pH and overall acceptance of the pickled shrimp after 6 weeks of fermentation.
ACKNOWLEDGMENTS
I would like to express my gratitude to Mrs Nguyen Hong Nga for providing raw shrimp in this investigation.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All data sets generated or analyzed during this study are included in the manuscript.
- Hill C, Guarner F, Reid G, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014; 11:506–514.
Crossref - Health Canada. Accepted claims about the nature of probiotic microorganisms in food. Health Canada. 2009. http://www.hc-sc.gc.ca/ fn-an/label-etiquet/claims-reclam/probiotics_ claims-allegations_probiotiques-eng.php%20
- Smug LN, Salminen S, Sanders ME, Ebner S. Yoghurt and probiotic bacteria in dietary guidelines of the member states of the European Union. Benef. Microbes. 2014; 5(1):61–66.
Crossref - Della Salute M. Commissione unica per la nutrizione e la dietetica. Guidelines on probiotics and prebiotics. 2013. Ministero della Salute. http://www.salute.gov.it/imgs/ C_17_pubblicazioni_1016_allegato.pdf
- Allen SJ, Martinez EG, Gregorio GV, Dans, L.F. Probiotics for treating acute infectious diarrhoea. Cochrane Database of Systematic Rev. 2010;11:003048.
Crossref - Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Rev. 2011; 3: 005496.
Crossref - Ritchie ML, Romanuk TN. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE. 2012; 7: e34938.
Crossref - Aponte GB, Mancilla CAB, Pariasca NYC, Galarza RAR. Probiotics for treating persistent diarrhoea in children. Cochrane Database of Systematic Rev. 2013; 8: 007401.
- Goldenberg JZ, Ma SSY, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database of Systematic Rev. 2013;5:006095.
Crossref - Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr. Pharm. Des. 2009;15(13):1428–1518.
Crossref - Maidens C, Childs C, Przemska A, Dayel IB, Yaqoob P. Modulation of vaccine response by concomitant probiotic administration. Br. J. Clin. Pharmacol. 2013;75(3):663–670.
Crossref - van Baarlen P, Troost F, van der Meer C, et al. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl Acad. Sci. USA. 2011; 108(Suppl. 1): 4562–4569.
Crossref - Kumar M. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 2013; 71(1): 23–34.
Crossref - Ying C, Leilei Y, Nanzhen Q, et al. Latilactobacillus curvatus: A candidate probiotic with excellent fermentation properties and health benefits. Foods. 2020; 9(10): 1366.
Crossref - Castellano P, Belfiore C, Fadda S, Vignolo G. A review of bacteriocinogenic lactic acid bacteria used as bioprotective cultures in fresh meat produced in Argentina. Meat Sci. 2008;79(3):483–499.
Crossref - Castro MP, Palavecino NZ, Herman C, Garro OA, Campos CA. Lactic acid bacteria isolated from artisanal dry sausages: Characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat Sci. 2011;87(4): 321–329.
Crossref - Rivas FP, Castro MP, Vallejo M, Marguet E, Campos CA. Sakacin Q produced by Lactobacillus curvatus ACU-1: Functionality characterization and antilisterial activity on cooked meat surface. Meat Sci. 2014; 97: 475–479.
Crossref - De Souza Barbosa M, Todorov SD, Ivanova I, Chobert JM, Haertle T, de Melo Franco BDG. Improving safety of salami by application of bacteriocins produced by an autochthonous Lactobacillus curvatus isolate. Food Microbiol. 2015; 46: 254–262.
Crossref - Mauriello G, Ercolini D, La Storia A, Casaburi A, Villani F. Development of polythene films for food packaging activated with an antilisterial bacteriocin from Lactobacillus curvatus 32Y. J. Appl. Microbiol. 2004; 97(2):314–322.
Crossref - Massani MB, Fernandez MR, Ariosti A, Eisenberg P, Vignolo G. Development and characterization of an active polyethylene film containing Lactobacillus curvatus CRL705 bacteriocins. Food Addit. Contam. Part A 2008; 25(11): 1424–1430.
Crossref - Fadda S, Lopez C, Vignolo G. Role of lactic acid bacteria during meat conditioning and fermentation: Peptides generated as sensorial and hygienic biomarkers. Meat Sci. 2010;86(1):66–79.
Crossref - Sun F, Kong B, Chen Q, Han Q, Diao X. N-nitrosoamine inhibition and quality preservation of Harbin dry sausages by inoculated with Lactobacillus pentosus, Lactobacillus curvatus and Lactobacillus sake.Food Control. 2017; 73(Part B): 1514-1521.
Crossref - Yoo SR, Kim YJ, Park DY, et al. Probiotics, L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity. 2013; 21(12): 2571–2578.
Crossref - Dahiya DK, Renuka, Puniya M, et al. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Front. Microbiol. 2017;8:563.
Crossref - Nakano K, Shiroma A, Tamotsu H, et al. First complete genome sequence of the skin-improving Lactobacillus curvatus strain FBA2, isolated from fermented vegetables, determined by pacbio single-molecule real-time technology. Genome Announc. 2016;4(5):e00884.
Crossref - Lee SH, Jung MY, Song JH, Lee M, Chang JY. Complete genome sequence of Lactobacillus curvatus strain WiKim38 isolated from Kimchi. Genome Announc. 2017; 5(18):e00273-17.
Crossref - Teran LC, Coeuret G, Raya R, Champomier-Verges MC, Chaillou S. Draft genome sequence of Lactobacillus curvatus FLEC03, a meat-borne isolate from beef carpaccio packaged in a modified atmosphere. Genome Announc. 2017; 5(26): e00584.
Crossref - Kyoui D, Mikami N, Yamamoto H, Kawarai T, Ogihara H. Complete genome sequence of Lactobacillus curvatus NFH-Km12, isolated from the Japanese traditional fish fermented food Kabura-zushi. Microbiol. Resour. Announc. 2018;7(1): 00823.
Crossref - Stentz R, Zagorec M. Ribose utilization in Lactobacillus sakei: Analysis of the regulation of the rbs operon and putative involvement of a new transporter.
J. Microbiol. Biotechnol. 1999;1:165–173. - Wiame E, Lamosa P, Santos H, Van Schaftingen E. Identification of glucoselysine-6-phosphate deglycase, an enzyme involved in the metabolism of the fructation product glucoselysine. Biochem. J. 2005;392(Pt 2): 263–269.
Crossref - Teran LC, Coeuret G, Raya R, Zagorec M, Champomier-Verges MC, Chaillou S. Phylogenomic analysis of Lactobacillus curvatus reveals two lineages distinguished by genes for fermenting plant-derived carbohydrates. Genome Biol. Evol. 2018;10(6): 1516–1525.
Crossref - Yew WS, Gerlt JA. Utilization of L-ascorbate by Escherichia coli K-12: Assignments of functions to products of the yjf-sga and yia-sgb operons. J. Bacteriol. 2002;184(1):302–306.
Crossref - Collado MC, Meriluoto J, Salminen S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007;45(4): 454–460.
Crossref - Garcia-Cayuela T, Korany AM, Bustos IP, et al. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014;57:44–50.
Crossref - Cunningham FE, Proctor VA, Goetsch SJ. Egg-white lysozyme as a food preservative: An overview. World Poultry Sci. J. 1991;47(2):141-163.
Crossref - Corzo G, Gilliland SE. Bile salt hydrolase activity of three strains of Lactobacillus acidophilus. J. Dairy Sci. 1999;82(3):472-480.
Crossref - Erkkila S, Petaja E. Screening of commercial meat starter cultures at low pH and in the presence of bile salts for potential probiotic use. Meat Sci. 2000; 55(3): 297–300.
Crossref - Piddock LJ. Multidrug-resistance efflux pumps—Not just for resistance. Nat. Rev. Microbiol. 2006;4: 629–636.
Crossref - Garcia-Ruiz A, Gonzalez de Llano D, Esteban-Fernandez A, Requena T, Bartolome B, Moreno-Arribas MV. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 2014; 44:220–225.
Crossref - Jiang Y, Ren F, Liu S, Zhao L, Guo H, Hou C. Enhanced acid tolerance in Bifidobacterium longum by adaptive evolution: Comparison of the genes between the acid-resistant variant and wild-type strain. J. Microbiol. Biotechnol. 2016;26(3):452–460.
Crossref - Hong SW, Kim JH, Bae HJ, et al. Selection and characterization of broad-spectrum antibacterial substance-producing Lactobacillus curvatus PA40 as a potential probiotic for feed additives. Anim. Sci. J. 2018;89(10):1459–1467.
Crossref - Gunalan B, Nina Tabitha S, Soundarapandian P, Anand T. Nutritive value of cultured white leg shrimp Litopenaeus vannamei. Int. J. Fisher. and Aqua. 2013; 5: 166-171.
- Daya JS, Ponniah AG, Khan I, Babu EPM, Ambasankar K, Vasagam KPK. Shrimps – a nutritional perspective. Current Sci. 2013; 104: 1487-1491.
- Nguyen PM, Pham TLP, Nguyen HT, Nguyen TVL. Technical factors influencing to production of galangal-pickled shrimp (Litopenaeus Vannamei). Oriental J. Chem. 2019;35:442-448.
Crossref - Durve VS, Bal DV. Studies on the chemical composition of the oyster Crassostrea gvphoides (Schlothecium). J. Zoologic. Soci. Ind. 1962;13:70-72.
- Giese AC. Canning of edible oyster meat. Physiological Rev. 1966;46:244-248.
Crossref - Ansari ZA, Parulekhar AH, Natondkhar SGP. Nutritional studies of fishes. Indian Journal of Marine Sciences. 1981;10:128-136.
- Abraham TJ, Rathnakumar K, Jeyachandran P. Microbiological characteristics of prawn pickle. Fishery Technol. 1996;33:111-115.
- Kumar S, Basu S. Preparation of prawn pickle and its storage characteristics. Journal of the Indian Fisheries Association. 2001;28:105-111.
- Van Reckem E, Geeraerts W, Charmpi C, Van der Veken D, De Vuyst L, Leroy F. Exploring the link between the geographical origin of European fermented foods and the diversity of their bacterial communities: The case of fermented meats. Front. Microbiol. 2019; 10: 2302.
Crossref - Jamila Patterson P, Ayyakannu K. Pickled product from a gastropod Babylonia spirata. Fishery Technol. 1997; 34:45-48.
- Ernestina M, Peralta, Hideo H, et al. Antioxidative activity of Philippine salt-fermented shrimp and variation of its constituents during fermentation. J. Oleo Sci. 2005; 54: 553-558.
Crossref - Papagianni M, Anastasiadou S. Pediocins: The bacteriocins of Pediococci. Sources, production, properties and applications. Microb. Cell Fact. 2009; 8:3.
Crossref - Vogel BF, Hansen LT, Mordhorst H, Gram L. The survival of Listeria monocytogenes during long term desiccation is facilitated by sodium chloride and organic material. Int. J. Food Microbiol. 2010; 140(2-3): 192–200.
Crossref - Zhang Y, Zhu L, Dong P, et al. Bio-protective potential of lactic acid bacteria: Effect of Lactobacillus sakei and Lactobacillus curvatus on changes of the microbial community in vacuum-packaged chilled beef. Asian Australas. J. Anim. Sci. 2018;31(4):585–594.
Crossref - Hu Y, Xia W, Ge C. Effect of mixed starter cultures fermentation on the characteristics of silver carp sausages. World J. Microb. Biot. 2007; 23: 1021-1031.
Crossref - Nie X, Lin S, Zhang Q. Proteolytic characterisation in grass carp sausage inoculated with Lactobacillus plantarum and Pediococcus pentosaceus. Food Chem. 2014;145:840–844.
Crossref - Kim SH, Kang KH, Kim SH, et al. Lactic acid bacteria directly degrade N-nitrosodimethylamine and increase the nitrite-scavenging ability in kimchi. Food Control. 2017; 71: 101–109.
Crossref - Dortu C, Huch M, Holzapfel WH, Franz CM, Thonart P. Anti-listerial activity of bacteriocin-producing Lactobacillus curvatus CWBI-B28 and Lactobacillus sakei CWBI-B1365 on raw beef and poultry meat. Lett. Appl. Microbiol. 2008;47(6):581–586.
Crossref - Fadda S, Sanz Y, Vignolo G, Aristoy MC, Toldra F, Oliver G. Hydrolysis of pork muscle sarcoplasmic proteins by Lactobacillus curvatus and Lactobacillus sake. Appl. Environ. Microbiol. 1999; 65(2):578–584.
Crossref - Sunisa S, Naiyana P, Sujira A, Worapong U. Development of green curry paste marinade for white shrimp (Litopenaeus vannamei). Songklanakarin J. Sci. Technol. 2008;30:35-40.
- Chandrashekhar TC. A method of processing and preservation of prawn pickle. Seafood Export Journal. 1970;11:15-18.
- Jawahar AT, Shetty TMR. Effect of sodium benzoate on the fermentative fish pickle. Fishery Technol. 1994; 31: 48-51.
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.