Staphylococcus aureus (S. aureus) formation presents a critical challenge in healthcare settings, profoundly affecting patient outcomes and treatment strategies. Biofilms, complex microbial communities encased in an extracellular matrix, exhibit increased resistance to antibiotics and host immune responses. The ability of S. aureus to form biofilms in medical devices, such as catheters, prosthetic joints, and cardiac implants contributes to persistent and recurrent infections including bloodstream infections, endocarditis, osteomyelitis, and surgical site infections. These infections are frequently associated with implant failure, delayed wound healing, and prolonged hospital stays. S. aureus strains embedded in biofilms also facilitate the spread of healthcare-associated infections (HAIs), posing a significant risk to vulnerable patient populations. Understanding the dynamics of biofilm-associated infections is crucial for developing targeted therapeutic interventions, including novel antibiotics, biofilm-disrupting agents, and immune-modulating strategies, to enhance treatment outcomes and strengthen infection control within healthcare environments. As the prevalence of antibiotic-resistant strains increases, innovative strategies such as biofilm-disrupting agents, antimicrobial-coated implants, and nanoparticle-based therapies are being explored to enhance treatment efficacy. This review explores the multifaceted impact of S. aureus biofilms on healthcare by evaluating specific infection types, clinical complications, and advancements in therapeutic strategies, with a focus on their economic burden, effects on treatment efficacy, and contribution to patient morbidity to improve healthcare outcomes.
Staphylococcus aureus Biofilm, Healthcare Associated Infections, Antibiotic Resistance, Spectrum of Genes, Infection Control Strategies
Staphylococcus aureus (S. aureus), a versatile bacterium that resides as a commensal and pathogen on human skin and nasal passages, spans from asymptomatic colonization to severe infections. Known for its virulence factors and antibiotic resistance, notably Methicillin-resistant Staphylococcus aureus (MRSA), it challenges healthcare.1 Biofilm formation, a sophisticated survival mechanism, complicates treatment by fostering resistance to antimicrobials and immune responses. Biofilms are the result of microorganisms adhering to surfaces and producing a protective matrix. Biofilm genes of S. aureus, such as icaADBC, agr, and sarA, regulate adhesion, matrix synthesis, and virulence, amplifying risks and resistance. In healthcare settings, biofilm related S. aureus infections on medical devices and tissues increase infection risks, complicating treatments, increasing costs, and affecting patient outcomes.2,3
Current treatments face challenges due to biofilm resilience, diagnostic limitations, and antibiotic resistance. Strategies involve antibiotic therapy, biofilm disruption agents, antibiotic coated implants, surgical intervention, but face limitations in combating entrenched biofilms. Innovations are focused on antibiotic development, biofilm disruption, diagnostics, and customized treatments, using nanoparticles, antimicrobial peptides, and advanced diagnostics such as confocal microscopy. Tailored treatment strategies, surface modifications, and personalized medicine aim to overcome biofilm complexities and optimize patient centered care.4 Surface engineering aims to prevent initial bacterial attachment and disrupt biofilm matrices in medical devices, significantly reducing S. aureus associated infections in healthcare settings. These advancements present promising avenues to combat biofilm related S. aureus infections by addressing their multifaceted challenges and enhancing treatment efficacy.5 The objective of this article is to explore the multifaceted challenges posed by S. aureus biofilm related infections, investigate current treatment strategies, and evaluate innovative approaches to overcome biofilm complexities, improve treatment efficacy, and reduce infection risks in healthcare settings.
Staphylococcus aureus
S. aureus, a Gram-positive bacterium, is a versatile human commensal and pathogen, residing on the skin and nasal passages. It exists in both asymptomatic colonization and virulent pathogenic states. Renowned for its diverse virulence factors, S. aureus can cause several infections, from minor skin conditions to severe invasive diseases. In particular, it can develop antibiotic resistance, including the notorious MRSA, which poses a substantial challenge in healthcare settings.3 S. aureus, a facultative anaerobic bacterium, commonly resides as a commensal on human epithelial surfaces, predominantly colonizing the nares and skin.6 Its adaptability and genetic versatility enable it to transition between commensalism and pathogenicity, dictated by host and environmental factors. Pathogenic potential is attributed to an extensive range of virulence factors, including surface adhesions, toxins, and enzymes that facilitate adherence, immune evasion, and tissue damage. S. aureus infections span a broad spectrum, from superficial skin infections such as folliculitis and impetigo to more serious invasive conditions, including bacteremia, pneumonia, osteomyelitis, and endocarditis.7 The ability to form biofilms on biotic and abiotic surfaces further complicates treatment strategies, enhancing resistance to antimicrobial agents and immune responses. The emergence of antibiotic resistant strains, particularly MRSA, is of considerable concern, posing a substantial public health threat.8 The evolution of resistance mechanisms in S. aureus has led to challenges in the treatment of infections, necessitating a multidisciplinary approach involving infection control measures, surveillance, and the development of novel therapeutic strategies.9
Biofilm formation
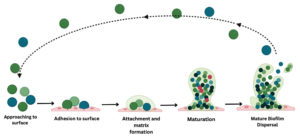
Biofilm formation is a sophisticated microbial survival strategy characterized by the aggregation and adherence of microorganisms to surfaces, embedded within a self produced matrix of extracellular polymeric substances (EPS).10 This dynamic and structured consortium of bacteria, housed in a polymeric matrix, endows them with communal resilience that exceeds the sum of their individual capacities (Figure 1).11
The initial phase of biofilm formation involves the reversible attachment of planktonic microorganisms to a substrate. Subsequently to this attachment, irreversible adhesion occurs, leading to the secretion of EPS constituents, including polysaccharides, proteins, and nucleic acids (Figure 1).12 These constituents collectively contribute to the formation of a three-dimensional (3D) matrix that encapsulates the microbial community.13 Biofilms confer numerous advantages on their constituent microorganisms, such as enhanced resistance to antimicrobial agents, protection from host immune responses, and increased tolerance to environmental stressors. This increased resilience arises from the matrix acting as a physical barrier, impeding antimicrobial diffusion, and shielding the bacterial community from external assaults (Figure 1).14
Biofilm formation has substantial implications in various fields, including medicine, industry, and environmental science. In the medical context, biofilms are known for their role in chronic infections, as they can form on biotic and abiotic surfaces, including medical devices.15 The intricate mechanisms that govern biofilm development continue to be an important focus of research, with the ultimate goal of devising strategies to mitigate or disrupt biofilm formation for therapeutic and industrial applications (Figure 1).16
Quorum sensing
Quorum sensing is a sophisticated communication mechanism used by bacteria to coordinate group behavior, such as biofilm formation, based on the density of the cell population. Through the secretion and detection of signalling molecules, known as autoinducers, bacteria can assess their local population density.17 When the concentration of these molecules reaches a threshold level, it triggers specific gene expression, regulating behaviours such as biofilm formation. This coordinated action enables bacteria to coordinate activities, including producing EPS crucial for biofilm development.18
Quorum sensing represents a complex signalling system that bacteria use to orchestrate collective behavior, notably biofilm formation, in response to fluctuation of cell density within their environment. This intricate communication mechanism involves the synthesis, release, and detection of small diffusible molecules, termed autoinducers, by bacterial cells.19 In biofilm formation, bacteria initially exist in a planktonic state, releasing autoinducers into their surroundings. These autoinducers accumulate as the microbial population grows, reaching a critical concentration. Once a threshold level is reached, the autoinducers are detected by bacterial cells, initiating a signalling cascade that leads to alterations in gene expression patterns. Activated genes regulate the production of various factors crucial for biofilm formation, including EPS.12 EPS, which comprises polysaccharides, proteins, and DNA, facilitates adherence to surfaces and intercellular cohesion, fostering the formation of a robust 3D biofilm structure. This synchronized response orchestrated by quorum sensing enables the bacterial communities to collectively switch from a planktonic state to a sessile biofilm forming state. Through this coordinated action, biofilm associated bacteria gain increased resistance to antimicrobial agents, host immune defences, and adverse environmental conditions.20,21
Genes in biofilm formation of S. aureus
S. aureus, a versatile pathogen, uses a spectrum of genes and regulatory mechanisms in the intricate biofilm formation process (Table 1).2
Table (1):
The role of genes in different stages of biofilm formation
Genes |
Description |
|---|---|
IcaADBC |
These genes encode the proteins responsible for the synthesis of intercellular polysaccharide adhesin (PIA), a major component of the extracellular matrix in S. aureus biofilms. PIA plays a critical role in mediating cell to cell adhesion and biofilm stability.22 |
atl and aae |
These genes contribute to autolysis and cell wall turnover, affecting the release of extracellular DNA (eDNA). eDNA is a crucial structural component within the biofilm matrix, which promotes cohesion and stability among bacterial cells.23,24 |
agr |
The accessory gene regulator system regulates the expression of various virulence factors in S. aureus. Influences biofilm formation by modulating the balance between cell adhesion and dispersal within the biofilm structure.25 |
sarA |
This gene encodes a global regulator that influences the expression of multiple genes involved in biofilm formation, including icaADBC and surface adhesins. SarA contributes to the general regulation of the related genes, which affects biofilm architecture and development of biofilms.26 |
fnbA and fnbB |
These genes encode fibronectin binding proteins that facilitate the initial adhesion of S. aureus to host tissues or abiotic surfaces. They play a crucial role in the initial stages of biofilm formation by promoting bacterial attachment.27 |
clfA and clfB |
Clumping factors A and B are surface proteins involved in bacterial adhesion to host cells and the extracellular matrix, which contribute to the initiation and aggregation of biofilms of bacterial cells.28 |
Biofilm associated risks in healthcare
The impact of S. aureus that forms biofilms in healthcare settings is a multifaceted problem with significant implications for patient care, treatment outcomes, and healthcare infrastructures. Biofilm related infections caused by S. aureus present a formidable challenge due to their persistence, resistance mechanisms, and capacity to cause a wide spectrum of infections (Table 2).29
Biofilms formed by S. aureus pose a grave risk in healthcare settings. Serving as reservoirs for persistent infections, these biofilms adhere to medical devices, compromised tissues, and implanted materials, increasing the likelihood of device related infections, surgical site infections, and Healthcare-associated infections (HAI) (Table 2).30 The resilience of S. aureus against antimicrobial agents and immune responses complicates treatment strategies, making infections chronic, recurrent, and difficult to eliminate. In addition, biofilm formation fuels antibiotic resistance mechanisms, altering gene expression and metabolic activity, amplifying resistance, and narrowing therapeutic options (Table 2).8 Clinically, these infections lead to prolonged hospital stays, increased morbidity, and increased mortality rates, encompassing serious complications like bloodstream infections, endocarditis, osteomyelitis, and soft tissue infections. Controlling biofilm forming S. aureus is challenging within healthcare facilities due to its persistence on surfaces and medical devices, mandating stringent hygiene protocols and thorough decontamination practices.31 The substantial financial burden imposed by these infections, attributed to prolonged hospitalizations, repeated treatments, and the need for specialized care, underscores the urgency of combating biofilm related S. aureus infections within healthcare systems (Table 2).32
Table (2):
Biofilm associated risks in healthcare
Risk |
Description |
|---|---|
Increased Infection |
Biofilms serve as persistent infection reservoirs in healthcare, adhering to medical devices, tissues, and implants, increasing the risks of device related infections and HAI.30 |
Treatment Complexity |
The resilience of biofilm embedded S. aureus complicates treatment, reducing antimicrobial efficacy, making infections chronic and challenging to eradicate.8 |
Antibiotic Resistance |
Biofilm formation induces antibiotic resistance in S. aureus, altering gene expression, reducing metabolic activity, and enhancing genetic exchange, limiting therapeutic options.8 |
Clinical Complications |
Biofilm related S. aureus infections lead to prolonged hospital stays, increased morbidity, mortality rates, and severe complications, including bloodstream infections and osteomyelitis.31 |
Infection Control |
The tenacity of biofilm forming S. aureus poses challenges in infection control within healthcare, requiring stringent hygiene protocols and thorough decontamination practices.31 |
Economic Impact |
Treatment of biofilm related infections incurs substantial financial burdens due to prolonged hospitalizations, repeated treatments, and the need for specialized care, escalating overall healthcare costs.32 |
Current treatment strategies
Antibiotics and antimicrobials
Traditional antibiotic therapy represents the cornerstone in the treatment of S. aureus infections; however, its efficacy against biofilm associated S. aureus infections is notably limited. The biofilm matrix is a formidable barrier that prevents antibiotic penetration and fosters bacterial resilience.33 Biofilm embedded S. aureus displays altered metabolic states and reduced susceptibility to antimicrobial agents compared to their planktonic counterparts. This altered phenotype within the biofilm community results in decreased antibiotic effectiveness, leading to failures in treatment and recurrent infections.34 Combination therapies involving multiple antibiotics or higher doses are often considered to combat biofilm related S. aureus infections. However, the achievement of satisfactory outcomes due to the complex nature of the biofilm architecture and the presence of persisting cells that exhibit heightened tolerance to antibiotics.35
Biofilm disruption agents
Biofilm disruptors are instrumental in combating biofilm associated S. aureus infections by specifically targeting the complex structure and resilience of biofilms. Disruptive agents dismantle defense mechanism, making bacteria more susceptible to conventional treatments.36 Disrupting matrix production by compounds such as Dispersin B, which cleave the structural components; inhibiting quorum sensing to interfere with bacterial communication; and using chelating agents that destabilize vital ions vital for biofilm stability.37 Various classes of compounds, including enzymes such as Dispersin B and DNase, synthetic small molecules, and natural compounds from plant extracts, are explored as potential biofilm disruptors, each showing promise in either degrading biofilm structures or interfering with bacterial communication pathways, ultimately rendering biofilms more vulnerable to treatment.38
Antibiotic coated implants
Antibiotic coated implants represent a strategic intervention to counter biofilm associated S. aureus infections, particularly prevalent in medical devices and implants. These surfaces present a substantial risk of bacterial colonization and subsequent biofilm development, culminating in persistent and difficult to treat infections.8 The fundamental concept involves the integration of antibiotics onto implant surfaces, creating a proactive defense mechanism against bacterial adherence and subsequent biofilm formation. Functioning by releasing antibiotics into surrounding tissues, these implants establish an antimicrobial environment that deters initial bacterial adhesion to the implant surface, consequently diminishing biofilm formation.36 The antibiotics released also combat any adherent bacteria, preventing the establishment and proliferation of biofilm associated S. aureus infections. Antibiotics and antimicrobial agents, including vancomycin, rifampin, gentamicin, and silver nanoparticles, serve as coatings for implants. The application methods vary, including physical adsorption, chemical bonding, or integration into the implant material during manufacturing.8,39
Antibiotic coated implants are widely used in orthopedic surgeries involving joint replacements, spinal implants, and prosthetic devices, where susceptibility to biofilm related infections is high. The efficacy of implants in reducing the incidence of biofilm-associated infections.40
Surgical intervention
In biofilm associated S. aureus infections, surgical intervention is a crucial therapeutic strategy. Surgical methods are used to physically eliminate the biofilm reservoir and infected tissues, particularly in cases involving implanted medical devices.29 Debridement, the removal of infected or necrotic tissues, often complements antibiotic therapy, facilitating the eradication of biofilm embedded bacteria. This surgical procedure reduces the bacterial load and disrupts the structure of the biofilm, creating an environment conducive to effective antimicrobial action. Complete device removal may be necessary for infections related to medical devices such as prosthetic implants or catheters.41 Current intervention strategies are designed to prevent the initial device from eliminating the source of biofilm colonization, preventing recurrent infections and offering a chance for successful treatment and recovery.42
Challenges
Biofilm associated S. aureus exhibits increased antibiotic resistance compared to planktonic bacteria, originating from altered metabolic states and persisting cells within biofilms. This resistance leads to treatment failures and recurrent infections, which pose a significant challenge. The robustness of the biofilms against host immune responses and antimicrobial agents further complicates treatment. The protective matrix structure enables bacterial survival even under harsh conditions, contributing to chronic infections and recalcitrance to treatment (Table 3).8 Detecting and diagnosing biofilm associated S. aureus infections remains challenging, as conventional diagnostic methods often overlook biofilm embedded bacteria. This limitation results in underestimating the severity of the infection and inappropriate treatment. The absence of standardized protocols for the treatment of these infections also contributes to varied treatment outcomes. Tailored therapeutic approaches that address biofilm specific characteristics are crucial, but lack widespread implementation (Table 3).29 Biofilm related S. aureus infections have substantial clinical implications, prolonging hospital stays, increasing healthcare costs, and increasing the risk of morbidity and mortality among patients. These infections are linked to persistent complications, including device related infections and chronic wounds, significantly affecting patient outcomes (Table 3).43
Table (3):
The challenges and clinical implications of biofilm associated S. aureus infections
Challenges |
Details |
|---|---|
Antibiotic Resistance |
Biofilm associated S. aureus exhibits increased antibiotic resistance, attributed to altered metabolic states and persisting cells, leading to treatment failures and recurrent infections.8 |
Biofilm Resilience |
The robustness of biofilms against host immune responses and antimicrobial agents poses a formidable challenge, with the matrix structure protecting bacterial communities, allowing survival in harsh conditions and contributing to chronic infections and treatment recalcitrance.8 |
Diagnostic Limitations |
Detecting and diagnosing biofilm associated S. aureus infections remains challenging, as conventional diagnostic methods often fail to identify biofilm embedded bacteria, leading to underestimation of infection severity and inappropriate treatment.29 |
Inadequate Treatment Strategies |
The lack of standardized protocols or guidelines for the management of biofilm related S. aureus infections contributes to varied treatment outcomes. Tailored therapeutic approaches considering biofilm specific characteristics are essential but currently lack widespread implementation.29 |
Clinical Implications |
Biofilm associated S. aureus infections prolong hospital stays, increase healthcare costs, and increase the risk of morbidity and mortality among patients. These infections are linked to persistent complications, such as device related infections or chronic wounds, affecting patient outcomes.43 |
Treatment advancement
Advancements in the treatment of biofilm associated S. aureus infections encompass various innovative strategies aimed at antibiotic resistance, diagnostic limitations, and personalized treatment approaches.44
Innovative antibiotic approaches
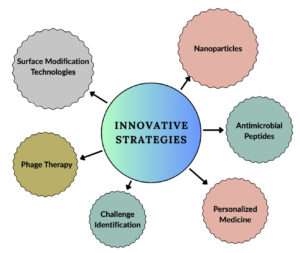
Efforts in antibiotic innovation aim to overcome the challenges posed by biofilm associated S. aureus infections. Novel antibiotic formulations, such as antibiotic nanoparticles or liposomal carriers, offer improved drug delivery mechanisms.45 These formulations enhance drug penetration into the biofilm matrix, where conventional antibiotics often struggle to reach. Combination therapies that involve multiple antibiotics or the synergistic use of antimicrobial agents are being explored to combat biofilm resistance and increase treatment efficacy.46 The exploration of bacteriophage therapy presents a promising alternative to conventional antibiotics. Bacteriophages, viruses that specifically target and lyse bacteria, can potentially disrupt biofilm embedded S. aureus cells. Phage therapy offers a tailored approach that could overcome the antibiotic resistance mechanisms inherent in biofilm related infections.47
Biofilm targeted therapies
Biofilm targeted therapeutic approaches focus on the disruption of biofilm architectures and increased susceptibility to antimicrobial agents. Within this domain, enzymes such as Dispersin B and DNase demonstrate the ability to dismantle the biofilm matrix, thereby facilitating the enhanced permeation of antimicrobial agents. These enzymatic agents work by degrading the structural components of the biofilm, allowing improved access and efficacy of antimicrobial compounds.48
Nanoparticles, specifically those that harbor silver, manifest inherent antimicrobial attributes that efficiently target and disrupt bacteria that are embedded within biofilms. The unique properties of these nanoparticles enable them to infiltrate biofilm structures, exerting antimicrobial effects and destabilizing the integrity of bacterial colonies, notably including those formed by S. aureus. The inclusion of silver within nanoparticles provides a multifaceted mechanism to combat biofilm related S. aureus infections.39 The utilization of antimicrobial peptides exhibits notable specificity against biofilm associated S. aureus. These peptides present a promising avenue for confronting the complexities posed by S. aureus biofilms. Their specific targeting and disruptive actions against the structure of the biofilm contribute to a potential therapeutic strategy to combat these persistent and challenging infections. The distinctiveness in the mode of action of antimicrobial peptides against biofilms marks them as a compelling area for further exploration and development in the context of biofilm associated infections associated with S. aureus (Figure 2).49
Improved diagnostics
Advanced imaging modalities, including confocal microscopy and sophisticated molecular diagnostic approaches, have emerged as pivotal contributors, facilitating a more nuanced identification of bacteria ensconced within biofilm structures. These refined techniques transcend the limitations of conventional diagnostic modalities, offering increased precision in discerning biofilm associated S. aureus infections.50
Confocal microscopy stands out as a cutting edge imaging technique that facilitates 3D visualization of biofilm architecture, allowing for a comprehensive assessment of the presence and distribution. This method surpasses conventional microscopy by providing detailed insights into the intricate matrix of biofilms, enhancing the ability to identify S. aureus within these complex structures. The application of confocal microscopy in diagnostics marks a significant advancement, enabling clinicians to visualize bacteria with unprecedented clarity.51 The integration of advanced molecular diagnostics represents a paradigm shift in the approach to detecting biofilm associated S. aureus infections. Molecular techniques, such as polymerase chain reaction (PCR) and nucleic acid sequencing, allow a more granular examination of the microbial genetic material. This level of scrutiny enables the identification of specific genetic markers associated with the formation of biofilms of S. aureus. Through biomarker identification and profiling, clinicians can refine diagnostic tools, ensuring greater sensitivity and specificity in the detection of infections intricately linked to biofilm structures (Figure 2).52
Figure 2. Innovative strategies for treatment of biofilm-associated S. aureus infections (Source: BioRender.com)
The elucidation of distinct biomarkers associated with biofilm related infections contributes to the development of diagnostic tools characterized by increased precision and early detection capabilities. These refined diagnostics transcend the limitations of traditional methods, allowing for a timely and precise identification of biofilm associated S. aureus infections. As a consequence, this not only facilitates faster therapeutic interventions but also mitigates the potential for prolonged and recurrent infections, thus increasing the overall efficacy of clinical management strategies (Figure 2).53
Tailored treatment strategies
Developing customized and personalized treatment strategies addresses the unique characteristics of biofilm related infections. The establishment of standard protocols or guidelines for the management of biofilm associated S. aureus infections is a current focus. These guidelines aim to optimize treatment outcomes by considering the complexity of biofilm structures and the varied responses observed in these infections.29,54 Standardized protocols and guidelines are being developed specifically to address infections associated with S. aureus biofilms. These comprehensive guidelines cover diagnostic criteria, treatment algorithms, and preventive strategies. Biofilm specific approaches involve custom treatments that consider the intricate nature of biofilm structures. Therapeutic regimens are strategically designed to address the resilience and the challenges posed by antibiotic resistance within these communities. Strategies may include combinations of antimicrobial agents, enzymes that alter biofilm matrices, or innovative nanoparticle based therapies, all aimed at improving drug penetration and efficacy against bacteria embedded within biofilms (Figure 2).55
Advancements in personalized medicine enable personalized treatment approaches based on patient specific factors. This includes a comprehensive evaluation of the patient’s medical history, the specific site of infection, and the unique characteristics of the biofilm. Such tailor made treatments consider variations in biofilm architecture, bacterial virulence, and patient immune status, thereby ensuring more precise and effective interventions.56 Additionally, tailored treatment strategies prioritize patient centered care, striving to improve patient outcomes and quality of life. Taking into account patient preferences, individual needs, and potential treatment side effects into account, healthcare providers can create more informed and collaborative treatment plans. This approach ultimately aims to increase patient satisfaction and adherence to therapies (Figure 2).57
Surface modification
Advanced surface engineering strategies not only aim to prevent initial bacterial attachment, but also attempt to disrupt the biofilm matrix upon its formation. Such approaches may involve the integration of surface coatings or functionalities that facilitate the release of antimicrobial agents upon detection of biofilm formation, thus actively inhibiting its maturation and facilitating the subsequent eradication of established biofilms. Advances in surface engineering for medical devices aim to significantly mitigate the incidence of S. aureus associated infections in healthcare settings.36 By impeding biofilm formation on these surfaces, the risk of device related infections is profoundly diminished, consequently improving patient outcomes, reducing the need for extensive antibiotic treatments, and curbing the economic burden associated with prolonged hospital stays and recurrent infections (Figure 2).58
S. aureus, recognized for its pathogenicity and capacity to develop MRSA, exhibits a difficult attribute in biofilm formation, complicating therapeutic interventions. Biofilms, an intricate microbial survival mechanism, present structured communities that are resistant to antimicrobial agents and immune responses. Essential genes such as icaADBC and agr orchestrate biofilm development, affecting cell adhesion and matrix synthesis. Their presence significantly increases the risks of infection, fosters antibiotic resistance, and imposes substantial economic burdens within healthcare settings. Innovative treatment strategies that target biofilm resilience, enhanced diagnostic precision, and personalized therapeutic approaches and overcome resistance to biofilms, emphasizing tailored interventions to accommodate the intricate nature of these infections.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AKB conceptualized and designed the study. NJB, GB and AP performed literature review. AKB wrote the manuscript. NJB, GB, AP, VA and MT revised the manuscript. AKB, NJB, AP and GB read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
Not applicable.
ETHICS STATEMENT
Not applicable.
- Gherardi G. Staphylococcus aureus Infection: Pathogenesis and Antimicrobial Resistance. Int J Mol Sci. 2023;24(9):8182.
Crossref - Peng Q, Tang X, Dong W, Sun N, Yuan W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics. 2022;12(1):12.
Crossref - Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin Microbiol Rev. 2015;28(3):603-661.
Crossref - Shrestha L, Fan HM, Tao HR, Huang JD. Recent Strategies to Combat Biofilms Using Antimicrobial Agents and Therapeutic Approaches. Pathogens. 2022;11(3):292.
Crossref - Balaure PC, Grumezescu AM. Recent Advances in Surface Nanoengineering for Biofilm Prevention and Control. Part II: Active, Combined Active and Passive, and Smart Bacteria-Responsive Antibiofilm Nanocoatings. Nanomaterials. 2020;10(8):1527.
Crossref - Balasubramanian D, Harper L, Shopsin B, Torres VJ. Staphylococcus aureus pathogenesis in diverse host environments. Pathog Dis. 2017;75(1).
Crossref - Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12(1):547-569.
Crossref - Tuon FF, Suss PH, Telles JP, Dantas LR, Borges NH, Ribeiro VST. Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics. 2023;12(1):87.
Crossref - Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309-318.
Crossref - Rather MA, Gupta K, Mandal M. Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz J Microbiol. 2021;52(4):1701-1718.
Crossref - Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322-332.
Crossref - Muhammad MH, Idris AL, Fan X, et al. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front Microbiol. 2020;11:928.
Crossref - Karygianni L, Ren Z, Koo H, Thurnheer T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020;28(8):668-681.
Crossref - Lopez D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2(7):a000398-a000398.
Crossref - Mirghani R, Saba T, Khaliq H, et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022;8(3):239-277.
Crossref - Asma ST, Imre K, Morar A, et al. An Overview of Biofilm Formation-Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life. 2022;12(8):1110.
Crossref - Rutherford ST, Bassler BL. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb Perspect Med. 2012;2(11):a012427-a012427.
Crossref - Luo Y, Yang Q, Zhang D, Yan W. Mechanisms and Control Strategies of Antibiotic Resistance in Pathological Biofilms. J Microbiol Biotechnol. 2021;31(1):1-7.
Crossref - Li YH, Tian X. Quorum Sensing and Bacterial Social Interactions in Biofilms. Sensors. 2012;12(3):2519-2538.
Crossref - Limoli DH, Jones CJ, Wozniak DJ. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Ghannoum M, Parsek M, Whiteley M, Mukherjee P, editors. Microbiol Spectr. 2015;3(3):3.3.29.
Crossref - Di Martino P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018;4(2):274-88.
Crossref - O’Gara JP. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270(2):179-188.
Crossref - Porayath C, Suresh MK, Biswas R, Nair BG, Mishra N, Pal S. Autolysin mediated adherence of Staphylococcus aureus with Fibronectin, Gelatin and Heparin. Int J Biol Macromol. 2018;110:179-184.
Crossref - Fagerlund A, Langsrud S, Heir E, Mikkelsen MI, Moretro T. Biofilm Matrix Composition Affects the Susceptibility of Food Associated Staphylococci to Cleaning and Disinfection Agents. Front Microbiol. 2016;7:856.
Crossref - Yang X, Dong F, Qian S, et al. Accessory gene regulator (agr) dysfunction was unusual in Staphylococcus aureus isolated from Chinese children. BMC Microbiol. 2019;19(1):95.
Crossref - Wolska KI, Grudniak AM, Rudnicka Z, Markowska K. Genetic control of bacterial biofilms. J Appl Genet. 2016;57(2):225-238.
Crossref - Speziale P, Pietrocola G. The Multivalent Role of Fibronectin-Binding Proteins A and B (FnBPA and FnBPB) of Staphylococcus aureus in Host Infections. Front Microbiol. 2020;11:2054.
Crossref - Herman-Bausier P, Labate C, Towell AM, Derclaye S, Geoghegan JA, Dufrene YF. Staphylococcus aureus clumping factor A is a force-sensitive molecular switch that activates bacterial adhesion. Proc Natl Acad Sci. 2018;115(21):5564-5569.
Crossref - Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence. 2011;2(5):445-459.
Crossref - Otto M. Staphylococcal Biofilms. In: Romeo, T. (eds) Bacterial Biofilms. Current Topics in Microbiology and Immunology, vol 322. Springer, Berlin, Heidelberg.
Crossref - Masters EA, Trombetta RP, De Mesy Bentley KL, et al. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy.” Bone Res. 2019;7(1):20.
Crossref - Subhadra B, Kim D, Woo K, Surendran S, Choi CH. Control of Biofilm Formation in Healthcare: Recent Advances Exploiting Quorum-Sensing Interference Strategies and Multidrug Efflux Pump Inhibitors. Materials. 2018;11(9):1676.
Crossref - Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther. 2015;13(12):1499-1516.
Crossref - Manandhar S, Singh A, Varma A, Pandey S, Shrivastava N. Biofilm Producing Clinical Staphylococcus aureus Isolates Augmented Prevalence of Antibiotic Resistant Cases in Tertiary Care Hospitals of Nepal. Front Microbiol. 2018;9:2749.
Crossref - Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon. 2019;5(8):e02192.
Crossref - Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9(1):522-554.
Crossref - Abdelhamid AG, Yousef AE. Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections. Antibiotics. 2023;12(6):1005.
Crossref - Khan J, Tarar SM, Gul I, Nawaz U, Arshad M. Challenges of antibiotic resistance biofilms and potential combating strategies: a review. 3 Biotech. 2021;11(4):169.
Crossref - Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver Nanoparticles and Their Antibacterial Applications. Int J Mol Sci. 2021;22(13):7202.
Crossref - Rodriguez-Merchan EC, Davidson DJ, Liddle AD. Recent Strategies to Combat Infections from Biofilm-Forming Bacteria on Orthopaedic Implants. Int J Mol Sci. 2021;22(19):10243.
Crossref - Jiang Y, Geng M, Bai L. Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms. 2020;8(8):1222.
Crossref - Donlan RM, Costerton JW. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin Microbiol Rev. 2002;15(2):167-193.
Crossref - Assefa M, Amare A. Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. Infect Drug Resist. 2022;15:5061-5068.
Crossref - Chopra H, Islam MdA, Sharun K, Emran TB, Al-Tawfiq JA, Dhama K. Recent advances in the treatment of biofilms induced surgical site infections. Int J Surg. 2023;109(1):65-67.
Crossref - Pinto RM, Lopes-de-Campos D, Martins MCL, Van Dijck P, Nunes C, Reis S. Impact of nanosystems in Staphylococcus aureus biofilms treatment. FEMS Microbiol Rev. 2019;43(6):622-641.
Crossref - Makhlouf Z, Ali AA, Al-Sayah MH. Liposomes-Based Drug Delivery Systems of Anti-Biofilm Agents to Combat Bacterial Biofilm Formation. Antibiotics (Basel). 2023;12(5):875.
Crossref - Opperman CJ, Wojno JM, Brink AJ. Treating bacterial infections with bacteriophages in the 21st century. S Afr J Infect Dis. 2022;37(1):346.
Crossref - Mishra S, Gupta A, Upadhye V, Singh SC, Sinha RP, Hader DP. Therapeutic Strategies against Biofilm Infections. Life. 2023;13(1):172.
Crossref - Yasir M, Willcox MDP, Dutta D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials. 2018;11(12):2468.
Crossref - Neu TR, Manz B, Volke F, Dynes JJ, Hitchcock AP, Lawrence JR. Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol Ecol. 2010;72(1):1-21.
Crossref - Schlafer S, Meyer RL. Confocal microscopy imaging of the biofilm matrix. J Microbiol Methods. 2017;138:50-59.
Crossref - Gerace E, Mancuso G, Midiri A, Poidomani S, Zummo S, Biondo C. Recent Advances in the Use of Molecular Methods for the Diagnosis of Bacterial Infections. Pathogens. 2022;11(6):663.
Crossref - Vestby LK, Gronseth T, Simm R, Nesse LL. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics. 2020;9(2):59.
Crossref - Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1-25.
Crossref - Waqas U, Farhan A, Haider A, Qumar U, Raza A. Advancements in biofilm formation and control in potable water distribution systems: A comprehensive review and analysis of chloramine decay in water systems. J Environ Chem Eng. 2023;11(6):111377.
Crossref - Ma R, Hu X, Zhang X, et al. Strategies to prevent, curb and eliminate biofilm formation based on the characteristics of various periods in one biofilm life cycle. Front Cell Infect Microbiol. 2022;12:1003033.
Crossref - Kwame A, Petrucka PM. A literature-based study of patient-centered care and communication in nurse-patient interactions: barriers, facilitators, and the way forward. BMC Nurs. 2021;20(1):158.
Crossref - Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control. 2019;8:76.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.