Accurate and rapid diagnostics are required for environmental monitoring, food safety, and medicine in the 21st century. Disposable sensors provide low-cost, user-friendly options for rapid measurement from clinical diagnostics to food science. Synthetic single-stranded DNA or RNA, termed aptamers, are relatively new tools with clear advantages in terms of structural, binding specificity, chemical flexibility, and new improvements in robustness in complex, regulated matrices. This review provides a comprehensive overview of nanomaterial-integrated aptamer-based biosensors, categorizing major diagnostic methods such as colorimetric, electrochemical, fluorescence, lateral flow, and CRISPR-based detection systems. Applications in clinical diagnostics, food safety, and environmental monitoring are addressed. Target accessibility, stability in vivo, and regulatory limitations are highlighted as the major challenges through the integration of recent studies. The current work aims to help researchers with designing and using long-lasting, aptamer-based nano biosensors for practical diagnostic applications.
Aptamer, Nanomaterial, Biosensor, Microbial Diagnostics
Single-stranded nucleotides of DNA or RNA that target a specific region in proteins, small molecules are known as aptamers, which have a strong affinity towards the target.1 They are artificially produced by a process called SELEX (Systematic Evolution of Ligands by EXponential enrichment), a sequential enrichment method used to isolate highly specific aptamer candidates from a whole set of aptamer sequences.2 In recent years, applications against complex targets, like microbial pathogens, have been diagnosed with multiple complex SELEX variants, notably cell-SELEX, microfluidic-SELEX, and toggle-SELEX, which drastically improved the efficacy and specificity of aptamer selection.3,4
Aptamers are more viable alternatives to antibodies in diagnostics and therapeutics because they possess strong affinity, remarkable specificity, and inherent stability. Aptamers can be chemically modified to introduce novel functionalities, enhancing their adaptability to different diagnostic strategies for identifying molecular targets. DNA and RNA aptamers contain multiple conformations that can provide higher-affinity binding, while they contain the best separated and composed common nucleic acid motifs. Unique structures also arise, including G-quadruplexes, stacked structures formed by four guanine bases linked via Hoogsteen-type hydrogen bonding. The first observed G-quadruplex was from a DNA-based aptamer designed to bind and suppress thrombin activity, exhibiting a compact, symmetrical structure that consisted of two guanosine tetrads and three loops.5 Predicting the secondary structures of aptamers is difficult, and particularly so for DNA aptamers containing G-quadruplexes or pseudoknots. Despite their structural intricacies, aptamers demonstrate excellent shape complementarity with protein epitopes, unlike conventional antibodies.
The structural flexibility of the aptamers plays a vital role when binding to the target, which can be enhanced by pairing the aptamers with functional nanomaterials like gold nanoparticles, carbon nanostructures, and quantum dots, which enhance the detection and improve biosensing applications, which is extensively studied for microbial diagnostics by enabling rapid, accurate and affordable detection methods in contrast with conventional antibody-based techniques.6,7
Aptamers utilize both well-defined nucleic acid motifs and structurally flexible regions to achieve high specificity and affinity for their targets. Upon interaction with their targets, aptamers can undergo conformational transitions while retaining specificity.8,9 Macugen stands as the first aptamer-derived therapeutic to receive FDA approval based on an aptamer to treat age-related macular degeneration.10,11 Recent efforts are focused on understanding aptamer structural features and how the properties of the aptamers can be used. Computational approaches for modeling aptamer 3D structures and simulating target interactions have proven valuable tertiary structures of aptamers and in docking them with targets, have also helped toward rational design.12-14
SELEX
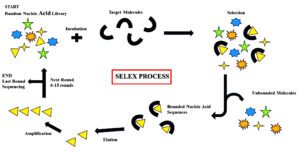
The SELEX process (Systematic Evolution of Ligands by Exponential Enrichment) forms the foundation of designing high-affinity nucleic acid aptamers to discriminate between a diverse array of target entities (Figure 1). The SELEX process was independently created by Tuerk and Gold initially and later by Ellington and Szostak in 1990. SELEX is a process of universal in vitro evolutionary technique used to select aptamers with increased binding affinity and specificity.15,16 The process is initiated with a highly randomized large-scale oligonucleotide library of 20 to 60 nucleotides with constant primer binding sites for subsequent amplification.17 The library will be incubated with the target molecules, ranging from small molecules to whole cells or complex biological structures.18 Unbound molecules are washed away, leaving affinity-binding aptamers bound with the target. Bounded sequences are eluted out and amplified by PCR, and thus progressively enrich the subsequent iterative rounds for high-affinity candidates.19
Recent technologies like microfluidics and high-throughput sequencing have been integrated to increase selection and efficiency at less time and labor costs conventionally incurred in SELEX.20 It has to be noted, however, that native aptamers are prone to degradation in vivo, and therefore, the addition of chemical modifications is needed to make them stable and increase their pharmacodynamics.21 The inherent flexibility of the SELEX process leaves space for the creation of new targets and secondary structures of aptamers, as seen in the evolution of aptamers that can bind to ATP but also to GTP.22
Types of aptamers
Aptamers are developed from DNA, RNA, or synthetic XNA molecules and have various applications in research, biosensing, biotechnology, and medicine. DNA aptamers are the most well-studied and used aptamers due to their chemical structure, stability, and low cost of production. These DNA aptamers can fold into a three-dimensional shape when they bind with and identify the target; their folding kinetics is considered to be fast, which occurs in milliseconds, and they also possess similar properties to small proteins, while they possess a more structured transition state with few intermediate formations.23 RNA aptamers are more tunable and flexible than DNA aptamers, which are more binding-capable, and they can bind to targets. Due to their compact form, they exhibit extremely low immunogenicity and are easy to modify. They also have many benefits over antibodies in diagnostics and therapeutics.24 RNA aptamers have also been promising in targeted delivery systems, such as the delivery of small interfering RNAs (siRNAs) to target cells.25 Xeno-nucleic acids (XNAs) represent the newest aptamer technology frontier. Chemically modified backbones or sugars contain synthetic analogs of the natural nucleic acids, the XNAs. These offer greater resistance to nucleases as well as enhanced biostability. XNA aptamers may be generated by dedicated selection protocols such as cross-chemistry SELEX (X-SELEX), using mutated polymerases to enable the inclusion of modified nucleotides. Among the various XNA alternatives, threose nucleic acid (TNA) aptamers dubbed “threomers” have drawn interest because of their antibody-like fold mimicry and more rapid protein-binding kinetics mediated by aromatic side chains.26
Aptamer-based diagnostic platforms
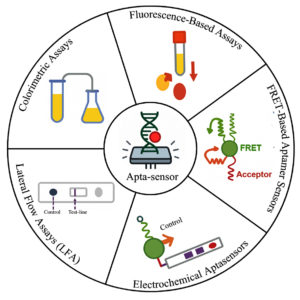
Colorimetric assays
Aptamer-based colorimetric biosensors have proven themselves to be potent agents for rapid, sensitive determination of a wide variety of targets from disease biomarkers to pathogens and small molecules (Table 1). The assays preferentially function by visible colorimetric change or change in absorbance, abolishing the requirement for complex instrumentation and allowing them to be deployed at the point of care (POC)27 constructed one of the earliest systems based on polydiacetylene vesicles for detecting E. coli O157:H7, while showed the application of gold nanoparticles (AuNPs) in the detection of thrombin and abrin toxin, respectively, as a sign of the versatility of aptamers with the help of nanomaterials.28 Colorimetric assays are also used in food safety with high specificity and sensitivity in the analysis of foodborne pathogens reported higher than 90% sensitivity and specificity for several pathogens using low volumes of samples.29
Table (1):
Timeline of Breakthrough Innovations in Aptamer-Based Diagnostics
Year |
Breakthrough |
Detection type |
Target Analyte |
LOD |
Ref. |
|---|---|---|---|---|---|
2020 |
Aptamer functionalized porous silicon and carbon nanotube-based biosensors for protein detection |
Electronic |
Thrombin, his-tagged proteins |
Single molecule, real-time detection |
78 |
2021 |
Electrochemical aptamer sensor for neuropeptide Y with rapid dynamic measurement |
Electrochemical |
Neuropeptide Y (NPY) |
162 pM (in buffer), 390 pM (in serum) |
79 |
2022 |
Aptamer-based targeted drug delivery platforms in cancer therapy |
Various (Therapeutic) |
Cancer biomarkers |
– |
80 |
2023 |
Aptamer-functionalized molecular electronic device for continuous thrombin detection |
Electronic/Electrochemical |
Thrombin |
Single molecule detection |
76 |
2024 |
Aptamer-based fluorescent sensor for rapid mercury ion detection in aqueous and living cells |
Fluorescent |
Mercury ions |
33 µg/L (~0.16 µM) |
81 |
2025 |
Peptide aptamer electrochemical sensors with antifouling layers for amino acid detection |
Electrochemical |
L-Arginine |
0.01 pM |
82 |
2025 |
Integration of functional nanomaterials (AuNPs, graphene, MOFs) to enhance aptamer sensors |
Electrochemical |
Prostate-specific antigen (PSA) |
0.05 fM |
4 |
Aptamer target interactions with AuNP-based systems often result in nanoparticle aggregation or disaggregation due to variations in surface plasmon resonance, which causes significant color shifts. These assays frequently rely on their unique physicochemical qualities, and in some cases, these nanoparticles act as enzyme mimics (i.e, nanozymes), revealing intrinsic peroxidase-like activity. The presence of target molecules is determined by a catalytic process in which oxidation of chromogenic substrates takes place, which has the capability of showing visible color changes.30,31
Fluorescence-based assays
Fluorescence assays are central to high-throughput screening platforms due to their sensitivity, automation facility, and wide range of fluorophores. Fluorescence assays have specific utility for the quantitation of enzymatic reactions, protein-protein and protein-DNA interactions, and cellular processes in real time. These technologies facilitate label integration directly into molecular targets with the maintenance of biological context and reduction of perturbation during measurement. In diagnostics and food safety, fluorescence technologies have sped up the identification of allergens, toxins, contaminants, and spoilage markers. Fluorescence microarrays and quantum dot biochips have been employed for the multiplex screening of trace residues and foodborne pathogens in parallel detection, ranging from picomolar to nanomolar.32,33 For instance, fluorophore-labeled probes have been utilized in a graphene oxide-based FRET platform to successfully detect aflatoxin B1 and Salmonella spp. in food samples with LODs below regulatory levels. Such technologies play a huge role in food traceability, authenticity, and public health guarantee.34
FRET-based aptamer sensors
Fluorescence resonance energy transfer (FRET) is a universal and broadly used approach towards the construction of aptamer-based biosensors, where it utilizes the non-radiative transfer of energy from a donor to an acceptor fluorophore to generate highly sensitive and specific sensing signals. FRET has been successfully used in many applications, such as genetically encoded RNA-based FRET biosensors for the real-time monitoring of molecular interactions in living cells.35 Further, upconverting phosphor-based FRET biosensors have shown superior sensitivity in the sensing of thrombin, a very significant coagulation disorder biomarker, by utilizing their unique photophysical properties to suppress background autofluorescence and boost signal-to-noise ratios.27 In addition, the versatility of FRET-based aptamer sensors is also utilized in drug discovery research and live-cell imaging, through which they facilitate visualizing dynamic biomolecular processes and high-throughput screening of therapeutic candidates, thus driving both basic biological research and translational medicine.36
Quantum Dot (QD)-aptamer conjugates
Quantum dot (QD)-aptamer conjugates are a novel family of biosensors that together take advantage of the unrivaled photostability and intense fluorescence of quantum dots and the high specificity and affinity of aptamer binding to provide them as powerful probes for cancer imaging and diagnosis. These conjugates have also been widely applied in both in vivo and in vitro detection of cancer cells, thereby facilitating specific identification and tracking of the tumor cells with improved sensitivity.37 QD-aptamer conjugates have also been incorporated into electrochemical cytosensors that facilitate selective identification of tumor cells by electrochemical signaling pathways and thus a quantitative and sensitive method of diagnosing tumors.38 In addition to single-target imaging, multiplexed imaging applications with various QD-aptamer conjugates simultaneously image several cancer biomarkers and enable cancer diagnosis and research to be more precise and complete.39
Signal amplification strategies
To achieve enhanced sensitivity for aptamer-based fluorescent biosensors, several new signal amplification strategies have been documented. They include the use of fluorescent nucleotide analogues whose binding to the target upon activation generates a sensitive and direct fluorescent signal directly correlated with the molecular recognition event.40 Another innovative method employs whispering-gallery mode lasing in microspheres, which enhances fluorescence signals through resonant light confinement to significantly raise detection limits.41 Cation exchange reactions within nanoclusters also exhibit effective amplification by controlling fluorescence properties based on target occurrence to offer a robust signal enhancement process.42 Besides, multisignal double-stranded DNA (dsDNA) probes have been developed to yield amplified diagnostic signals through multiple fluorescent emissions, thereby improving sensitivity and enabling more accurate biomarker quantification.43 In sum, these fluorescence signal amplification methods provide powerful agents for improving the applications.
Multiplex detection strategies
Multiplex fluorescent aptasensors are effective analytical tools for the simultaneous determination of multiple analytes, thereby enhancing diagnostic assay throughput and efficiency (Figure 2). For instance, FRET-based systems coupled with graphene oxide quenching have been successfully employed in detecting a variety of antibiotics, enabling sensitive and selective multi-target analysis.44 In addition, hybrid structures with quantum dots, aptamers, and (AuNPs) have also been developed to detect a range of ions and small molecules with higher specificity using the unique optical features and signaling transduction abilities of these nanomaterials.45 Colorimetric aptasensors using AuNPs further enhance the multiplex detection capability by providing simple and visual readouts, which are particularly valuable for rapid testing in the field.46 Other than fluorescence and colorimetry, novel multiplex detection formats also include entirely electrochemical sensors, SERS-based systems, and paper- or microfluidic chip-based systems. These newer technologies hold significant potential for high-sensitivity foodborne contaminant screening by integrating miniaturization, portability, and multiplex capability to meet the requirements of analytical needs in the real world.34
Electrochemical aptasensors
Electrochemical aptamer-based biosensors, dubbed electrochemical aptasensors, have been extensively used as extremely sensitive detection devices in clinical diagnostics, environmental inspection, and food safety assessment.47 These sensors function through target-induced conformational, configurational, or conductance modifications of the aptamer on the electrode surface, and the inclusion of nanomaterials in electrodes has substantially enhanced sensor sensitivity, electron transfer efficiency, and overall performance. The electrochemical readouts are divided into label-free and label-based techniques. The label-free techniques include cyclic voltammetry (CV), differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS), which track target binding in real-time without supplementary labels, whereas label-based techniques use enzymes, redox mediators, or nanoparticles to amplify the electrochemical signal upon target binding, thereby increasing sensitivity.48 These methods facilitate real-time monitoring of compounds in intricate biological fluids, providing continuous, in vivo detection of medications, metabolites, and heavy metals at high temporal resolution.49
Lateral Flow Assays (LFA)
Aptamer-based lateral flow assays (LFAs) have emerged as robust platforms for point-of-use, low-cost, and fast diagnostics by combining the molecular recognition capability of aptamers with the convenience of lateral flow technology. Their capacity for chemical modification and conjugation into gold nanoparticle (AuNP)-based formats might enable high-performance assays for mycotoxins, environmental micropollutants, and low-molecular-weight molecules.50 Optimized concentration of aptamer, appropriate choice of membrane, and adequate blocking are critical parameters in the achievement of ideal specificity and sensitivity. Other innovative methods, like Linkage Inversion Assembled Nano-Aptasensors (LIANA), which improve the conjugation between the nanoparticles and aptamer using structured linker assembly, and Crossover-SELEX, which is a variant of SELEX, aim to enhance the target specificity and affinity through iterative selection among various targets and conditions, have substantially improved aptamer-nanoparticle conjugation and target binding efficacy in lateral flow assay (LFA) systems.51 The devices enable fluid manipulation, metering, mixing, transport, and detection within compact platforms optimized for particular applications.52 Digital microfluidics (DMF) is particularly noted to offer multiplexing with the potential for portable point-of-care diagnostic devices.53 They have low sample demands and provide rapid on-site detection and are therefore highly suitable for pathogen surveillance and clinical diagnostics.54
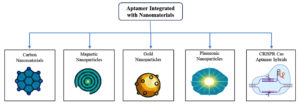
Integration with nanomaterials
Aptamer-nanoparticle hybrid biosensors have garnered huge attention for their excellent sensitivity, target specificity, and rapid detection rates for a wide variety of analytes.55 Aptamers have high affinity that serve as effective bioreceptors if they are immobilized on nanomaterials such as gold nanoparticles (AuNPs), carbon nanomaterials (CNMs), magnetic nanoparticles (MNPs), and plasmonic nanomaterials.56 Aptamer-nanoparticle conjugates are also being developed as multifunctional platforms for diagnostics and therapeutics owing to their distinctive blend of high specificity, tunability, and biocompatibility.57 The benefits of these hybrid systems include diminished immunogenicity, heterogeneous surface functionalization, and target molecular recognition (Figure 3).58 Optimization strategies for these conjugates include control of nanoparticle size, chemical bonds, and multivalent aptamer architecture for increasing their functional yield. Overall, their integration is a valuable progression toward the creation of targeted and individualized therapeutic platforms with low off-target toxicity.59
Gold Nanoparticles (AuNPs)
Aptamer-functionalized gold nanoparticles (Apt-AuNPs) are used for their biocompatibility, ease of surface functionalization, and strong surface plasmon resonance properties.60 These platforms have demonstrated exceptional utility in both colorimetric sensing and targeted drug delivery, offering rapid and sensitive detection capabilities with minimal instrumentation.61 For example, aptamer-induced aggregation of AuNPs has enabled simple colorimetric detection of organophosphate pesticides like malathion, with limits of detection as low as 1.48 µg/L within 40 minutes. Moreover, thiol-modified aptamers immobilized on AuNPs have shown strong stability and successful detection of glycated hemoglobin (HbA1c) directly in whole blood samples.62
Carbon Nanomaterials (CNMs)
Graphene and carbon nanotubes (CNTs) significantly improve biosensor sensitivity by offering a high electroactive surface area, excellent conductivity, and ease of aptamer immobilization.63 CNT-based aptasensors have reached femtomolar detection limits for targets like bisphenol A.57,64 Recent advances include reduced graphene oxide field-effect transistor (rGO-FET) sensors detecting NT-proBNP in artificial saliva at concentrations as low as 41 fg/mL.65 Nonetheless, the complex three-dimensional structures of CNTs can present challenges in reproducibility and aptamer stability.66
Magnetic Nanoparticles (MNPs)
Magnetic nanoparticle-based aptamer biosensors offer efficient separation and concentration of targets in complex biological and environmental matrices.67 These platforms have been used to detect as few as 10 cancer cells, with recent systems achieving detection of heat shock proteins like HSP70 with LODs as low as 0.1 ng/mL and multiple reuse cycles,68 use in point-of-care diagnostics is expanding, supporting rapid, portable biomarker detection with high clinical relevance.54
Plasmonic nanomaterials
Plasmonic nanomaterials, particularly AuNPs and AgNPs, enhance aptamer-based detection through surface plasmon resonance (SPR), self-catalytic growth, and metal-enhanced fluorescence.69 Multispectral aptasensors utilizing plasmonic nanomaterials have demonstrated heightened bulk and surface sensitivity, enabling rapid detection of bacterial pathogens like E. coli O157:H7.70 However, challenges in achieving multiplexing and clinical reproducibility remain significant.71
CRISPR Cas-based aptamer hybrids
The CRISPR-Cas systems have drawn attention to the development of more sophisticated diagnostic platforms. The plasticity and high sensitivity of aptamers and the programmable nuclease function of CRISPR-Cas effectors provide sensitive detection of small molecules with a wide variety of biomarkers72,73 constructed a molecular radar strategy using CRISPR-Cas12a for highly sensitive ATP detection24 also constructed a CRISPR/Cas12a immunoassay with an aptamer-compatible platform, which is compatible with conventional ELISA platforms, and achieved diagnostic versatility on non-nucleic acid targets. Applications also include clinical diagnostics, like Alzheimer’s disease, for which designed a CRISPR-based aptasensor for the detection of Aβ40 and Aβ42 cerebrospinal fluid biomarkers with improved performance compared to standard ELISA.74 Aptamer-CRISPR biosensors are noted for their simplicity, rapidity, and cost-effectiveness when compared to conventional assays.75
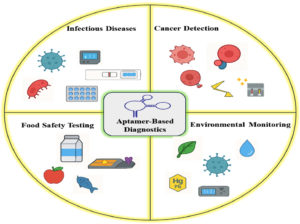
Aptamer-based diagnostics application fields
Aptamer-based diagnostics have been acknowledged as powerful devices in a wide range of applications since they possess high specificity, high affinity, chemical stability, and easier synthesis than antibodies (Figure 4).76 Despite problems of implementation with deeply rooted antibody use in commerce and poor public awareness, advances have opened up their usage across infectious disease detection, cancer diagnosis, environmental monitoring, and food testing safety. Diversiform platforms such as ELISA-like assays, lateral flow tests, microfluidics, and aptasensors have enhanced sensitivity and specificity for various diagnostic applications.77
Infectious disease diagnostics
Aptamers are known to be far better than antibodies in bacterial and viral diagnosis; they have easier synthesis, modifiability, and wider target ranges.83 Aptasensors, ELISA-type, and lateral flow devices are selective sensors that can detect pathogens like Salmonella typhimurium at 600 CFU/mL concentrations.84,85 Aptamers are utilized as bio-recognition units in aptasensors for point-of-care virus detection of HIV, HBV, HCV, SARS, influenza, and Ebola.86,87 Aptamers can interfere with virus-host interaction or intracellular protein binding to stop viral replication.83 Aptamer biosensors combine optical, electrochemical, and mass-sensitive sensing modes for high-affinity and selective detection of bacterial proteins, surface antigens, and toxins.88 Single-cell level detection is difficult, although combining nanomaterials and advanced SELEX methods holds the promise of increased sensitivity.22
Cancer diagnostics
Aptamers, “chemical antibodies”, have been employed extensively in cancer biomarker detection and diagnosis of tumors because of their high selectivity, affinity, ease of synthesis, and chemical stability.89 Cell-SELEX with proteomic analysis allows the simultaneous measurement of numerous cancer biomarkers.16 Aptamer-based biosensors (aptasensors) by electrochemical, optical, and fluorescence methods enable cancer early diagnosis to be performed rapidly, sensitively, and economically.90 If combined with nanotechnology, there is further optimization of the sensor’s performance. Aptasensors are also future candidates for point-of-care diagnosis and non-invasive tumor imaging, bypassing the limitations of traditional approaches.90 Aptamers in therapeutics are used as drug delivery targeting ligands, where diagnostic and therapeutic functions are sheltered.91 Fluorescence, colorimetric, and multimodal imaging probes have been engineered in recent times for cancer detection and treatment approaches.2
Environmental monitoring
Aptamer biosensors are powerful, selective, and responsive environmental toxin sensors, for example, heavy metals, agrochemicals, and waterborne pathogens.92,93 SMART aptasensors are Specific, Measurable, Accurate, Robust, and Time-saving sensors that enable rapid and sensitive analysis for food, water, and biological samples.72 Nanomaterial doping and heterogeneous transduction methods, optical, electrochemical, and mass-sensitive, mean specificity and sensitivity enhancement. Detection of pollutants such as pharmaceutical residues, endocrine disruptors, pesticides, and cyanotoxins is achievable by the biosensors with detection values upgraded to the femtomolar.94 Wearable next-generation technologies and lab-on-a-chip platforms enable potential multifunctional environmental monitoring.93 Problems remain in sensor sensitivity in sophisticated environmental matrices and online monitoring.92
Food safety testing
Aptasensors also exhibit vast potential for the rapid, sensitive, and economical identification of contaminants and foodborne pathogens.61 Aptamers are employed in these biosensors to differentiate bacteria, toxins, antibiotics, and allergens in electrochemical, optical, fluorescence, and surface-enhanced Raman scattering detection modes.95 Recent developments incorporate multiplexed arrays of detection and transcription aptasensors for label-free, culture-free whole pathogen detection.96 Screen-printed electrodes-based electrochemical aptasensors provide enhanced analytical performance in food matrices of multi-component.95 Advantages of aptamer-based diagnostics in food safety are ease of modification, chemical stability, and in vitro synthesis over antibodies.95 Future research remains to grapple with problems of strong detection in multiplexed samples and to offer useful applications along the food supply chain.96
Constraints and challenges of aptamer-based diagnostics
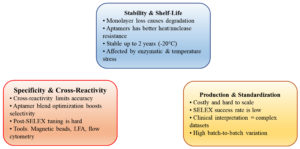
Concerns of stability and shelf-life
Aptasensors, particularly electrochemical-based aptamer (E-AB) biosensors, have been refined with an enhanced understanding of their degradation processes. In contrast to earlier presumptions, signal decay is no longer largely due to nuclease hydrolysis, as late research indicates. Rather, sensor degradation now largely results from the loss of monolayer components on the electrode surface.97 Thermostable analogs such as TNA aptamers are also superior to their conventional DNA and FANA counterparts concerning nuclease resistance and heat stability.98 Storage stability has also been demonstrated that DNA aptamers are stable for more than two years under appropriate storage.99 Electrochemical sensors preserved at -20 °C remain active for six months even without preservation.94 Other methods, such as adapted SELEX and engineered polymerases, have increased the nucleic acid and peptide aptamer stability (Figure 5).98 Structural characteristics such as loop size and center sequence are also important for stability.97 Taken together, these innovations supply essential information for constructing robust, stable diagnostic platforms.
Challenges with specificity and cross-reactivity
Aptamers offer a cost-efficient, targeted competitor to antibodies for biosensing applications.100 But cross-reactivity is still at the root of diagnostic accuracy. Methods like aptamer blend optimization, where aptamers with different affinities are mixed, increase sensor selectivity without being confounded by interference.101 Cross-recognition methods, like those used with lateral flow tests for detecting small molecules, demonstrate aptamers’ utility across a wide range of targets.102 Nevertheless, challenges with library design, post-SELEX modification, and screening sensitivity persist.94 Technologies such as magnetic bead capture, flow cytometry quantification, and integration of transduction technology have resulted in ultrasensitive diagnostic devices that have reached commercialization.75 Further development of these technologies is poised to make aptamers ready for widespread use for biomarker discovery and disease monitoring.
Production and standardization
While aptamers are replete with promise due to their specificity and modularity, practical problems of manufacture and standardization have to be addressed. Limitations such as modest in vivo efficacy, sparse safety data, and costliness of manufacture restrict their large-scale application.103 Furthermore, large-scale synthesis creates monstrous datasets, rendering clinical interpretation a problem.50 Besides low batch-to-batch variation and ease of handling,104 the SELEX success rate is still less than optimal. Improved approaches include microenvironmental tailoring and optimized post-SELEX procedures.105 Overcoming these technological and scientific challenges is crucial to expanding the scope of applications for aptamer technologies in therapeutics, diagnostics, and drug delivery.
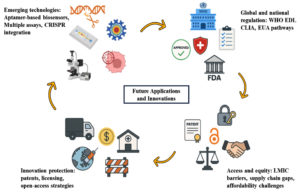
Diagnostic innovation: market, regulation, IP, and accessibility
Market readiness and technological feasibility
New diagnostic technologies propel forward to meet global health requirements, especially in resource-limited settings. Point-of-care (POC) diagnostics provide rapid clinical decision-making with minimal human interaction and are particularly valued for infectious and cardiovascular illnesses.106 Multiplex analysis is enabled by high-throughput platforms, such as addressable laser bead immunoassays, microarrays, and nanobarcode particles, though clinical adoption requires validation and standardization.107 Aptamer-based platforms have expanded diagnostic capability significantly, particularly when integrated with CRISPR/Cas platforms to enhance specificity and biosensing ability.72 Aptamer-functionalized QDs have enabled multiplex imaging and drug targeting in cancer cells with improved tumor imaging and therapeutic performance.39
Regulatory frameworks and diagnostic approvals
In vitro diagnostic (IVD) devices play a central role in disease detection and management. The Essential Diagnostics List (EDL) of the World Health Organization identifies the world’s need for quality, affordable diagnostics.108 Selecting quality diagnostics to use for low-resource settings involves process approaches, from defining use cases, market research, and monitoring performance.109 In the United States, control is maintained by the FDA and CMS according to CLIA guidelines.110 Emergency Use Authorization (EUA) that was initially initiated in 2004 has been instrumental during health crises like COVID-19 by facilitating access to priority diagnostics and therapeutics.111,112
Intellectual property and licensing considerations
Intellectual property (IP) policies are central to encouraging innovation and supporting access to the relevant diagnostics. Effective IP systems support biomarker research, technology commercialization, and international health partnerships.113 Open innovation strategies, including CAMBIA and the Innovative Medicines Initiative, are transforming corporate IP cultures through expanded accessibility.114 The CRISPR-Cas9 context has brought diagnostic IP licensing complexity into the limelight. Institutional ownership-commercial interest disputes have ignited controversy regarding ethical licensing models and semi-commons strategies that ensure equitable access and continued innovation.115,116
Cost-effectiveness, scalability, and access
New diagnostics have shown promise for cost savings and clinical benefit in multiple situations. Some examples include results of triple testing for breast cancer and MRI usage for scaphoid fracture, both reducing unnecessary procedures and cost.117 Although NGS is more sensitive in oncology, this is coupled with higher upfront costs, balanced by more effective clinical trial pairing and results (Figure 6).118 As advanced as technology has become, access in LMICs is still inhibited by the environment, supply chain limitations, infrastructure, and affordability.119 Stock-outs at rural health clinics and poor supply chain coordination limit diagnostic delivery.120 Enhancing access can be optimized by the likes of harmonized standards, dynamic supply chain models, resourced health systems, and quality assurance (Table 2).121
Table (2):
Summary of aspects, key challenges, and future prospects influencing the market readiness of aptamer-based diagnostics
Aspects |
Key Points |
Challenges |
Future Scope |
|---|---|---|---|
Market Readiness |
Rapid growth in point-of-care and multiplex aptamer diagnostics (microarrays, QDs, CRISPR). |
Limited clinical validation and standardization. |
AI integration and portable platforms for faster, real-time deployment. |
Regulation |
WHO Essential Diagnostics List guides priorities; FDA/CLIA and EUA facilitate approvals. |
Regulatory diversity delays global accessibility. |
Development of harmonized, adaptive international approval systems. |
Intellectual Property |
IP encourages innovation; open models (CAMBIA, IMI) improve diagnostic accessibility. |
Restrictive licensing limits equitable access, especially in LMICs. |
Adoption of flexible or semi-commons IP models balancing innovation and access. |
Accessibility & Cost |
New diagnostics reduce costs and improve clinical outcomes. |
High upfront costs and poor infrastructure in LMICs. |
Promotion of local manufacturing and optimized supply chain models |
Artificial intelligence integration in aptamer-based diagnostics
Artificial intelligence (AI) significantly improves aptamer-based diagnostics by enhancing the development, efficiency, and statistical interpretation of aptamer systems that employ machine learning with deep learning algorithms, allowing the prediction and generation of aptamer sequences that have high affinity and specificity towards the target. Integrating sequence and structure-based parameters permits these computational models to precisely forecast aptamer-target interactions, thus minimizing experimental efforts and accelerating discovery and enhancing the analytical capabilities of aptamer-based biosensors, particularly when combined with nanoparticle-functionalized or electrochemical sensing platforms, thus improving signal processing, sensitivity, and accuracy.122,123 Advanced predictive modeling involving architectures such as transformers, variational autoencoders, and reinforcement learning algorithms has simplified in silico affinity optimization, assisting in the rational design of aptamers customized for personalized and point-of-care diagnostics.124
AI-driven workflows enable rapid interpretation of high-throughput sequencing data by accelerating clinical decision-making and improving diagnostic reliability with the integration of AI with modern diagnostics, including microfluidic device platforms, biosensors, and Internet of Medical Things (IoMT), which presents major opportunities for real-time, data-driven, and accessible healthcare solutions. Despite all developments, significant challenges in maintaining the stability of aptamers, large-scale production, and the need for regulatory standardization. Future research has to be carried forward on the development of standardized and transport AI procedures that assure us reproducibility, scalability, and fidelity for clinical requirements. This combination enables the growth of rapid, cost-effective diagnostic systems, which implies significant advancements in precision medicine.123,125
Aptamer-based biosensors are becoming a universal and robust platform for contemporary diagnostics because of their tunable structure, easy chemical synthesis, and elevated specificity. Following conjugation with nanomaterials like gold nanoparticles, carbon nanostructures, quantum dots, and metal-organic frameworks, their functional performance is greatly improved in stability, signal transduction, and sensitivity. This review assessed significant detection approaches, colorimetric, electrochemical, fluorescence-based, lateral flow, and CRISPR-based systems, zealously illustrating how each modality is advantaged by the aptamer-nanomaterial synergy. Particular attention was placed on target recognition mechanisms, amplification approaches, and biosensor format innovation, making these systems available for point-of-care and on-site analysis. Technologies concerned have been extensively applied in the identification of pathogenic microorganisms, viruses, toxins, small molecules, and relevant biomarkers in medicine, the environment, and food safety. Although significant strides have been made, issues of aptamer stability in biological matrices, matrix interference, and mass manufacturing remain. Future work must focus on building stable in vivo aptamer systems, incorporating them into digital platforms and AI platforms, and multiplexed sensing. With the ongoing chain of interdisciplinary breakthroughs, nanomaterial-aided aptamer sensors are bound to bridge the gap between molecular diagnostics in the laboratory and cost-effective, real-world health care and monitoring solutions.
ACKNOWLEDGMENTS
The authors thank SRM Institute of Science and Technology Management for providing the facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Hassibian S, Amin M, Taghdisi SM, et al. Aptamers: Design, Theory, and Applications to Diagnosis and Therapy for Diseases. MedComm. 2025;6(5):e70180.
Crossref - Sun H, Zhu X, Lu PY, Rosato RR, Tan W, Zu Y. Oligonucleotide Aptamers: New Tools for Targeted Cancer Therapy. Mol Ther Nucleic Acids. 2014;3(8):e182.
Crossref - Saito S. SELEX-based DNA Aptamer Selection: A Perspective from the Advancement of Separation Techniques. Anal Sci. 2021;37(1):17-26.
Crossref - Raina J, Kaur G, Singh I. Recent progress in nanomaterial-based aptamers as biosensors for point of care detection of Hg2+ ions and its environmental applications. Talanta. 2024;277:126372.
Crossref - Kotkowiak W, Pasternak A. Beyond G-Quadruplexes-The Effect of Junction with Additional Structural Motifs on Aptamers Properties. Int J Mol Sci. 2021;22(18):9948.
Crossref - Wei Y, Tao Z, Wan L, et al. Aptamer-based Cas14a1 biosensor for amplification-free live pathogenic detection. Biosens Bioelectron. 2022;211:114282.
Crossref - Zheng X, Huang Z, Zhang Q, Li G, Song M, Peng R. Aptamer-functionalized nucleic acid nanotechnology for biosensing, bioimaging and cancer therapy. Nanoscale. 2025;17(2):687-704.
Crossref - Guan B, Zhang X. Aptamers as Versatile Ligands for Biomedical and Pharmaceutical Applications. Int J Nanomed. 2020;15:1059-1071.
Crossref - Kalra P, Dhiman A, Cho WC, Bruno JG, Sharma TK. Simple Methods and Rational Design for Enhancing Aptamer Sensitivity and Specificity. Front Mol Biosci. 2018;5:41.
Crossref - Parashar A. Aptamers in Therapeutics. J Clin Diagn Res. 2016;10(6):BE01-BE06.
Crossref - Que-Gewirth NS, Sullenger BA. Gene therapy progress and prospects: RNA aptamers. Gene Ther. 2007;14(4):283-291.
Crossref - Buglak AA, Samokhvalov AV, Zherdev AV, Dzantiev BB. Methods and Applications of In Silico Aptamer Design and Modeling. Int J Mol Sci. 2020;21(22):8420.
Crossref - Lee SJ, Cho J, Lee BH, Hwang D, Park JW. Design and Prediction of Aptamers Assisted by In Silico Methods. Biomedicines. 2023;11(2):356.
Crossref - Zhang N, Chen Z, Liu D, et al. Structural Biology for the Molecular Insight between Aptamers and Target Proteins. Int J Mol Sci. 2021;22(8):4093.
Crossref - Ni S, Zhuo Z, Pan Y, et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl Mater Interfaces. 2021;13(8):9500-9519.
Crossref - Darmostuk M, Rimpelova S, Gbelcova H, Ruml T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol Adv. 2015;33(6):1141-1161.
Crossref - Ilgu M, Fazlioglu R, Ozturk M, Ozsurekci Y, Nilsen-Hamilton M. Aptamers for Diagnostics with Applications for Infectious Diseases. Recent Advances in Analytical Chemistry. IntechOpen. 2019.
Crossref - Radom F, Jurek PM, Mazurek MP, Otlewski J, Jelen F. Aptamers: molecules of great potential. Biotechnol Adv. 2013;31(8):1260-1274.
Crossref - Byun J. Recent Progress and Opportunities for Nucleic Acid Aptamers. Life. 2021;11(3):193.
Crossref - Liu Y, Wang N, Chan CW, et al. The Application of Microfluidic Technologies in Aptamer Selection. Front Cell Dev Biol. 2021;9:730035.
Crossref - Lipi F, Chen S, Chakravarthy M, Rakesh S, Veedu RN. In vitro evolution of chemically-modified nucleic acid aptamers: Pros and cons, and comprehensive selection strategies. RNA Biol. 2016;13(12):1232-1245.
Crossref - HUANG Z, SZOSTAK JW. Evolution of aptamers with a new specificity and new secondary structures from an ATP aptamer. RNA. 2003;9(12):1456-1463.
Crossref - Lawrence C, Vallee-Belisle A, Pfeil SH, de Mornay D, Lipman EA, Plaxco KW. A comparison of the folding kinetics of a small, artificially selected DNA aptamer with those of equivalently simple naturally occurring proteins. Protein Sci. 2014;23(1):56-66.
Crossref - Li CY, Zheng B, Liu YH, et al. A boosting upconversion luminescent resonance energy transfer and biomimetic periodic chip integrated CRISPR/Cas12a biosensor for functional DNA regulated transduction of non-nucleic acid targets. Biosens Bioelectron. 2020;169:112650.
Crossref - Germer K, Leonard M, Zhang X. RNA aptamers and their therapeutic and diagnostic applications. Int J Biochem Mol Biol. 2013;4(1):27-40.
- McCloskey CM, Li Q, Yik EJ, et al. Evolution of Functionally Enhanced a-L-Threofuranosyl Nucleic Acid Aptamers. ACS Synth Biol. 2021;10(11):3190-3199.
Crossref - Wu W, Zhang J, Zheng M, et al. An Aptamer-Based Biosensor for Colorimetric Detection of Escherichia coli O157:H7. PloS One. 2012;7(11):e48999.
Crossref - Chen Y, Xiang J, Liu B, Chen Z, Zuo X. Gold nanoparticle-engineered electrochemical aptamer biosensor for ultrasensitive detection of thrombin. Anal Methods. 2020;12(29):3729-3733.
Crossref - Wei X, Zhou W, Sanjay ST, et al. Multiplexed Instrument-Free Bar-Chart SpinChip Integrated with Nanoparticle-Mediated Magnetic Aptasensors for Visual Quantitative Detection of Multiple Pathogens. Anal Chem. 2018;90(16):9888-9896.
Crossref - Vilela D, Gonzalez MC, Escarpa A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal Chim Acta. 2012;751:24-43.
Crossref - Davydova A, Vorobyeva M. Aptamer-Based Biosensors for the Colorimetric Detection of Blood Biomarkers: Paving the Way to Clinical Laboratory Testing. Biomedicines. 2022;10(7):1606.
Crossref - Liu X, Hussain M, Dai J, et al. Programmable Biosensors Based on RNA-Guided CRISPR/Cas Endonuclease. Biol Proced Online. 2022;24(1):2.
Crossref - Mu Y, Chen Z, Zhan J, Zhang J. Recent Advances in Aptamer-Based Sensors for In Vitro Detection of Small Molecules. Anal Sens. 2024;4(6):e202400027.
Crossref - Weng X, Zhang C, Jiang H. Advances in microfluidic nanobiosensors for the detection of foodborne pathogens. LWT. 2021;151:112172.
Crossref - Jepsen MDE, Sparvath SM, Nielsen TB, et al. Development of a genetically encodable FRET system using fluorescent RNA aptamers. Nat Commun. 2018;9(1):18.
Crossref - Ghosh S, Chen Y, Sebastian J, George A, Dutta M, Stroscio MA. A study on the response of FRET based DNA aptasensors in intracellular environment. Sci Rep. 2020;10:13250.
Crossref - Liu CH, Grodzinski P. Nanotechnology for Cancer Imaging: Advances, Challenges, and Clinical Opportunities. Radiol Imaging Cancer. 2021;3(3):e200052.
Crossref - Shabalina AV, Sharko DO, Glazyrin YE, et al. Development of Electrochemical Aptasensor for Lung Cancer Diagnostics in Human Blood. Sensors. 2021;21(23):7851.
Crossref - Kang WJ, Chae JR, Cho YL, Lee JD, Kim S. Multiplex imaging of single tumor cells using quantum-dot-conjugated aptamers. Small Weinh Bergstr Ger. 2009;5(22):2519-2522.
Crossref - Katilius E, Katiliene Z, Woodbury NW. Signaling aptamers created using fluorescent nucleotide analogues. Anal Chem. 2006;78(18):6484-6489.
Crossref - Kim S, Kuroda A, Fujitsuka M, Majima T. Amplifying fluorescence signal contrast of aptamer-modified microspheres inspired by whispering-gallery mode lasing. RSC Adv. 2018;8(37):20822-20828.
Crossref - Sheng Z, Hu D, Zhang P, et al. Cation exchange in aptamer-conjugated CdSe nanoclusters: a novel fluorescence signal amplification for cancer cell detection. Chem Commun. 2012;48(35):4202-4204.
Crossref - Liu Z, Liu Y, Sun Y, Chen G, Chen Y. Double-stranded DNA-scaffolded fluorescent probes for fluorescence imaging of cell-surface molecules. RSC Adv. 2017;7(83):52581-52587.
Crossref - Youn H, Lee K, Her J, et al. Aptasensor for multiplex detection of antibiotics based on FRET strategy combined with aptamer/graphene oxide complex. Sci Rep. 2019;9(1):7659.
Crossref - Kim YS, Jurng J. Gold nanoparticle-based homogeneous fluorescent aptasensor for multiplex detection. Analyst. 2011;136(18):3720-3724.
Crossref - Niu S, Lv Z, Liu J, Bai W, Yang S, Chen A. Colorimetric Aptasensor Using Unmodified Gold Nanoparticles for Homogeneous Multiplex Detection. PLoS ONE. 2014;9(10):e109263.
Crossref - Shaban SM, Kim DH. Recent Advances in Aptamer Sensors. Sensors. 2021;21(3):979.
Crossref - Majdinasab M, Marty JL. Recent Advances in Electrochemical Aptasensors for Detection of Biomarkers. Pharmaceuticals. 2022;15(8):995.
Crossref - Kurup CP, Mohd-Naim NF, Ahmed MU. Recent trends in nanomaterial-based signal amplification in electrochemical aptasensors. Crit Rev Biotechnol. 2022;42(5):794-812.
Crossref - Jiang L, Qian J, Yang X, et al. Amplified impedimetric aptasensor based on gold nanoparticles covalently bound graphene sheet for the picomolar detection of ochratoxin A. Anal Chim Acta. 2014;806:128-135.
Crossref - Velu R, DeRosa MC. Lateral flow assays for Ochratoxin A using metal nanoparticles: comparison of “adsorption-desorption” approach to linkage inversion assembled nano-aptasensors (LIANA). Analyst. 2018;143(19):4566-4574.
Crossref - Mark D, Haeberle S, Roth G, Stetten F von, Zengerle R. Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev. 2010;39(3):1153-1182.
Crossref - Samiei E, Tabrizian M, Hoorfar M. A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab Chip. 2016;16(13):2376-2396.
Crossref - Foudeh AM, Didar TF, Veres T, Tabrizian M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip. 2012;12(18):3249-3266.
Crossref - Xu R, Ouyang L, Chen H, Zhang G, Zhe J. Recent Advances in Biomolecular Detection Based on Aptamers and Nanoparticles. Biosensors. 2023;13(4):474.
Crossref - Chiu TC, Huang CC. Aptamer-Functionalized Nano-Biosensors. Sensors. 2009;9(12):10356-10388.
Crossref - Lee JH, Yigit MV, Mazumdar D, Lu Y. Molecular Diagnostic and Drug Delivery Agents based on Aptamer-Nanomaterial Conjugates. Adv Drug Deliv Rev. 2010;62(6):592-605.
Crossref - Jo H, Ban C. Aptamer-nanoparticle complexes as powerful diagnostic and therapeutic tools. Exp Mol Med. 2016;48(5):e230.
Crossref - Liao J, Liu B, Liu J, Zhang J, Chen K, Liu H. Cell-specific aptamers and their conjugation with nanomaterials for targeted drug delivery. Expert Opin Drug Deliv. 2015;12(3):493-506.
Crossref - Deng G, Zha H, Luo H, Zhou Y. Aptamer-conjugated gold nanoparticles and their diagnostic and therapeutic roles in cancer. Front Bioeng Biotechnol. 2023;11.
Crossref - Nnachi RC, Sui N, Ke B, et al. Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ Int. 2022;166:107357.
Crossref - Li P, Zhan H, Tao S, Xie Z, Huang J. Bio-inspired aptamers decorated gold nanoparticles enable visualized detection of malathion. Front Bioeng Biotechnol. 2023;11:1165724.
Crossref - Evtugyn G, Porfireva A, Shamagsumova R, Hianik T. Advances in Electrochemical Aptasensors Based on Carbon Nanomaterials. Chemosensors. 2020;8(4):96.
Crossref - Nammahachak N, Aup-Ngoen KK, Asanithi P, et al. Hydrothermal synthesis of carbon quantum dots with size tunability via heterogeneous nucleation. RSC Adv. 2022;12(49):31729-31733.
Crossref - Kumaran A, Jude Serpes N, Gupta T, et al. Advancements in CRISPR-Based Biosensing for Next-Gen Point of Care Diagnostic Application. Biosensors. 2023;13(2):202.
Crossref - Zou Y, Griveau S, Ringuedי A, Bedioui F, Richard C, Slim C. Functionalized Multi-Walled Carbon Nanotube-Based Aptasensors for Diclofenac Detection. Front Chem. 2022;9:812909.
Crossref - Behyar MB, Nilghaz A, Ebrahimi R, Hasanzadeh M, Shadjou N. Advancements in magnetic aptasensors: Recent progress and future trends in biosensor technology. TrAC Trends Anal Chem. 2024;172:117549.
Crossref - Zhou J, Li Z, Olajide JS, Wang G. CRISPR/Cas-based nucleic acid detection strategies: Trends and challenges. Heliyon. 2024;10(4):e26179.
Crossref - Sundaresan SM, Fothergill SM, Tabish TA, Ryan M, Xie F. Aptamer biosensing based on metal enhanced fluorescence platform: A promising diagnostic tool. Appl Phys Rev. 2021;8(4):041311.
Crossref - Banerjee T, Panchal N, Sutton C, et al. Tunable Magneto-Plasmonic Nanosensor for Sensitive Detection of Foodborne Pathogens. Biosensors. 2023;13(1):109.
Crossref - Liu L, Hong J, Wang W, et al. Fluorescent aptasensor for detection of live foodborne pathogens based on multicolor perovskite-quantum-dot-encoded DNA probes and dual-stirring-bar-assisted signal amplification. J Pharm Anal. 2022;12(6):913-922.
Crossref - Kadam US, Cho Y, Park TY, Hong JC. Aptamer-based CRISPR-Cas powered diagnostics of diverse biomarkers and small molecule targets. Appl Biol Chem. 2023;66(1):13.
Crossref - Niu C, Wang C, Li F, Zheng X, Xing X, Zhang C. Aptamer assisted CRISPR-Cas12a strategy for small molecule diagnostics. Biosens Bioelectron. 2021;183:113196.
Crossref - Jiao C, Sharma S, Dugar G, et al. Noncanonical crRNAs derived from host transcripts enable multiplexable RNA detection by Cas9. Science. 2021;372(6545):941-948.
Crossref - Zhao X, Li S, Liu G, et al. A versatile biosensing platform coupling CRISPR-Cas12a and aptamers for detection of diverse analytes. Sci Bull. 2021;66(1):69-77.
Crossref - Yoo H, Jo H, Oh SS. Detection and beyond: challenges and advances in aptamer-based biosensors. Mater Adv. 2020;1(8):2663-2687.
Crossref - Di Nardo F, Chiarello M, Cavalera S, Baggiani C, Anfossi L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors. 2021;21(15):5185.
Crossref - Moretta R, De Stefano L, Terracciano M, Rea I. Porous Silicon Optical Devices: Recent Advances in Biosensing Applications. Sensors. 2021;21(4):1336.
Crossref - Seibold JM, Abeykoon SW, Ross AE, White RJ. Development of an Electrochemical, Aptamer-Based Sensor for Dynamic Detection of Neuropeptide Y. ACS Sens. 2023;8(12):4504-4511.
Crossref - Wei Z, Zhou Y, Wang R, Wang J, Chen Z. Aptamers as Smart Ligands for Targeted Drug Delivery in Cancer Therapy. Pharmaceutics. 2022;14(12):2561.
Crossref - Chaghazardi M, Kashanian S, Nazari M, Shariati-Rad M, Joseph Y, Rahimi P. Mercury (Ii) Sensing Using a Simple Turn-On Fluorescent Graphene Oxide Based Aptasensor in Serum and Water Samples. Social Science Research Network. 2023.
Crossref - Wang W, He Y, He S, et al. A Brief Review of Aptamer-Based Biosensors in Recent Years. Biosensors. 2025;15(2):120.
Crossref - Kim TH, Lee SW. Aptamers for Anti-Viral Therapeutics and Diagnostics. Int J Mol Sci. 2021;22(8):4168.
Crossref - Labib M, Zamay AS, Kolovskaya OS, et al. Aptamer-Based Impedimetric Sensor for Bacterial Typing. Anal Chem. 2012;84(19):8114-8117.
Crossref - Tang Z, Gao M, Gong F, et al. Quantum Dot Reporters Designed for CRISPR-Based Detection of Viral Nucleic Acids. Anal Chem. 2024;96(40):16017-16026.
Crossref - Wandtke T, Wo niak J, Kopinski P. Aptamers in diagnostics and treatment of viral infections. Viruses. 2015;7(2):751-780.
Crossref - Gonzalez VM, Martin ME, Fernandez G, Garcia-Sacristan A. Use of Aptamers as Diagnostics Tools and Antiviral Agents for Human Viruses. Pharm Basel Switz. 2016;9(4):78.
Crossref - Leguillier V, Heddi B, Vidic J. Recent Advances in Aptamer-Based Biosensors for Bacterial Detection. Biosensors. 2024;14(5):210.
Crossref - Mercier MC, Dontenwill M, Choulier L. Selection of Nucleic Acid Aptamers Targeting Tumor Cell-Surface Protein Biomarkers. Cancers. 2017;9(6):69.
Crossref - Mo T, Liu X, Luo Y, et al. Aptamer-based biosensors and application in tumor theranostics. Cancer Sci. 2022;113(1):7-16.
Crossref - Wu X, Chen J, Wu M, Zhao JX. Aptamers: Active Targeting Ligands for Cancer Diagnosis and Therapy. Theranostics. 2015;5(4):322-344.
Crossref - Nguyen VT, Kwon YS, Gu MB. Aptamer-based environmental biosensors for small molecule contaminants. Curr Opin Biotechnol. 2017;45:15-23.
Crossref - McConnell EM, Nguyen J, Li Y. Aptamer-Based Biosensors for Environmental Monitoring. Front Chem. 2020;8.
Crossref - Flores-Contreras EA, Gonzalez-Gonzalez RB, Gonzalez-Gonzalez E, Melchor-Martinez EM, Parra-Saldivar R, Iqbal HMN. Detection of Emerging Pollutants Using Aptamer-Based Biosensors: Recent Advances, Challenges, and Outlook. Biosensors. 2022;12(12):1078.
Crossref - Mishra GK, Sharma V, Mishra RK. Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review. Biosensors. 2018;8(2):28.
Crossref - Zhang K, Li H, Wang W, Cao J, Gan N, Han H. Application of Multiplexed Aptasensors in Food Contaminants Detection. ACS Sens. 2020;5(12):3721-3738.
Crossref - Shaver A, Kundu N, Young BE, Vieira PA, Sczepanski JT, Arroyo-Curras N. Nuclease Hydrolysis Does Not Drive the Rapid Signaling Decay of DNA Aptamer-Based Electrochemical Sensors in Biological Fluids. Langmuir. 2021;37(17):5213-5221.
Crossref - Dunn MR, McCloskey CM, Buckley P, Rhea K, Chaput JC. Generating Biologically Stable TNA Aptamers that Function with High Affinity and Thermal Stability. J Am Chem Soc. 2020;142(17):7721-7724.
Crossref - Zang Y, Lei J, Ju H. Principles and applications of photoelectrochemical sensing strategies based on biofunctionalized nanostructures. Biosens Bioelectron. 2017;96:8-16.
Crossref - Thiviyanathan V, Gorenstein DG. Aptamers and the Next Generation of Diagnostic Reagents. Proteomics Clin Appl. 2012;6(11-12):563-573.
Crossref - Liu B, Zhuang J, Wei G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ Sci Nano. 2020;7(8):2195-2213.
Crossref - Kaiser L, Weisser J, Kohl M, Deigner HP. Small molecule detection with aptamer based lateral flow assays: Applying aptamer-C-reactive protein cross-recognition for ampicillin detection. Sci Rep. 2018;8(1):5628.
Crossref - Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16(3):181-202.
Crossref - Wang Q, Yin Q, Fan Y, et al. Double quantum dots-nanoporphyrin fluorescence-visualized paper-based sensors for detecting organophosphorus pesticides. Talanta. 2019;199:46-53.
Crossref - Qi S, Duan N, Khan IM, et al. Strategies to manipulate the performance of aptamers in SELEX, post-SELEX and microenvironment. Biotechnol Adv. 2022;55:107902.
Crossref - Chang M, Song T, Liu X, Lin Q, He B, Ren J. Cellulose-based Biosensor for Bio-molecules Detection in Medical Diagnosis: A Mini-Review. Curr Med Chem. 2020;27(28):4593-4612.
Crossref - Fritzler MJ, Fritzler ML. The Emergence of Multiplexed Technologies as Diagnostic Platforms in Systemic Autoimmune Diseases. Curr Med Chem. 2006;13(21):2503-2512.
Crossref - Moussy FG, Berumen AV, Pai M. The WHO list of essential in vitro diagnostics: Development and next steps. EBioMedicine. 2018;37:1-2.
Crossref - Kosack CS, Page AL, Klatser PR. A guide to aid the selection of diagnostic tests. Bull World Health Organ. 2017;95(9):639-645.
Crossref - Sarata AK, Johnson JA. Regulation of Clinical Tests: In Vitro Diagnostic (IVD) Devices, Laboratory Developed Tests (LDTs), and Genetic Tests. Congressional Research Service; 2014
- Tran NT, Danner E, Li X, et al. Precise CRISPR-Cas-mediated gene repair with minimal off-target and unintended on-target mutations in human hematopoietic stem cells. Sci Adv. 2022;8(22):eabm9106.
Crossref - Quinn SC, Jamison AM, Freimuth V. Communicating Effectively About Emergency Use Authorization and Vaccines in the COVID-19 Pandemic. Am J Public Health. 2021;111(3):355-358.
Crossref - Glorikian H, Warburg RJ, Moore K, Malinowski J. Intellectual property considerations for molecular diagnostic development with emphasis on companion diagnostics. Expert Opin Ther Pat. 2018;28(2):123-128.
Crossref - Thornblad T, Hedner T. The impact of open IP platforms on IP-strategy norms in life sciences. Int J Technol Intell Plan. 2012.
Crossref - Panagopoulos A, Sideri K. Prospect patents and CRISPR; rivalry and ethical licensing in a semi-commons environment. J Law Biosci. 2021;8(2):lsab031.
Crossref - Graff GD, Sherkow JS. Models of Technology Transfer for Genome-Editing Technologies. Annu Rev Genomics Hum Genet. 2020;21:509-534.
Crossref - Karl JW, Swart E, Strauch RJ. Diagnosis of Occult Scaphoid Fractures: A Cost-Effectiveness Analysis. J Bone Joint Surg Am. 2015;97(22):1860-1868.
Crossref - de Alava E, Pareja MJ, Carcedo D, Arrabal N, Garcia JF, Bernabe-Caro R. Cost-effectiveness analysis of molecular diagnosis by next-generation sequencing versus sequential single testing in metastatic non-small cell lung cancer patients from a south Spanish hospital perspective. Expert Rev Pharmacoecon Outcomes Res. 2022;22(6):1033-1042.
Crossref - McNerney R. Diagnostics for Developing Countries. Diagnostics. 2015;5(2):200-209.
Crossref - Kuupiel D, Bawontuo V, Drain PK, Gwala N, Mashamba-Thompson TP. Supply chain management and accessibility to point-of-care testing in resource-limited settings: a systematic scoping review. BMC Health Serv Res. 2019;19(1):519.
Crossref - Engel N, Wachter K, Pai M, et al. Addressing the challenges of diagnostics demand and supply: insights from an online global health discussion platform. BMJ Glob Health. 2016;1(4):132.
Crossref - Chen Z, Hu L, Zhang BT, et al. Artificial Intelligence in Aptamer-Target Binding Prediction. Int J Mol Sci. 2021;22(7):3605.
Crossref - Fasogbon IV, Ondari EN, Tusubira D, et al. Advances and future directions of aptamer-functionalized nanoparticles for point-of-care diseases diagnosis. Biol Methods Protoc. 2025;10(1):bpaf046.
Crossref - Wang Z, Dong Y, Sui X, et al. An artificial intelligence-assisted microfluidic colorimetric wearable sensor system for monitoring of key tear biomarkers. Npj Flex Electron. 2024;8(1):35.
Crossref - Kumar S, Mohan A, Sharma NR, et al. Computational Frontiers in Aptamer-Based Nanomedicine for Precision Therapeutics: A Comprehensive Review. ACS Omega. 2024;9(25):26838-26862.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.