Lactic acid bacteria (LAB) and Bifidobacterium make exopolysaccharides (EPS), which positively affect the physicochemical and sensory properties of fermented food. The isolated EPS is also useful for improving viscosity, stability, and food textures, and also finds applications in the medical field. Thus, there is an increasing research focus on enhancing EPS production by these bacteria. Altering the growth media composition, by varying carbon and mineral sources, is a tested approach for such a purpose. Cultivation conditions like temperature, pH, and shaking also significantly influence EPS production in a strain-specific manner. Given the plausible role of EPS in stress tolerance, elevating EPS yield by exposure to certain stressors, such as bile, has been achieved. Advanced strategies such as evolutionary engineering and cross-kingdom ecological interactions of LAB, especially with yeast, also appear to be promising techniques for enhancing bacterial EPS yield and quality. This review elucidates recent research on all the above-mentioned ways of enhancing EPS production and the possible utility of such bacteria in industrial applications.
Adaptive Laboratory Evolution, Biomaterial, EPS Biosynthesis, Food Fermentation, Food Texture, Lactobacilli, Stress Response
Lactic acid bacteria (LAB) and Bifidobacterium spp. belong to the generally recognized as safe (GRAS) category of microbes. They are associated with numerous habitats, including fermented foods and the human GI tract. Exopolysaccharides (EPS) are essential products secreted by these bacteria into the extracellular environment. EPS plays a key role in helping bacteria adapt to numerous environmental stimuli, including serving as a physical barrier for protection against bacteriophages, extreme pH, and inhibitory chemicals. Additionally, the water-holding ability of EPS helps bacteria from desiccation. EPS is an essential component of biofilms and thus aids in intra- and inter-kingdom communication.1
Foods fermented with these bacteria, such as dairy products, often contain EPS produced in situ. Bacterial EPS are also exogenously added to processed foods as emulsifiers and thickening agents.2 Thus, the human gut receives bacterial EPS via such foods. These bacteria are important components of the human gut microbiome and also produce EPS in the gut. EPS can provide a substrate for fermentation by a portion of the microbiota in the distal colon, acting as a prebiotic and supporting the growth of beneficial bacteria.3 In addition to its involvement in gut microbial communications, EPS influences intestinal adhesion of microbes, provides a substrate for producing bioactive short-chain fatty acids, imparts immunomodulatory effects, and thus affects the overall gut microbiome and host health.4
Bacterial EPS plays a pivotal role in food applications due to its remarkable properties that enhance rheology, texture, mouthfeel, and overall sensory experience. Specific EPS-producing strains of LAB, such as those belonging to the Lactobacillus genus, are widely employed in yogurt production to improve the viscosity and texture of the yogurt.5 Similarly, in cheese-making, EPS enhances the overall quality and texture, elevating the product’s appeal.6 Its water-holding ability also makes EPS useful as a biomaterial in wound-healing applications7 and as a hydrating agent in the cosmetic industry.8
Bacterial EPS can be secreted in two forms: slime polysaccharides, loosely attached or expelled into the environment surrounding the bacteria, and capsular polysaccharides, which form a capsule around the bacteria by adhering to the cell wall.9 Based on their structures, EPS can be classified as homopolysaccharides (HoPS) comprising a single type of monosaccharide, such as glucose and fructose, and heteropolysaccharides (HePS), composed of different monosaccharides. Some of the well-known producers of HoPS, such as fructan, β-D-glucan, and dextran, are Lactobacillus and Leuconostoc spp.10 On the other hand, HePS are more commonly produced by Lactococcus and Lactobacillus spp.11 Many LAB strains that combine these polysaccharides are also known.12 Bacterial production of HoPS is generally higher than that of HePS because the simpler pathway requires fewer cellular resources.
The bioactivity and physicochemical properties of bacterial EPS depend on their structural properties, including molecular weight, monosaccharide composition, branching, linkages, and chain length.13 For example, EPS with low molecular weight and negative charge often have higher immuno-stimulating properties compared to high molecular weight EPS.14 High antioxidant activity was also observed in EPS with a low molecular weight.15 Other factors, such as specific functional groups and glycosidic linkages, are also thought to affect the anticancer and anti-inflammatory activity of EPS produced by Lactobacillus spp.16 The different monosaccharides are seen to have different impacts on the bioactivity of the EPS. EPS rich in glucose and galactose have been reported to have strong immunomodulatory activities; those with mannose possess anti-inflammatory activities, and those composed of rhamnose and glucose have shown antimicrobial activity.17
The backbone structure affects the water solubility of EPS, mainly due to the quantity and distribution of various linkages. Additionally, biological modifications by enzymes can affect solubility by adding or eliminating functional groups such as acetyl, pyruvyl, succinyl, and glyceryl groups.18 Furthermore, in vitro chemical modifications, such as acetylation and sulfonation, have been observed to enhance the solubility of the polysaccharide.19 Similarly, the rheological property of EPS is also influenced by its glycosidic linkages, functional groups, molecular weight, and sugar content.20
The interplay of various factors, such as environmental, nutritional, and genetic aspects, can govern bacterial EPS structural characteristics and biosynthesis. The optimization of media, including the carbon and nitrogen sources, along with other parameters, such as temperature and pH, leads to modulation in the yield and structural properties of the EPS. Given the critical interactions of various factors involved, a thorough understanding of the mechanisms behind EPS biosynthesis is essential. Increased EPS production has been found to improve the texture and rheological characteristics of fermented foods in the food industry and stimulate the development of bioactive compounds. Boosting EPS production by optimizing the culture conditions would expand their functionality for industrial use. This mini-review examines the various ways in which the properties and yield of EPS produced by Lactobacillus and Bifidobacterium spp. could be improved. Since genetically engineered bacteria are not allowed in foods, studies using such strategies for improving EPS production have not been covered in this article.
Effect of Media Composition
The chemical composition of the culture media is one of the obvious factors that impact the yield and the quality of the EPS. The carbon source is the most influential media component affecting bacterial EPS production. A fundamental reason is that homopolysaccharides, such as glucan and fructan, are synthesised from sucrose, while heteropolysaccharides are primarily synthesised from glucose.21 Some studies showing the varying EPS yields with different sugars in the medium, such as glucose, fructose, mannose, sucrose, galactose, cellobiose, and lactose, have been carried out in various genera of LAB and Bifidobacterium spp., such as Lacticaseibacillus, Lactiplantibacillus, Lactobacillus, Lactococcus, Limosilactobacillus, Weissella, Leuconostoc and Bifidobacterium (Table). One common finding across all such studies is that there is no universal rule regarding which sugar, when included in the medium, leads to higher EPS production in all Lactobacillus spp. Such a conclusion also emerges from the fact that the most variable genomic regions in Lactobacillus spp. are those involved in carbohydrate utilization, involving genes for sugar transport, energy metabolism and biosynthesis of structural components.22 Thus, it is imperative that for any given strain, the sugar giving the highest EPS yield be experimentally determined. Indeed, such an approach can itself result novel mechanistic insights into EPS biosynthesis. For example, while glucans are derived from sucrose, Mayer et al.23 found glucan production in Lactobacillus johnsonii FI9785 in the absence of sucrose. Further studies using genetic tools led to the discovery of a novel bactoprenol glycosyltransferase and flippase-dependent EPS biosynthesis pathway.
Table:
Studies on optimization of media composition and cultivation conditions for improving the EPS production by lactic acid bacteria and bifidobacteria
No. |
Bacteria |
Type of EPS |
Experimental variables |
Optimum conditions |
References |
|---|---|---|---|---|---|
1 |
L. rhamnosus C83 |
HePS |
Carbon sources and temperatures (20-37 °C) |
40 g/L mannose and 20 g/L glucose +fructose; 25 °C |
75 |
2 |
L. delbrueckii subsp. bulgaricus CNRZ 1187 and CNRZ 416 |
HePS |
Glucose concentrations |
10 g/L glucose |
76 |
3 |
L. rhamnosus RW-9595M |
Not specified |
Nitrogen sources, amino acids and salts |
Whey permeate with yeast extract supplemented with salts and amino acids |
28 |
4 |
L. helveticus ATCC 15807 |
HoPS |
Carbon sources, vitamins, nucleotide bases and pH (4.5 & 6.2) |
Lactose, biotin & thiamine, and adenine at pH 4.5 |
26 |
5 |
L. delbrueckii subsp. bulgaricus B3 & G12, and S. thermophilus W22 |
Not specified |
pH (4.0–7.0), temperatures (30- 45 °C) and cultivation time (5–48 h) |
pH of 6.2 at 45°C for B3 & G12 and pH of 6.8 at 45 °C for W22 |
43 |
6 |
L. pentosus LPS26 |
HoPS |
pH (5.0 and 6.0) and temperatures (20-30 °C) |
pH 6.0 at 20 °C |
42 |
7 |
L. lactis subsp. cremosris (JFR1) |
HoPS |
pH (5.5-6.5) |
pH 5.5 |
40 |
8 |
L. fermentum F6 |
HoPS |
Carbon sources, nitrogen sources, pH (5.0-7.0), and temperatures (25-42 °C) |
2% (W/V) of glucose, 0.5% (W/V) of whey protein concentrate, pH 6.5 at 37 °C in skim milk |
38 |
9 |
B. longum subsp. infantis CCUG 52486 and Bifiinfantis NCIMB 702205 |
Not specified |
Nitrogen sources and temperatures (25-42 °C) |
1.5% of casein hydrolysate at 37 °C |
77 |
10 |
S. thermophilus BN1 |
Not specified |
Temperatures (37 and 42 °C) |
37 °C |
41 |
11 |
L. plantarum NTMI05 |
HePS |
Carbon, organic, and inorganic nitrogen sources |
RSM method – 20 g/L glucose, 25 g/L yeast extract and 2 g/L ammonium sulphate |
78 |
12 |
W. confusa OF126 |
HoPS |
Sucrose concentrations, pH (6.0-8.0), temperature (20-40 °C) and cultivation time (12-96 h) |
RSM method- 24 g/L sucrose; pH of 7.0; 30 °C; and 48.5 h |
79 |
13 |
S. thermophilus 1275 |
Not specified |
pH (4.5-6) and temperatures (30-42 °C) |
pH of 5.5 at 40 °C |
39 |
14 |
Weissella cibaria 10 M |
Dextran |
Temperatures (6-30 °C) |
A cold shift from 30 °C to 6 °C |
44 |
15 |
L. mesenteroides NRRL B-512F |
Dextran |
Sucrose concentration, permeate powder, and yeast extract |
20 g/L sucrose, 15 g/L milk permeate, and 15 g/L yeast extract |
25 |
16 |
L. lactis AV1 |
Dextran |
Carbon sources and temperatures (20-37 °C) |
0.8% sucrose, and 20 °C |
45 |
17 |
W. confusa XG-3 |
Not specified |
Carbon sources, pH (2.0-12) and inorganic sources |
80.1 g/L sucrose, pH of 5.8, and 3.7 g/L sodium acetate |
80 |
18 |
L. rhamnosus LOCK 0943, LOCK 0935, and OM-1. |
Not specified |
Carbon and nitrogen sources |
20 g/L fructose, glucose and sucrose for LOCK 0935; 20 g/L fructose for LOCK 0943; and 20 g/L sucrose for OM-1; no significant difference found in nitrogen sources |
81 |
19 |
L. plantarum MF460, MF556 |
Not specified |
Carbon sources, nitrogen sources, pH (5.0-7.0), temperature (20-37 °C), cultivation time (48-120 h), and mineral salts |
Glucose; bacto-peptone; pH 6.0; 30 °C after 48 h for MF460 and addition of calcium carbonate for MF556 |
31 |
20 |
L. mesenteroides SF3 |
Dextran |
Sucrose concentrations (10%-35%), temperatures (25-37 °C), and cultivation time (8-24 h) |
RSM method- 10% sucrose; 25 °C; and 16 h |
82 |
21 |
L. plantarum K25 |
HoPS |
Inorganic salt (0-100 mg/L) |
20 mg/L of CaCl2 |
32 |
22 |
Leuconostoc citreum BD1707 |
Levan |
Sucrose concentrations (50-250 g/L), pH (4.5- 8.5), temperatures (15-35 °C), and Cultivation time (0-96 h) |
RSM method- 172 g/L sucrose; 26 °C; and 112 h |
83 |
23 |
S. thermophilus 937 |
HoPS |
Amino acids |
1 mM histidine, isoleucine and glutamate each |
27 |
24 |
L. paracasei 2333 and
L. rhamnosus 1019 |
HePS |
Carbon sources |
Maltose and lactose, respectively, for each strain |
84 |
25 |
L. plantarum RO30 |
Not specified |
Carbon sources, organic, and inorganic nitrogen sources |
OFAT method – 20 g/L of sucrose, 25 g/L of beef extract and 4 g/L of ammonium sulfate RSM method – 40 g/L of sucrose, 25 g/L of beef extract, 5.5 of pH, at 30 °C, for 72 h |
36 |
In addition to the EPS yield, the carbon source also influences the properties of EPS made by the bacteria. Polak-Berecka et al.24 showed that varying carbon sources in the media can not only affect the chain length and branching of the EPS produced by Lactobacillus rhamnosus E/N but also influence the viscosity of the polymers. Esmaeilnejad-Moghadam et al.25 found that fermentation of milk permeate by Leuconostoc mesenteroides NRRL B-512F leads to the production of dextran with a lower molecular weight and higher solubility than that made upon fermentation of MRS broth. This effect of permeate was attributed to the presence of lactose, which inhibits the dextransucrase enzymes, resulting in shorter dextran chains.
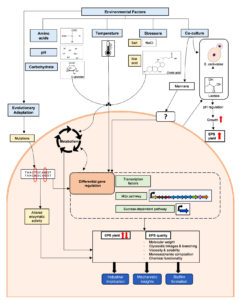
Apart from sugars, most other media components are not the direct precursors for EPS biosynthesis. However, since they affect other biochemical and physiological processes in bacteria, their presence in the media can influence EPS production. This is especially applicable to HePS, which are made via a Wzy-dependent pathway that relies on several expensive cellular resources such as sugar nucleotides. Obviously, any media components that support the biosynthesis of sugar nucleotides can result in higher EPS production. In Lactobacillus helveticus ATCC 15807, the provision of adenine in the growth media significantly stimulated the synthesis of EPS.26 The adenine could help in generating ATP, sparing the nucleotides for EPS biosynthesis. Wa et al.27 found that the inclusion of amino acids, such as histidine, glutamate, and isoleucine, in the defined media resulted in a 2-fold increase in EPS production in Streptococcus thermophilus 937. This phenomenon was shown to be driven by increased activities of enzymes involved in the synthesis of sugar nucleotides, along with upregulation of the eps gene cluster (Figure). A similar effect of amino acids on stimulating the EPS production has been reported earlier in L. rhamnosus RW-9595M.28 While the exact mechanism of how amino acids upregulate the EPS gene cluster is not known, studies from other bacteria can provide some insights. Trouillon et al.29 found that certain amino acids caused differential expression of as many as 32 transcription factors in Escherichia coli. Provided that HePS biosynthesis in LAB is controlled by various transcriptional regulators, including those belonging to LytR,30 the impact of amino acids on their expression is highly plausible and needs to be investigated.
Figure. Mechanism of the influence of various environmental factors on the EPS production by Lactobacillus and Bifidobacterium spp.
Since milk is the traditional medium for cultivating LAB and is naturally rich in calcium, the influence of calcium on the physiology of LAB has drawn significant scientific interest. Studies suggest varying effects of calcium on the EPS production by different Lactobacillus spp. strains. Midik et al.31 found that calcium carbonate marginally affects EPS production only in a few LAB strains. In the case of Lactiplantibacillus plantarum K25, calcium chloride significantly enhanced EPS production while also affecting structural features such as molecular weight and monosaccharide composition, along with causing the upregulation of EPS biosynthesis genes.32 The total content of rhamnose in EPS was observed to be increased in the presence of calcium, possibly because of upregulation of cps4F (capsular polysaccharide biosynthesis gene) and rfbD (encodes rhamnose pathway enzyme). Such an influence of calcium could be driven via transcriptional regulators, which were found to be differentially regulated by calcium. The modulation of EPS biosynthesis by calcium is also intertwined with its effect on the related phenomenon in probiotics, such as biofilm formation, adhesion to eukaryotic cells, and possible regulation of mucus-binding proteins.33,34
While varying individual media components and studying the influence on EPS generation and their properties provide useful mechanistic insights into EPS biosynthesis, industries are ultimately interested in getting the maximum EPS yield from LAB. Thus, varying all possible media components for identifying the best combination giving the highest EPS yield has been a common theme of many studies (Table). Kefiran is one such commercially important EPS made primarily by Lactobacillus kefiranofaciens. Its production was optimized by testing varying carbon and nitrogen sources, vitamins, minerals, fermentation temperature, and agitation speed.35 Assessing and optimizing various culture parameters in all possible combinations manually can be a laborious task. Thus, statistical methods such as one-factor-at-a-time experimentation,36 response surface methodology,25 and Plackett-Burman design37 have also been found useful in media optimization for modulating EPS production by Lactobacillus spp. strains.
Because of the production of lactic acid, fermentation of the given medium by Lactobacillus spp. strains lead to a drop in pH. Such acidification is integral for their growth as it suppresses competitive microbes, but also affects many physiological processes in LAB, including EPS production. However, the direction of such an effect shows genus-wise variation, highlighting possible physiological and metabolic differences. For example, while Limosilactobacillus fermentum F6 yielded the highest quantity of EPS at pH 6.5, at lower pH,38 L. helveticus ATCC 15807 depicted higher EPS synthesis at pH 4.5 than at pH 6.2. In the case of S. thermophilus ASCC 1275, the expression of genes involved in EPS production was upregulated as the pH decreased to 5.5, which can lead to higher EPS biosynthesis.39 EPS can increase the viscosity of the cultivation medium and is one of the most crucial determinants of its textural applications. Viscosity appears to be positively correlated to the acidity of the cultivation medium of the EPS-producing LAB.38,40 In case of Lactococcus lactis subsp. cremoris JFR1, such an increase in the viscosity of the cultivation medium at low pH was possibly because of complex interactions between EPS and proteins in the medium.40 Contrastingly, the solution made from purified EPS obtained from a low pH medium had lower viscosity in spite of a higher molecular weight. Thus, it is important to evaluate the impact of EPS on the rheological properties of foods by distinctly considering whether the EPS is produced in situ or added exogenously, as EPS produced during fermentation is due to the adaptive response to the acidification of the medium, while an exogenous source of EPS is obtained under specified culture conditions. Also, by taking into account the specific physicochemical properties and composition of the final matrix.
Effect of cultivation conditions
In addition to the composition of the culture medium, the conditions under which bacteria are cultivated play a crucial role in the biosynthesis of various metabolites. Cultivation temperature is one such factor that impacts bacterial physiology and influences EPS production in different directions. In S. thermophilus and L. pentosus LPS26, EPS production was higher when the bacteria were cultivated respectively at 37 °C and 20 °C, which were temperatures lower than the optimal growth temperatures of these bacteria.41,42 On the other hand, in Lactobacillus delbrueckii subsp. bulgaricus, cultivation at higher temperatures (45 °C) resulted in an increase in EPS yields.43 The studies mentioned above indicate that the production of EPS in LAB is influenced by temperature, and this effect is strain-specific. Temperature has a more nuanced effect on in situ EPS production in foods. Dextran is an α-glucan synthesized by some LAB through dextransucrase catalytic activity. Weissella spp. naturally produce such dextrans in the sourdough environment, and this phenomenon affects the dough texture and bread quality. Because of the crucial involvement of EPS in possibly dictating consumer acceptance of sourdough bread, the influence of fermentation temperature on the EPS quality of sourdough has been investigated. When the temperature during sourdough fermentation by Weissella cibaria 10 M was shifted from 30 °C to 6-25 °C, the expression of dextransucrase involved in dextran biosynthesis was enhanced. Furthermore, sourdough fermentation under low temperatures also supported high dextran production without an excess pH drop (Table).44 Leuconostoc spp. is also a well-known dextran producer, especially in non-dairy environments like fermented plants. Similar to the above observation for W. cibaria, cultivation of Leuconostoc lactis AV1n at a low temperature of 20 °C resulted in a 10-fold increase in dextran production compared to 37 °C. This effect was plausibly driven by the higher activity of dextransucrase since dsrLL, the gene encoding this enzyme, was also found to be upregulated in the presence of sucrose.45 These examples highlight the fact that cold shift can be used as a strategy for enhancing dextran production in these two bacteria. However, such a phenomenon might be strain-specific, and more studies are required to understand if the production of other EPS types is also affected by such a cold shift.
Bifidobacterium and Lactobacillus spp. strains are obligate and facultative anaerobes, respectively. In the case of Bifidobacterium spp., specific gases are used to limit the oxygen availability and enhance the growth. Ninomiya et al.46 found that Bifidobacterium longum JBL05 cultivated under 20% CO2 yields maximum EPS production. This effect is likely mediated via promoting the Bifidobacterium spp. growth instead of the direct regulation of EPS production by CO2. Lactobacillus spp. can tolerate oxygen, and several of them can even respire when exogenously provided with heme and menaquinone, since they cannot biosynthesize these electron chain components.47 Respiration positively affects several important biotechnological properties of Lactobacillus spp., including biomass yield, stress tolerance, long-term storage survival, and production of flavoring chemicals.48 Surprisingly, the effect of respiration on the EPS production of Lactobacillus spp. has been scarcely studied. Li et al.49 found that recombinant Lactobacillus casei LC2W overexpressing NADH oxidase produces 75% higher EPS under aerobic cultivation than the wild-type strain. The probable mechanism involves increased availability of oxidized NAD and lesser accumulation of lactate. Such an influence of aerobic metabolism needs to be studied in numerous other EPS-producing LAB strains.
Effect of stressors
Considering that one of the main functions of EPS is to protect bacteria from environmental stress, such stressful conditions significantly affect the yield and characteristics of the EPS produced. LAB frequently encounters osmotic stress in food environments containing salts added as preservatives. An increased salt concentration can impact bacterial survival by causing water loss. Often, the EPS production under such stress acts as a barrier that retains water and protects the cells from dehydration. However, the direction of the effect of salt on EPS synthesis varies across different strains. For instance, in Lactobacillus sakei TMW 1.411, high saline stress reduced EPS production. Cultivation at 10 °C without salt resulted in a dextran yield of 6.7 g/L, whereas the addition of 9.5% NaCl drastically decreased the yield to just 0.5 g/L.50 This reduction is likely to be because of the extracellular nature of dextransucrase, leading to its inactivation by the high salt content of the medium. In contrast, when Lactobacillus confusus TISTR 1498 was cultivated using solid-state fermentation on an agar surface, a medium containing 4.97% NaCl led to an increased yield of EPS compared to its production in the absence of salt.51 Such an effect might be due to the adaptive response of bacteria to the salt-induced dehydration, considering a biological role of EPS in desiccation prevention. The contrasting effect of salt stress observed in the above studies also has a few important implications. Firstly, the differences could be due to the obvious differences in the species tested, which, based on the current classification, belong to different genera (Latilactobacillus and Weissella, respectively). Furthermore, the usage of different media (broth and agar, respectively) could confound the effect of salt on EPS production. Lastly and most importantly, such contrasting findings indicate the need to undertake a comprehensive study to understand the effect of such stressors on EPS production by diverse LAB in various media.
Bile and low pH impose another natural stress on commensal and food-originating bacteria in the GI tract and have been studied for their impact on bacteria from numerous facets. Lactobacillus and Bifidobacterium spp. depict strain-wise variation in the bile and acid resistance, and in general, EPS production is correlated to resistance to these stressors.52-54 In agreement with these observations, bile salts have also been shown to promote biosynthesis of EPS in LAB such as Bifidobacterium animalis subsp. lactis, L. delbrueckii subsp. lactis, and L. rhamnosus GG.55-57 While some of the above studies also suggest upregulation of EPS biosynthesis genes upon bile exposure, Koskenniemi et al.58 showed, using transcriptomic and proteomic approaches, the downregulation of EPS biosynthetic genes in L. rhamnosus GG in the presence of bile. This could be a signalling mechanism wherein the removal of EPS production would be inhibited by gastric bile. Since EPS generally interferes with the intestinal adhesion of Lactobacillus spp.,59 the bile-induced silencing of EPS could allow for the adhesion of Lactobacillus spp. to the intestinal epithelial cells. Thus, the response of Lactobacillus and Bifidobacterium spp. in terms of EPS production to bile appears to be multifaceted and species-specific, and thus needs to be studied in greater detail on a diverse set of bacteria.
Evolutionary modulations in EPS production
Adaptive laboratory evolution (ALE) involves cultivating microbes under specific environmental conditions for extended periods to enhance their fitness under those stressful conditions. This improvement is achieved through the natural selection of the fittest mutants, which exhibit traits beneficial for industrial applications.60 Changes in environmental conditions can lead to alterations in the genetic makeup of bacterial populations, influencing both the quality and quantity of metabolites produced. Lactobacillus and Bifidobacterium spp. have been subjected to ALE to improve their industrial robustness, with altered EPS production observed in many such studies.
Considering the role of EPS in the prevention of desiccation, adaptation to such dehydrating conditions can lead to an enhancement in the EPS production. For instance, during efforts to enhance the freeze-drying tolerance and storage stability of L. mesenteroides WiKim33 by exposing it to heat and osmotic shock, researchers observed increased EPS production and a remarkable 331% increase in biofilm thickness.61 Although there is no evident role of EPS in thermo-protection in LAB, evolutionary adaptation to higher temperature has been found to enhance the firmness of milk fermented by L. delbrueckii subsp. bulgaricus. Since the mutant was unable to acidify the milk, the enhanced firmness was attributed to higher EPS production by the mutant.62 This suggests the possibility of a metabolic trade-off, wherein under thermal stress, EPS synthesis might be favoured as compared to lactic acid production, underlining the complex metabolic coordination by LAB.
In contrast to the above examples of a positive correlation between stress adaptation and EPS production, evolutionary acid adaptation in B. longum does not result in an increase in EPS production. Jiang et al.63 found that adapting B. longum BBMN68 to low pH resulted in a mutant with reduced EPS accumulation compared to the ancestral strain. This reduction was attributed to a point mutation in the cpsD encoding a galactosyl transferase involved in EPS biosynthesis, leading to amino acid substitutions. This change is speculated to alter the monosaccharide composition of the EPS. At the same time, under acidic conditions
(pH 5), cpsD was upregulated in the wild-type strains and downregulated in the acid-adapted strains.63 Thus, the change in gene expression of the eps cluster might explain the tolerance to acid stress in the adapted strains.
Another evidence of the genomic impact of stress adaptation on EPS biosynthesis has been obtained in L. rhamnosus GG. Kwon et al.64 subjected L. rhamnosus GG to freeze-thaw growth for 150 cycles to improve freeze-thaw tolerance. Genome resequencing of the adapted strain revealed a loss-of-function mutation in wze, which encodes for a tyrosine kinase involved in EPS regulation. Although EPS production was not analyzed, such mutations likely result in continuous elongation of capsular polysaccharides on the cell.
Apart from these studies, the mechanistic details of evolutionary EPS modulation have not been much studied in Lactobacillus and Bifidobacterium spp. However, there appears to be a complex interplay between evolutionary adaptation and transient stress response which affects EPS production in these bacteria. Unlike Lactobacillus spp., the transcriptional regulators of EPS biosynthesis have not been studied in detail in Bifidobacterium spp.65 The EPS gene clusters in both these bacteria have numerous genes involved in EPS biosynthesis, export, and their regulation. Thus, studies on how all these functions are affected under stress exposure and evolutionary adaptation using a multi-omics approach are required.
In addition to the apparent physiological role of EPS in stress tolerance, the physicochemical properties of EPS can themselves be useful in developing and identifying EPS overproducing mutant strains. Since EPS production prevents bacterial sedimentation in low-viscosity environments, Martin et al.59 identified slower-sedimenting isolates of L. rhamnosus CNCM I-3690 as high-EPS producers. Although these strains exhibited diminished anti-inflammatory effects, reduced adhesion to colonic epithelial cells, and decreased probiotic efficacy in vivo, they provide a useful tool for EPS generation for technological applications. Additionally, such studies demonstrate the potential of evolutionary selection using other physicochemical properties of EPS for identifying EPS-overproducing strains.
Effect of co-culture
Lactobacillus spp. produce lactic acid as their primary metabolic end product. While they are relatively adapted to lactate-rich environments, high lactate concentrations can inhibit their growth and reduce yields of valuable products like EPS. This issue has been particularly noted during kefiran polysaccharide production by L. kefiranofaciens KPB-167B, a key microbe in kefir grains.66
One of the most thoughtful ways this limitation was overcome was by utilizing the microbial diversity of kefir. Kefir grains contain yeasts like Saccharomyces cerevisiae, which utilize lactate. Co-culturing L. kefiranofaciens JCM6985 with S. cerevisiae reduces lactate accumulation, facilitating higher bacterial growth and increased kefiran yields.67 This strategy was further optimized using fed-batch co-culture techniques that balanced lactate production by L. kefiranofaciens JCM6985 with lactate consumption by S. cerevisiae, achieving even higher kefiran yields.68 The cross-taxa induction of EPS is also known to occur in L. kefiranofaciens OSU-BDGOA1 by Kluyveromyces marxianus, which is another yeast found in kefir grains69 and in S. thermophilus 1275 (EPS-producing strain) by S. thermophilus 1303 (a non-EPS-producing strain).70 The increased yield of EPS may result from the complementary relationship between the microbes in terms of exchange of metabolites, similar to such interactions known in yogurt.71 Apart from lactate consumption and allowing for the growth of LAB, the stimulation of EPS production by yeast also takes place via a direct mechanism. Specifically, S. cerevisiae was found to upregulate the EPS biosynthesis genes in Lacticaseibacillus paracasei ATCC 33472 and L. rhamnosus RW-9595M.73
In case of L. paracasei, this phenomenon was further shown to be driven by recognition of mannans on the yeast surface (Figure). Although such physical interaction of yeast mannans is speculated to occur via L. paracasei surface proteins, which were upregulated by mannans, the detailed mechanism remains to be studied. Intriguingly, EPS obtained from L. plantarum ATCC 8014 cocultured with S. cerevisiae was found to have lower solubility, water uptake, and DPPH radical scavenging activity.74 The EPS produced under both conditions was found to have glucose residues with α-(1→6) linkage. The other structural features affected by co-culture, along with the mechanism of such a change in the properties of EPS, have not been studied. It would be interesting to delve deeper into the cross-taxa interactions by reverse genetics and biophysical approaches, can not only provide a detailed understanding of this phenomenon but also provide a means of its application for further modulating EPS production.
Researchers are increasingly exploring ways to enhance the production of EPS by LAB and Bifidobacterium spp. due to their potential health benefits and applications in food, medicine, and environmental biotechnology. Regardless of the tremendous progress in characterization and production of EPS, much of the underlying regulatory mechanisms and strategies that may be useful for enhancing the total yield remain less explored.
LAB and Bifidobacterium spp. have EPS biosynthetic gene clusters which are strain-specific, with carbon sources and amino acids acting as stimuli for their upregulation. The modulation of gene expression affects the quantity of EPS produced while altering its composition, which might lead to modulation in its bioactivity, indicating the requirement of precise nutrient optimization for targeted EPS synthesis. More studies and a better understanding of combining the metabolomic knowledge of the bacterial nutrient sensing regulatory network with its EPS activation clusters are required.
Adaptive laboratory evolution (ALE) has proven effective for improving microbial resilience under stress conditions while amplifying EPS yield and structure. A better understanding of the molecular, transcriptomic and genome levels could help enhance the conditions to maximize the EPS synthesis by the strains. While considerable research has focused on environmental stresses that promote EPS synthesis, evolutionary approaches remain underexplored but hold promise for industrial applications.
Co-culturing strategies have potential for diversifying structural properties and improving EPS yields through synergistic microbial interactions. A synergetic balance is created by co-cultures, such as the lactate synthesized by LAB, and utilized by the yeast. This influences bacterial growth along with EPS production, yielding biopolymers in higher quantities compared to monocultures. The polysaccharide synthesized by such a method would result in a novel polymer with functional characteristics to be employed for various industrial purposes, such as in targeted delivery systems, texture modifications, etc. EPS with refined rheological properties could be utilized for food emulsification and as environmental absorbents.
Future research should focus on optimizing evolutionary strategies while investigating molecular mechanisms underlying co-culture-induced changes in EPS bioactivity. Model systems should be used to experimentally validate the link between the EPS structure and its functional outcomes, which can establish a basis for targeted engineering. Integrating the techniques of media optimization, adaptive evolution, and co-culture systems strategically could not only enhance the quality and quantity of EPS but also expand its applicability across industries.
ACKNOWLEDGMENTS
The author (AG) would like to thank Symbiosis International (Deemed University) for the Junior Research Fellowship.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Singh S, Datta S, Narayanan KB, Rajnish KN. Bacterial exo-polysaccharides in biofilms: role in antimicrobial resistance and treatments. J Genet Eng Biotechnol. 2021;19(1):140.
Crossref - Cerning J. Production of exopolysaccharides by lactic acid bacteria and dairy propionibacteria. Le lait. 1995:75(4-5):463-472.
Crossref - Lv H, Teng Q, Chen J, et al. Probiotic potential of a novel exopolysaccharide produced by Bifidobacterium animalis subsp. Lactis SF. LWT. 2024;193.
Crossref - Oerlemans MMP, Akkerman R, Ferrari M, Walvoort MTC, de Vos P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J Funct Foods. 2021;76:104289.
Crossref - Gentes MC, Turgeon SL, St-Gelais D. Impact of starch and exopolysaccharide-producing lactic acid bacteria on the properties of set and stirred yoghurts. Int Dairy J. 2016;55:79-86.
Crossref - Wang J, Wu T, Fang X, Yang Z. Manufacture of low-fat Cheddar cheese by exopolysaccharide-producing Lactobacillus plantarum JLK0142 and its functional properties. J Dairy Sci. 2019;102(5):3825-3838.
Crossref - Zaghloul EH, Ibrahim MIA. Production and characterization of exopolysaccharide from newly isolated marine probiotic Lactiplantibacillus plantarum EI6 with in vitro wound healing activity. Front Microbiol. 2022;13:903363.
Crossref - Waoo AA, Singh S, Pandey A, et al. Microbial exopolysaccharides in the biomedical and pharmaceutical industries. Heliyon. 2023;9(8):e18613.
Crossref - Malaka R. Bacterial exopolysaccharides production and their roles for human life. IOP Conf Ser Earth Environ Sci. 2021;788.
Crossref - Salazar N, Gueimonde M, de los Reyes-Gavilan CG, Ruas-Madiedo P. Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Crit Rev Food Sci Nutr. 2016;56(9):1440-1453.
Crossref - De Vuyst L, De Vin F, Vaningelgem F, Degeest B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int Dairy J. 2001;11(9):687-707.
Crossref - Dertli E, Colquhoun IJ, Gunning AP, et al. Structure and biosynthesis of two exopolysaccharides produced by Lactobacillus johnsonii FI9785. J Biol Chem. 2013;288(44):31938-31951.
Crossref - Ruas-Madiedo P, Tuinier R, Kanning M, Zoon P. Role of Exopolysaccharides Produced by Lactococcus lactis subsp. Cremoris on the Viscosity of Fermented Milks. Int Dairy J. 2002;12(8):689-695.
Crossref - Hidalgo-Cantabrana C, Lopez P, Gueimonde M, et al. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob Proteins. 2012;4(4):227-237.
Crossref - Lakra AK, Ramatchandirane M, Kumar S, Suchiang K, Arul V. Physico-chemical characterization and aging effects of fructan exopolysaccharide produced by Weissella cibaria MD2 on Caenorhabditis elegans. LWT. 2021;143:111100.
Crossref - Li N, Huang Y, Liu Z, You C, Guo B. Regulation of EPS production in Lactobacillus casei LC2W through metabolic engineering. Lett Appl Microbiol. 2015;61(6):555-561.
Crossref - Wang B, Sun X, Xu M, Wang F, Liu W, Wu B. Structural characterization and partial properties of dextran produced by Leuconostoc mesenteroides RSG7 from pepino. Front Microbiol. 2023;14:1108120.
Crossref - Whitfield GB, Marmont LS, Howell PL. Enzymatic modifications of exopolysaccharides enhance bacterial persistence. Front Microbiol. 2015;6:471.
Crossref - Soumya MP, Nampoothiri KM. Evaluation of improved biological properties of chemically modified exopolysaccharides from Lactobacillus plantarum BR2. 3 Biotech. 2023;13(9):308.
Crossref - Zhou Y, Cui Y, Suo C, Wang Q, Qu X. Structure, physicochemical characterization, and antioxidant activity of the highly arabinose-branched exopolysaccharide EPS-M2 from Streptococcus thermophilus CS6. Int J Biol Macromol. 2021;192:716-727.
Crossref - Notararigo S, Nacher-Vazquez M, Ibarburu I, et al. Comparative analysis of production and purification of homo- and hetero-polysaccharides produced by lactic acid bacteria. Carbohydrate Polymers. 2013;93(1):57-64.
Crossref - Ceapa C, Lambert J, van Limpt K, et al. Correlation of Lactobacillus rhamnosus genotypes and carbohydrate utilization signatures determined by phenotype profiling. Appl Environ Microbiol. 2015;81(16):5458-5470.
Crossref - Mayer MJ, D’amato A, Colquhoun IJ, Le Gall G, Narbad A. Identification of genes required for glucan exopolysaccharide production in Lactobacillus johnsonii suggests a novel biosynthesis mechanism. Appl Environ Microbiol. 2020;86(8):e02808-19.
Crossref - Polak-Berecka M, Choma A, Wasko A, Gorska S, Gamian A, Cybulska J. Physicochemical characterization of exopolysaccharides produced by Lactobacillus rhamnosus on various carbon sources. Carbohydrate Polymers. 2015;117:501-509.
Crossref - Esmaeilnejad-Moghadam B, Mokarram RR, Hejazi MA, Khiabani MS, Keivaninahr F. Low molecular weight dextran production by Leuconostoc mesenteroides strains: optimization of a new culture medium and the rheological assessments. Bioactive Carbohydrates and Dietary Fibre. 2019;18:100181.
Crossref - Torino MI, Hebert EM, Mozzi F, Font De Valdez G. Growth and exopolysaccharide production by Lactobacillus helveticus ATCC 15807 in an adenine-supplemented chemically defined medium. J Appl Microbiol. 2005;99(5):1123-1129.
Crossref - Wa Y, Zhang C, Sun G, et al. Effect of amino acids on free exopolysaccharide biosynthesis by Streptococcus thermophilus 937 in chemically defined medium. J Dairy Sci. 2022;105(8):6460-6468.
Crossref - Macedo MG, Lacroix C, Gardner NJ, Champagne CP. Effect of Medium Supplementation on Exopolysaccharide Production by Lactobacillus rhamnosus RW-9595M in Whey Permeate. Int Dairy J. 2002;12(5):419-426.
Crossref - Trouillon J, Doubleday PF, Sauer U. Genomic footprinting uncovers global transcription factor responses to amino acids in Escherichia coli. Cell Systems. 2023;14(10):860-871.e4.
Crossref - Dertli E, Mayer MJ, Colquhoun IJ, Narbad A. EpsA is an essential gene in exopolysaccharide production in Lactobacillus johnsonii FI9785. Microb Biotechnol. 2016;9(4):496-501.
Crossref - Midik F, Tokatlı M, Elmacı SB, Ozcelik F. Influence of different culture conditions on exopolysaccharide production by indigenous lactic acid bacteria isolated from pickles. Arch Microbiol. 2020;202(4):875-885.
Crossref - Jiang Y, Zhang M, Zhang Y, Zulewska J, Yang Z. Calcium (Ca2+)-regulated exopolysaccharide biosynthesis in probiotic Lactobacillus plantarum K25 as analyzed by an omics approach. J Dairy Sci. 2021;104(3):2693-2708.
Crossref - Huynh U, King J, Zastrow ML. Calcium modulates growth and biofilm formation of Lactobacillus acidophilus ATCC 4356 and Lactiplantibacillus plantarum ATCC 14917. Sci Rep. 2025;15(1).
Crossref - Singh KS, Kumar S, Mohanty AK, Grover S, Kaushik JK. Mechanistic insights into the host-microbe interaction and pathogen exclusion mediated by the mucus-binding protein of Lactobacillus plantarum. Sci Rep. 2018;8(1):14198.
Crossref - Zajsek K, Gorsek A, Kolar M. Cultivating conditions effects on kefiran production by the mixed culture of lactic acid bacteria imbedded within kefir grains. Food Chem. 2013;139(1-4):970-977.
Crossref - Elmansy EA, Elkady EM, Asker MS, Abdallah NA, Khalil BE, Amer SK. Improved production of Lactiplantibacillus plantarum RO30 exopolysaccharide (REPS) by optimization of process parameters through statistical experimental designs. BMC Microbiol. 2023;23(1):361.
Crossref - Chen L, Gu Q, Zhou T. Statistical optimization of novel medium to maximize the yield of exopolysaccharide from Lacticaseibacillus rhamnosus ZFM216 and Its immunomodulatory activity. Front Nutr. 2022;9:924495.
Crossref - Zhang Y, Li S, Zhang C, Luo Y, Zhang H, Yang Z. Growth and exopolysaccharide production by Lactobacillus fermentum F6 in skim milk. Afr J Biotechnol. 2011;10(11):2080-2091.
Crossref - Wu Q, Shah NP. Comparative mRNA-seq analysis reveals the improved EPS production machinery in Streptococcus thermophilus ASCC 1275 during optimized milk fermentation. Front Microbiol. 2018;9:445.
Crossref - Ayala-Hernandez I, Hassan A, Goff HD, Mira de Orduna R, Corredig M. Production, isolation and characterization of exopolysaccharides produced by Lactococcus lactis subsp. cremoris JFR1 and their interaction with milk proteins: effect of pH and media composition. Int Dairy J. 2008;18(12):1109-1118.
Crossref - Rabha B, Nadra RS, Ahmed B. Effect of some fermentation substrate and growth temperature on exopolysaccharide production by Streptococcus thermophilus BN1. Int J Biosci Biochem Bioinformat. 2012;2(1):44-47.
Crossref - Sanchez JI, Martinez B, Guillen R, Jimenez-Diaz R, Rodriguez A. Culture conditions determine the balance between two different exopolysaccharides produced by Lactobacillus pentosus LPS26. Appl Environ Microbiol. 2006;72(12):7495-7502.
Crossref - Aslim B, Yuksekdagc ZN, Beyatli Y, Mercan N. Exopolysaccharide production by Lactobacillus delbruckii subsp. bulgaricus and Streptococcus thermophilus strains under different growth conditions. World J Microbiol Biotechnol. 2005;21(5):673-677.
Crossref - Hu Y, Ganzle MG. Effect of temperature on production of oligosaccharides and dextran by Weissella cibaria 10/ M. Int J Food Microbiol. 2018;280:27-34.
Crossref - Besrour-Aouam N, Mohedano ML, Fhoula I, et al. Different modes of regulation of the expression of dextransucrase in Leuconostoc lactis AV1n and Lactobacillus sakei MN. Front Microbiol. 2019;10:959.
Crossref - Ninomiya K, Matsuda K, Kawahata T, et al. Effect of CO2 concentration on the growth and exopolysaccharide production of Bifidobacterium longum cultivated under anaerobic conditions. J Biosci Bioeng. 2009;107(5):535-537.
Crossref - Pedersen MB, Gaudu P, Lechardeur D, Petit MA, Gruss A. Aerobic respiration metabolism in lactic acid bacteria and uses in biotechnology. Annu Rev Food Sci Technol. 2012;3(1):37-58.
Crossref - Zotta T, Guidone A, Ianniello RG, Parente E, Ricciardi A. Temperature and respiration affect the growth and stress resistance of Lactobacillus plantarum C17. J Appl Microbiol. 2013;115(3):848-858.
Crossref - Li Q, Liu X, Dong M, Zhou J, Wang Y. Aggregation and adhesion abilities of 18 lactic acid bacteria strains isolated from traditional fermented food. Int J Agric Pol Res. 2015;3(2):84-92.
Crossref - Prechtl RM, Wefers D, Jakob F, Vogel RF. Cold and salt stress modulate amount, molecular and macromolecular structure of a Lactobacillus sakei dextran. Food Hydrocolloids. 2018;82:73-81.
Crossref - Seesuriyachan P, Kuntiya A, Hanmoungjai P, Techapun C, Chaiyaso T, Leksawasdi N. Optimization of exopolysaccharide overproduction by Lactobacillus confusus in solid state fermentation under high salinity stress. Biosci Biotechnol Biochem. 2012;76(5):912-917.
Crossref - Fukao M, Zendo T, Inoue T, et al. Plasmid-encoded glycosyltransferase operon is responsible for exopolysaccharide production, cell aggregation, and bile resistance in a probiotic strain, Lactobacillus brevis KB290. J Biosci Bioeng. 2019;128(4):391-397.
Crossref - Sanhueza E, Paredes-Osses E, Gonzalez CL, Garcia A. Effect of pH in the survival of Lactobacillus salivarius strain UCO_979C wild type and the ph acid acclimated variant. Electron J Biotechnol. 2015;18(5):343-346.
Crossref - Suzuki S, Kimoto-Nira H, Suganuma H, Suzuki C, Saito T, Yajima N. Cellular fatty acid composition and exopolysaccharide contribute to bile tolerance in Lactobacillus brevis strains isolated from fermented Japanese pickles. Can J Microbiol. 2014;60(4):183-191.
Crossref - Amund OD, Ouoba LII, Sutherland JP, Ghoddusi HB. Assessing the effects of exposure to environmental stress on some functional properties of Bifidobacterium animalis ssp. lactis. Benef Microb. 2014;5(4):461-469.
Crossref - Burns P, Sanchez B, Vinderola G, et al. Inside the Adaptation Process of Lactobacillus Delbrueckii subsp. lactis to Bile. Int J Food Microbiol. 2010;142(1-2);132-141.
Crossref - Zhang L, Chichlowski M, Gross G, et al. Milk fat globule membrane protects Lactobacillus rhamnosus GG from bile stress by regulating exopolysaccharide production and biofilm formation. J Agric Food Chem. 2020;68(24):6646-6655.
Crossref - Koskenniemi K, Laakso K, Koponen J, et al. Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol Cell Proteomics. 2011;10(2):002741.
Crossref - Martin R, Cabello AB, Kulakauskas S, et al. Over production of exopolysaccharide by Lacticaseibacillus rhamnosus CNCM I-3690 strain cutbacks its beneficial effect on the host. Sci Rep. 2023:13(1).
Crossref - Sandberg TE, Salazar MJ, Weng LL, Palsson BO, Feist M. The Emergence of Adaptive Laboratory Evolution as an Efficient Tool for 1 Biological Discovery and Industrial Biotechnology. Metab Eng. 2019;56;1-16.
Crossref - Kim YY, Kim JC, Kim S, Yang JE, Kim HM, Park HW. Heterotypic stress-induced adaptive evolution enhances freeze-drying tolerance and storage stability of Leuconostoc mesenteroides WiKim33. Food Res Int. 2024;175:113731.
Crossref - Liang J, Yoo MJY, Seale B, Grazioli G. Nutritional and volatile characterisation of milk inoculated with thermo-tolerant Lactobacillus bulgaricus through adaptive laboratory evolution. Foods. 2021;10(12):2944.
Crossref - Jiang Y, Ren F, Liu S, Zhao L, Guo H, Hou C. Enhanced acid tolerance in Bifidobacterium longum by adaptive evolution: Comparison of the genes between the acid-resistant variant and wild-type strain. J Microbiol Biotechnol. 2015;26(3):452-460.
Crossref - Kwon YW, Bae JH, Kim SA, Han NS. Development of freeze-thaw tolerant Lactobacillus rhamnosus GG by adaptive laboratory evolution. Front Microbiol. 2018;9:1-10.
Crossref - Hidalgo-Cantabrana C, Sanchez B, Milani C, Ventura M, Margolles A, Ruas-Madiedo P. Genomic overview and biological functions of exopolysaccharide biosynthesis in Bifidobacterium spp. Appl Environ Microbiol. 2014;80(1):9-18.
Crossref - Yokoi H, Watanabe T. Optimum Culture Conditions for Production of Kefiran by Lactobacillus sp. KPB-167B Isolated from Kefir Grains. J Ferment Bioeng. 1992;74(5):327-329.
Crossref - Cheirsilp B, Shimizu H, Shioya S. Enhanced kefiran production by mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. J Biotechnol. 2003;100(1):43-53.
Crossref - Tada S, Katakura Y, Ninomiya K, Shioya S. Fed-batch coculture of Lactobacillus kefiranofaciens with Saccharomyces cerevisiae for effective production of kefiran. J Biosci Bioeng. 2007;103(6):557-562.
Crossref - Gonzalez-Orozco BD, Kosmerl E, Jimenez-Flores R, Alvarez VB. Enhanced probiotic potential of Lactobacillus kefiranofaciens OSU-BDGOA1 through co-culture with Kluyveromyces marxianus bdgo-ym6. Front Microbiol. 2023;14:1236634.
Crossref - Zisu B, Shah NP. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. J Dairy Sci. 2003;86(11):3405-3415.
Crossref - Sieuwerts S, Molenaar D, Van Hijum SAFT, et al. Mixed-Culture transcriptome analysis reveals the molecular basis of mixed-culture growth in Streptococcus thermophilus and Lactobacillus bulgaricus. Appl Environ Microbiol. 2010;76(23):7775-7784.
Crossref - Yamasaki-Yashiki S, Sawada H, Kino-Oka M, Katakura Y. Analysis of gene expression profiles of Lactobacillus paracasei induced by direct contact with Saccharomyces Cerevisiae through recognition of yeast mannan. Biosci Microbiota Food Health. 2017;36(1):17-25.
Crossref - Bertsch A, Roy D, LaPointe G. Enhanced exopolysaccharide production by Lactobacillus rhamnosus in co-culture with Saccharomyces cerevisiae. Appl Sci. 2019;9(19).
Crossref - Bustamama ANFA, Dauda NS, Azam ZM, et al. Production and functional characteristics of exopolysaccharide by Lactobacillus plantarum co-cultivation with Saccharomyces cerevisiae. AsPac J Mol Biol Biotechnol. 2023;31(1):14-25.
Crossref - Gamar L, Blondeau K, Simonet JM. Physiological approach to extracellular polysaccharide production by Lactobacillus rhamnosus strain C83. J Appl Microbiol. 1997;83(3):281-287.
Crossref - Petry S, Furlan S, Crepeau MJ, Cerning J, Desmazeaud M. Factors affecting exocellular polysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus grown in a chemically defined medium. Appl Environ Microbiol. 2000;66(8), 3427-3431.
Crossref - Prasanna PHP, Grandison AS, Charalampopoulos D. Effect of dairy-based protein sources and temperature on growth, acidification and exopolysaccharide production of Bifidobacterium strains in skim milk. Food Res Int. 2012;47(1):6-12.
Crossref - Imran MYM, Reehana N, Jayaraj KA, Ahamed, AAP, Dhanasekaran D, Thajuddin N, Alharbi NS, Muralitharan G. Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int J Biol Macromol. 2016;93:731-745.
Crossref - Adesulu-Dahunsi AT, Sanni AI, Jeyaram K, Ojediran JO, Ogunsakin AO, Banwo K. Extracellular polysaccharide from Weissella confusa OF126: Production, optimization, and characterization. Int J Biol Macromol. 2018;111:514-525.
Crossref - Zhao D, Liu L, Jiang J, Guo S, Ping W, Ge J. The response surface optimization of exopolysaccharide produced by Weissella confusa XG-3 and its rheological property. Prep Biochem Biotechnol. 2020;50(10):1014-1022.
Crossref - Oleksy-Sobczak M, Klewicka E. Optimization of media composition to maximize the yield of exopolysaccharides production by Lactobacillus rhamnosus strains. Probiotics & Antimicro Prot. 2020;12(2):774-783.
Crossref - Yáñez-Fernández J, Ovando MGH, Ramírez LP, Ramírez-Sotelo G, Guarin CA, Castro-Rodríguez DC. Factorial design to optimize dextran production by the native strain Leuconostoc mesenteroides SF3. ACS Omega. 2021;6(46):31203-31210.
Crossref - Han J, Feng H, Wang X, Liu Z, Wu Z. Levan from Leuconostoc citreum BD1707: production optimization and changes in molecular weight distribution during cultivation. BMC Biotechnol. 2021;21(1):14.
Crossref - Fuso A, Bancalari E, Castellone V, Caligiani A, Gatti M, Bottari B. Feeding lactic acid bacteria with different sugars: effect on exopolysaccharides (EPS) production and their molecular characteristics. Foods. 2023;12(1).
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.