ISSN: 0973-7510
E-ISSN: 2581-690X
Clostridium piliforme is an anaerobic, spore-forming, obligate intracellular bacterial pathogen that causes Tyzzer’s disease in laboratory, wild, and domestic animals. C. piliforme has significant economic implications for laboratory and commercial animal facilities due to its impact on research integrity, increased veterinary and management costs, and the need for enhanced biosecurity measures. In the present study, 100 rabbit faecal samples were collected, and nested PCR was performed using C. piliforme specific 16S rRNA primers. C. piliforme was detected in all five rabbit farms, with an overall prevalence of 40%. A statistically significant difference in prevalence was observed among farms. Male faecal samples accounted for only 25% of the total, and although more females (45.33%) were found to be infected, this difference was not statistically significant. Rabbits older than 5 months (42.05%) were more frequently infected than those younger than 5 months (25%), though this was not statistically significant. Breed, weight, rearing system, and feeding type did not influence prevalence. Because 16S rRNA primers may yield nonspecific amplicons, their use in detecting C. piliforme in samples, particularly faeces, should be interpreted in conjunction with clinical signs or gross lesions. Identification of C. piliforme specific gene target primers is urgently needed for effective screening of susceptible populations.
Clostridium piliforme, Rabbit, Nested PCR, Molecular Detection
Rabbits, valued for their roles in agriculture, research, and as companion animals, face numerous health challenges. Among them is Tyzzer’s disease, caused by C. piliforme. This elusive and often fatal pathogen poses a significant threat to the health and productivity of domestic rabbit populations. C. piliforme is an anaerobic, spore-forming, obligate intracellular bacterium that causes disease in laboratory, wild, and domestic animals.1,2 It has also been reported in an HIV-positive human patient.3
C. piliforme primarily targets the liver and intestinal tissues of rabbits. The severity of Tyzzer’s disease lies not only in its acute presentation but also in its ability to cause sudden, devastating outbreaks, leading to substantial economic losses and compromised animal welfare. The bacterium’s environmental persistence further complicates disease management.
Culturing C. piliforme is difficult, as it does not grow on routine bacteriological media. It can, however, be propagated in embryonated hen’s eggs or mammalian cell culture.1 Diagnosis is therefore generally based on histopathological examination, immunological techniques, or PCR. Histopathology allows identification of the bacterium but is laborious and time-consuming. Immunological techniques are widely used for colony surveillance because they are relatively rapid and inexpensive. However, false-positive or false-negative results due to antigen cross-reactivity limit their utility for confirmatory diagnosis in field cases. Molecular diagnosis of C. piliforme is highly specific and comparatively cost-effective.4-6 Niepceron and Licois7 developed nested PCR (nPCR) with improved sensitivity and specificity.
Despite the economic and animal welfare consequences of Tyzzer’s disease, knowledge of its prevalence, epidemiology, and risk factors remains incomplete. The present study therefore aimed to determine the prevalence, epidemiology, and associated risk factors of C. piliforme infection in rabbits in India.
Sample collection
A total of 100 fresh faecal samples were collected from both organized farms and backyard rabbitries in two locations in Tamil Nadu and three in Uttar Pradesh. Data on age, sex, feeding pattern, and other characteristics were collected and are summarized in Table 1.
Table (1):
Details of rabbit faecal samples collection
Place |
No. of samples |
Male |
Female |
Breed* |
Average weight (kg) |
Age group |
Farming system |
|---|---|---|---|---|---|---|---|
Chengalpattu |
26 |
4 |
22 |
NZW and SC |
2.49 |
4 months to 6.2 years |
Organised |
Chennai |
20 |
8 |
12 |
SC |
5.71 |
3 months to 3.1 years |
Organised |
Fatehgarh |
14 |
5 |
9 |
NZW |
2.25 |
7 months to 6.2 years |
Backyard |
Shamsabad |
20 |
4 |
16 |
NZW |
2.25 |
5 months to 6.2 years |
Backyard |
Mohammadabad |
20 |
4 |
16 |
NZW |
2.24 |
6 months to 6.6 years |
Backyard |
*NWZ – New Zealand White; SC – Soviet Chinchilla
Fresh faecal samples were collected directly from the rectum or from freshly voided pellets. Samples were hygienically collected with blunt thumb forceps, placed in airtight plastic containers with proper labelling, transported to the laboratory on ice, and stored at -20 °C until processing.
DNA extraction
DNA extraction followed the method of Wang et al.8 and Osmundson et al.9 who used NaOH to extract DNA from plant and fungal tissues, respectively, for PCR templates. The protocol was modified as follows: 250 mg of faecal sample was homogenized in 1 ml of Milli-Q water and centrifuged at 3000 rpm for 5 min at 20 °C. The supernatant was collected and adjusted to 1.25 ml with Milli-Q water, then centrifuged at 8000 rpm for 3 min at 20 °C. The supernatant was discarded, 10 µl of 0.5 M NaOH was added, and the mixture was mixed thoroughly. Then, 490 µl of Milli-Q water was added, followed by centrifugation at 8000 rpm for 3 min at 20 °C. The extracted DNA was stored at -20 °C. The 260/280 absorbance ratio of the DNA ranged from 1.57 to 1.85, with concentrations of 52-107 ng/µL.
Detection of C. piliforme by nested PCR
First-round PCR was performed in a 10 µl reaction volume containing 100-150 ng of DNA, 0.5 µM universal 16S rRNA primers (Forward: 5′-AGAGTTTGATCCTGGCTCAG-3′, Reverse: 5′ -TACGGYTACCTTGTTACGACTT-3′),10 and 2X PCR master mix (Ampliqon, Denmark). Cycling conditions were 95 °C for 3 min, followed by 30 cycles of 95 °C for 30 sec, 60 °C for 30 sec, and 72 °C for 45 sec, with a final extension at 72 °C for 5 min.
The second round of nPCR was performed using 1 µl of a 5-fold diluted first-round product and 0.5 µM C. piliforme-specific primers: PiliF (5′-TGGGATAACATCGAGAAATC-3′) and PiliR (5′-ACGTAGYCTGTCAATGGT-3′).7 Nuclease-free water was used as the no-template control. PCR cycling was as above, except annealing was at 58 °C for 30 cycles. Amplified products were electrophoresed in 1.5% agarose gel containing 0.5 µg/ml ethidium bromide and run at 100 V for 30-40 min (Medox Biotech India Pvt. Ltd.). Gels were visualized and documented using a gel documentation system (Bio-Rad, Inc., Laboratories, USA). To prevent cross-contamination, PCR reactions without template were prepared in a separate facility from DNA extraction and electrophoresis areas.
Statistical analysis
PCR results and associated metadata were analysed using appropriate statistical tests to assess the prevalence of C. piliforme in farm and laboratory rabbits. Analyses were performed using GraphPad Prism 5.0. Associations of age, sex, weight, farming type, and feeding with infection were evaluated using Chi-square or Fisher’s exact tests. Significant parameters were further compared with Fisher’s exact test and Bonferroni correction. P-values were interpreted as follows: p < 0.05 = least significant, p < 0.01 = significant, and p < 0.001 = highly significant.
In total, 100 rabbit faecal samples were collected from two farms located in two districts of Tamil Nadu (Chennai and Chengalpattu) and three farms (Fatehgarh, Shamsabad, and Mohammadabad) in the Farrukhabad district of Uttar Pradesh.
Detection of C. piliforme by nested-PCR
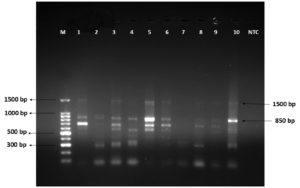
The first-round PCR was performed using universal 16S rRNA gene primers, which yielded a 1500 bp amplification product. Nested PCR was then carried out using C. piliforme specific primers, and positive faecal samples produced an 850 bp amplicon (Figure). A faint band at 1500 bp was observed, probably due to the presence of primers and products from the first-round PCR. In addition, some non-specific amplification products were detected along with specific products in certain rabbit faecal samples.
Figure. Agarose gel electrophoresis showing the results of nested PCR amplified products of Clostridium piliforme
Prevalence of C. piliforme in rabbits
Of the 100 rabbit faecal samples, 40 tested positive for C. piliforme, resulting in an overall prevalence of 40%. Details of C. piliforme prevalence with respect to other variables are shown in Table 2. The prevalence varied significantly between farms, with a P value of 0.0074 (<0.05). The highest prevalence (70%) was observed in Mohammadabad, whereas the lowest prevalence (19.23%) was recorded in Chengalpattu. Because a significant overall association was detected (p = 0.0074), a post-hoc Fisher’s exact test with Bonferroni correction was performed. Although a significant association was found between Fatehgarh and Mohammadabad in the unadjusted test (p = 0.03), this was not significant after Bonferroni adjustment (p = 0.35), as shown in Table 3. The strongest association between farms was observed for Mohammadabad versus Chengalpattu, both in the unadjusted (p = 0.00) and adjusted (p = 0.01) analyses.
Table (2):
Prevalence of Clostridium piliforme in rabbits with associated risk factors
| Variables | No. positive/No. tested (%) | Statistical test used | p-value |
|---|---|---|---|
| Farm | |||
| Chennai | 10/20 (50) | Chi-square | 0.0074* |
| Chengalpattu | 5/26 (19.23) | ||
| Fatehgarh | 14/20 (70) | ||
| Shamsabad | 7/20 (35) | ||
| Mohammadabad | 4/14 (28.57) | ||
| Sex | |||
| Male | 6/25 (24) | Two-sided Fisher’s exact test | 0.0649 |
| Female | 34/75 (45.33) | ||
| Age | |||
| <5 months | 3/12 (25) | Two-sided Fisher’s exact test | 0.3525 |
| >5 months | 37/88 (42.05) | ||
| Breed | |||
| New Zealand White | 28/71 (39.44) | Two-sided Fisher’s exact test | 1.0000 |
| Soviet Chinchilla | 12/29 (41.38) | ||
| Weight | |||
| <3 Kg | 25/58 (43.10) | Two-sided Fisher’s exact test | 1.0000 |
| >3 Kg | 15/42 (35.71) | ||
| Farm Management | |||
| Organized farm | 15/46 (32.60) | Two-sided Fisher’s exact test | 0.2194 |
| Backyard farming | 25/54 (46.30) | ||
| Type of feeding | |||
| Dry Powder form with grasses | 10/20 (50) | Chi-square | 0.0694 |
| Wet Mash form with grasses | 5/26 (19.23) | ||
| Vegetables with grasses | 25/54 (46.30) | ||
*p-value <0.01 – Significant
Table (3):
Post-hoc pairwise comparisons between places (Fisher) with Bonferroni adjustment
Comparison |
Odds Ratio (A vs B) |
Unadjusted |
Bonferroni |
Significant (Bonferroni 0.05) |
|---|---|---|---|---|
Fatehgarh vs Chengalpattu |
1.68 |
0.69 |
1 |
False |
Fatehgarh vs Chennai |
0.4 |
0.30 |
1 |
False |
Fatehgarh vs Mohammadabad |
0.17 |
0.03 |
0.35 |
False |
Fatehgarh vs Shamsabad |
0.74 |
1 |
1 |
False |
Chengalpattu vs Chennai |
0.24 |
0.06 |
0.55 |
False |
Chengalpattu vs Mohammadabad |
0.10 |
0.00 |
0.01 |
True |
Chengalpattu vs Shamsabad |
0.44 |
0.31 |
1 |
False |
Chennai vs Mohammadabad |
0.43 |
0.33 |
1 |
False |
Chennai vs Shamsabad |
1.86 |
0.52 |
1 |
False |
Mohammadabad vs Shamsabad |
4.33 |
0.06 |
0.56 |
False |
From the two organized farms in Tamil Nadu, 46 rabbit faecal samples were collected, of which 15 tested positive by nPCR, giving a prevalence of 32.60%. All three rabbit farms in Uttar Pradesh were backyard farms, from which 54 samples were collected; 25 of these tested positive by nPCR, resulting in a prevalence of 46.30%. However, the prevalence of C. piliforme infection was not statistically significant when compared between organized and backyard farms.
Faecal samples from male rabbits accounted for only 25% of the sample size. Although a higher proportion of females (45.33%) were infected with C. piliforme, this was not statistically significant. Similarly, rabbits older than five months (42.05%) showed a higher prevalence of infection than those younger than five months (25%), but this difference was also not statistically significant.
In this study, two rabbit breeds—Soviet Chinchilla and New Zealand White were examined for C. piliforme prevalence. The majority of samples (71%) were from New Zealand White rabbits. Neither breed nor body weight had a significant effect on the occurrence of infection. Rabbits fed wet mash were less commonly infected (19.23%) than those fed dry powder (50%) or vegetables (46.30%), but these differences were not statistically significant.
In this study, an overall C. piliforme prevalence of 40% was recorded in the faeces of clinically normal rabbits. Very high prevalence rates have been reported in healthy mice (83.01%) and rats (90.9%) by PCR in a laboratory animal facility in Turkey.11 In contrast, a study in three provinces of China detected C. piliforme antibodies in only 5.08% of clean-grade animals and 9.96% of specific pathogen-free animals using LAMP-LFD analysis.12
Fingerhood et al.13 detected C. piliforme by PCR in colon (5/10), liver (5/10), and heart (1/10) samples from pre-weaned orphaned kittens in the USA. Oliveira et al.14 detected C. piliforme by PCR in skin, liver, heart, intestines, and brain samples from a kitten that died after exhibiting nervous signs and diarrhoea. Similarly, Brooks et al.15 demonstrated the C. piliforme genome by PCR in liver samples of two farm-raised white-tailed deer fawns (Odocoileus virginianus), suggesting a probable association between copper toxicity and C. piliforme infection. Garcia et al.16 detected C. piliforme in all 24 liver samples (100%), 20 of 25 colon samples (83.33%), and 5 of 25 heart samples (20%) from foals suspected to have died of Tyzzer’s disease by PCR. These findings suggest that the sensitivity of PCR depends on the type of sample collected from infected animals.
Furukawa et al.6 detected C. piliforme DNA by PCR in 3 of 5 (60%) faecal samples collected from naturally infected rabbits. The authors explained that bile salts and bilirubin in faecal extracts reduced the sensitivity of PCR, and the detection limit of their assay was 10 bacteria per reaction.
Pritt et al.17 were unable to detect C. piliforme genomic DNA by PCR in caecum samples from 20 randomly selected seropositive rabbits without clinical signs. This indicates that serological tests cannot be considered confirmatory for the presence of C. piliforme in laboratory or commercial farm animals, as antigens used in these tests may cross-react with antibodies produced against non-pathogenic bacteria.18 Kirtland et al.19 evaluated the utility of plasma sorbitol dehydrogenase measurement for identifying subclinical or early Tyzzer’s disease in farm populations and potential carrier animals but concluded that it lacked sensitivity and specificity.
Disparity in the prevalence of C. piliforme reported in previous studies may be attributed to the health status of the animals (diseased vs. healthy) and the type of samples analyzed (affected organs vs. faeces). These studies also demonstrated that C. piliforme infections are often subclinical and cleared by immunocompetent hosts following seroconversion.20
The significant prevalence difference observed between two farms: Mohammadabad (70%) and Chengalpattu (19.2%), even after Bonferroni correction, may be due to environmental and management-related differences such as farming system (backyard vs. organized) and feeding practices (wet mash vs. vegetables).
Garcia et al.16 reported that all but one foal suspected to have died of C. piliforme were younger than 45 days. Artukovic et al.21 also observed that weaned rabbits were more predisposed to C. piliforme infection than adult rabbits. Tyzzer’s disease was confirmed in a 45-day-old kitten and in 4 to 5-day-old white-tailed deer fawns by Oliveira et al.14 and Brooks et al.,15 respectively. Tyzzer’s disease primarily affects well-nourished young animals, particularly those on protein-rich diets.22 In contrast, in the present study, although not statistically significant, C. piliforme was detected by nPCR in 42% of faecal samples collected from rabbits older than 5 months, while only 25% of samples from rabbits younger than 5 months were positive. Similarly, Ulker et al.11 reported a higher prevalence of C. piliforme in adult mice and rats compared with young animals. The differences in prevalence rates between age groups in various studies may be due to sample selection from healthy versus diseased animals.
Garcia et al.,16 Fingerhood et al.,13 and Brooks et al.15 studied foals, kittens, and deer fawns, respectively, that were suspected to have died of C. piliforme. In contrast, Ulker et al.11 and the present study examined faecal samples collected from clinically normal animals. Brooks et al.15 suggested that C. piliforme infection in deer fawns was probably associated with liver damage caused by copper toxicity. Co-infections of C. piliforme with canine distemper virus were also reported in two domestic dog puppies and a grey fox kit.23 Fingerhood et al.13 observed that 15 pre-weaned kittens that died of Tyzzer’s disease had co-morbidities such as sepsis, coccidiosis, and feline parvovirus (FPV). Oliveira et al.14 similarly reported co-infection with FPV in the ileum of a kitten that died of Tyzzer’s disease. In their studies, adult animals (deer and cats) were clinically healthy, suggesting that C. piliforme infection causes mortality in animals that are either co-infected or immunosuppressed. The high positivity rate in healthy adult rabbits in the present study suggests that they may act as carriers, as Tyzzer’s disease is generally transmitted through the faeco-oral route24 and C. piliforme spores can survive in the environment for at least 5 years.25
In the present study, breed, sex, weight, and type of rearing or feeding did not have a statistically significant association with C. piliforme infection in rabbits. It has been recorded that Tyzzer’s disease in animals is mainly predisposed by immunosuppression, stress, high environmental temperatures, overcrowding, poor sanitation, dietary changes, and the administration of sulfonamides or corticosteroids.25,26
In this study, C. piliforme DNA was amplified as an 850 bp amplicon by nested PCR. A larger-sized band of around 1500 bp was also observed. A similar larger band was reported by Niepceron and Licois,7 who explained that this was probably due to the presence of outer primers from the first-round PCR. They attempted the second-round PCR with a higher annealing temperature to achieve a single 850 bp band without by-products, but this resulted in a tenfold reduction in sensitivity in caecal samples.
A few non-specific amplification products were also observed in the second-round PCR. Such non-specific amplicons have been reported in mouse, rat, and hamster faeces,6 in rabbit caecum,7 in deer fawn liver samples,15 and in DNA extracted from cell-culture–propagated C. piliforme isolates.18 This may be due to the use of 16S rRNA primers. Because faeces contain diverse bacteria, cross-reactions between primers and the 16S rRNA regions of closely related clostridial organisms in the gastrointestinal tract may generate erroneous products. Furukawa et al.6 noted that diagnosis becomes challenging if obscure bands appear at the specific region when using faecal samples. Thus, although detection of C. piliforme in faeces by PCR is simple, rapid, and non-invasive, the lack of sensitivity and specificity cautions against its use as a sole confirmatory test in live animals.
Primers targeting specific genes of C. piliforme could not be designed because no complete genome sequence is currently available. Confirmation of C. piliforme infection in suspected cases is therefore achieved not by a single test but by a combination of methods. Recent studies have used clinical signs, gross lesions, histopathological changes, detection of clostridia in tissues, immunohistochemistry, and 16S rRNA gene PCR for confirmation in dead animals.13,14,23 However, no foolproof test exists for screening or diagnosis of C. piliforme in live animals, and only a combination of serological testing and PCR is recommended. Hence, the presence of C. piliforme in the present study should be further confirmed by sequencing the PCR products, and results should always be interpreted in correlation with serology and clinical signs/gross lesions.
Recently, Uprety et al.4 used shotgun metagenomics to obtain partial genome sequences of C. piliforme from the liver of a foal that died of Tyzzer’s disease. They identified partial sequences of virulence factor genes such as alveolysin, exo-α-sialidase, and those involved in flagellar and spore formation, thereby providing the first genetic evidence of virulence factors for C. piliforme. They also showed that flagellin protein sequences had low identity with those of C. colinum, the next closest phylogenetic species, indicating their potential utility in serological assays and vaccine development.
Immunocompromised humans are susceptible to C. piliforme infection, as demonstrated by a case detected in an HIV patient in the USA.3 A serological study detected antibodies against C. piliforme in 85.7% of laboratory animal handlers, 40.5% of personnel involved in laboratory animal care, and 22.0% of unrelated personnel.27 The drastic decline of North American muskrats over the last 50 years may be attributed to Tyzzer’s disease,28 and the susceptibility of a wide range of mammals to C. piliforme infection has been documented, especially in animals with co-morbidities.13-15,23 These studies highlight the potential public health risk of C. piliforme infection for laboratory animal handlers and immunocompromised individuals.
In conclusion, the presence of C. piliforme in rabbits could affect the health and research outcomes of these animals, as well as the health of caretakers and research personnel. Therefore, proper monitoring, preventive measures, and potential treatments should be implemented to maintain animal well-being and research integrity. Use of 16S rRNA-based PCR for detecting C. piliforme in samples, particularly faeces, should always be correlated with serological tests and clinical or pathological findings. The identification of C. piliforme specific gene target primers is urgently needed for screening susceptible animal populations.
ACKNOWLEDGMENTS
The authors are thankful to the Tamil Nadu Veterinary and Animal Sciences University, Chennai, India, for providing the necessary infrastructure to carry out the research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
ACM conceptualized, designed the study and consolidated the data. KGT acquired funding and performed administrative work. SR, SJ, PJ, PR and MRS performed sample collection. PR carried out laboratory work. PJ performed statistical analysis. PR drafted the original manuscript. ACM, SR, SJ, PJ, and MRS wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Uzal FA, Arroyo LG, Navarro MA, Gomez DE, Asin J, Henderson E. Bacterial and viral enterocolitis in horses: a review. J Vet Diagn Invest. 2022;34(3):354-375.
Crossref - Navarro MA, Uzal FA. Pathobiology and diagnosis of clostridial hepatitis in animals. J Vet Diagn Invest. 2020;32(2):192-202.
Crossref - Smith KJ, Skelton HG, Hilyard EJ, et al. Bacillus piliformis infection (Tyzzer’s disease) in a patient infected with HIV-1: confirmation with 16S ribosomal RNA sequence analysis. J Am Acad Dermatol. 1996;34(2):343-348.
Crossref - Uprety T, Swan M, Kennedy L, et al. Retrospective investigation of 43 necropsy cases of Tyzzer disease in foals and partial genome sequence of Clostridium piliforme by shotgun metagenomics. Vet Microbiol. 2025;304:110489.
Crossref - Goto K, Itoh T, Takakura A, Kunita S, Terada E, Kagiyama N. A serological survey on Bacillus piliformis infection in laboratory rabbits in Japan. Exp Anim. 1991;40(2):231-233.
Crossref - Furukawa T, Furumoto K, Fujieda M, Okada E. Detection by PCR of the Tyzzer’s disease organism (Clostridium piliforme) in faeces. Exp Anim. 2002;51(5):513-516.
Crossref - Niepceron A, Licois D. Development of a high-sensitivity nested PCR assay for the detection of Clostridium piliforme in clinical samples. Vet J. 2010;185(2):222-224.
Crossref - Wang H, Qi M, Cutler AJ. A simple method of preparing plant samples for PCR. Nucleic Acids Res. 1993;21(17):4153-4154.
Crossref - Osmundson TW, Eyre CA, Hayden KM, Dhillon J, Garbelotto MM. Back to basics: an evaluation of NaOH and alternative rapid DNA extraction protocols for DNA barcoding, genotyping, and disease diagnostics from fungal and oomycete samples. Mol Ecol Resour. 2013;13(1):66-74.
Crossref - Heuer H, Krsek M, Baker P, Smalla, K, Wellington EM. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol. 1997;63(8):3233-3241.
Crossref - Ulker U, Kyzyl S, Cecen EM, Aydyn E. Molecular Diagnosis of Clostridium piliforme, the Causative Agent of Tyzzer’s Disease in Mice and Rats. Journal of Laboratory Animal Science and Practices. 2024;4(2):78-82.
Crossref - Tao J, Yan H, Chen S, et al. Establishment and application of a loop-mediated isothermal amplification-lateral flow dipstick (LAMP-LFD) method for detecting Clostridium piliforme. Vet Med Sci. 2024;10(1):e1318.
Crossref - Fingerhood S, Mendonna FS, Uzal FA, et al. Tyzzer disease in 19 preweaned orphaned kittens. J Vet Diagn Invest. 2023; 35(2):212-216.
Crossref - Oliveira ES, Queiroz CRR, Santos DO, et al. Neurologic and cutaneous infection by Clostridium piliforme in a kitten with systemic Tyzzer disease. J Vet Diagn Invest. 2023;35(3):322-326.
Crossref - Brooks JW, Whary MT, Hattel AL, et al. Clostridium piliforme infection in two farm-raised white-tailed deer fawns (Odocoileus virginianus) and association with copper toxicosis. Vet Pathol. 2006;43(5):765-768.
Crossref - Garcia JA, Navarro MA, Fresneda K, Uzal FA. Clostridium piliforme infection (Tyzzer disease) in horses: retrospective study of 25 cases and literature review. J Vet Diagn Invest. 2022;34(3):421-428.
Crossref - Pritt S, Henderson KS, Shek WR. Evaluation of available diagnostic methods for Clostridium piliforme in laboratory rabbits (Oryctolagus cuniculus). Lab Anim. 2010;44(1):14-19.
Crossref - Feldman SH, Kiavand A, Seidelin M, Reiske HR. Ribosomal RNA sequences of Clostridium piliforme isolated from rodent and rabbit: re-examining the phylogeny of the Tyzzer’s disease agent and development of a diagnostic polymerase chain reaction assay. J Am Assoc Lab Anim Sci. 2006;45(5):65-73.
- Kirtland A, Pusterla N, Bozorgmanesh R. Successful management of an outbreak of Tyzzer’s disease on a Thoroughbred breeding farm in central Kentucky;use of sorbitol dehydrogenase to identify sub-clinical cases. Equine Vet Edu. 2023;35:e311-e324.
Crossref - Motzel SL, Riley LK. Subclinical infection and transmission of Tyzzer’s disease in rats. Lab Anim Sci. 1992;42(5):439-43.
- Artukovic B, Grabarevic Z, Kurilj AG, et al. Clinical and pathological findings of an outbreak of Tyzzer’s disease in a rabbit colony in Croatia. Veterinarski Arhiv. 2010;80(6):761-770.
- Sharma S, Sharma A, Singh AK. Tyzzer’s Disease in Laboratory Animals. Acta Sci Vet Sci. 2022;4(8):188-189.
Crossref - Jacobson SA, Ferro PJ, Navarro MA, Uzal FA, Edwards EE. Clostridium piliforme and canine distemper virus coinfection in 2 domestic dog littermates and a gray fox kit. J Vet Diagn Invest. 2022;34(5):894-897.
Crossref - Itoh T, Kagiyama N, Fujiwara K. Production of Tyzzer’s disease in rats by ingestion of bacterial spores. Jpn J Exp Med. 1989;59(1):9-15.
- Barthold SW, Griffey SM, Percy DH. Pathology of laboratory rodents and rabbits. 4th ed. Chichester, UK. Wiley. 2016:1-118.
Crossref - Ikegami T, Shirota K, Une Y, et al. Naturally occurring Tyzzer’s disease in a calf. Vet Pathol. 1999;36(3):253-255.
Crossref - Jufang Y, Jiaming T. Serological investigation on the infection of Tyzzer in the population. Shanghai Laboratory Animal Science. 1998;Z1:210.
- Ganoe LS, Brown JD, Yabsley MJ, Lovallo MJ, Walter WD. A Review of Pathogens, Diseases, and Contaminants of Muskrats (Ondatra zibethicus) in North America. Front Vet Sci. 2020;7:233.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.