ISSN: 0973-7510
E-ISSN: 2581-690X
Heavy metal pollution, primarily due to hexavalent chromium (Cr(VI)), poses a significant threat to ecosystems and human health. Chromium is widely used in industries such as tanning, electroplating, and dye manufacturing, which results in the discharge of toxic residues into the environment. Cr(VI) is highly soluble, mobile, and carcinogenic, making its removal from contaminated sites a pressing environmental challenge. Among various remediation strategies, bioremediation using bacteria provides a cost-effective, eco-friendly, and sustainable approach. This study focuses on the initial phase of a broader bioremediation project that involves developing a bacterial consortium for chromium detoxification. Here, we report the isolation, screening, and characterization of a potent chromium-tolerant bacterial isolate obtained from wastewater. Several morphologically distinct bacterial strains were isolated using selective enrichment techniques on chromium-supplemented media. The isolates were screened for their chromium tolerance and resistance levels based on minimum inhibitory concentration (MIC) assays. Among them, two isolates exhibited exceptional resistance to Cr(VI), maintaining high (Full) growth at concentrations as high as 250 ppm. Morphological and biochemical profiling revealed distinct features, and 16S rRNA gene sequencing confirmed their identity as members of the Bacillus subtilis and Lysinibacillus macroides groups. These isolates hold promise for further development into a functional part of a microbial consortium aimed at efficient chromium bioremediation. Future work will focus on optimization studies, consortium compatibility, and real-world application trials.
Ecosystem, Bioremediation, Wastewater, Chromium, Bacillus subtilis, Lysinibacillus, Bioremediation, Heavy Metal Detoxification
Chromium (Cr) is a naturally occurring transition metal that has gained notoriety due to its extensive industrial use and associated environmental hazards. Industries such as leather tanning, electroplating, textile dyeing, and metallurgy utilize chromium compounds extensively, especially in the hexavalent form [Cr(VI)].1,2 Cr(VI) is highly soluble, mobile in aquatic systems, and known for its carcinogenic, mutagenic, and teratogenic effects on living organisms.3 Continuous discharge of untreated or inadequately treated chromium-laden effluents has resulted in the accumulation of toxic metals in soil and water bodies, posing severe ecological and public health risks.2
Recent literature has emphasized the diverse strategies employed by bacteria to survive in heavy metal-contaminated environments4 provided a comprehensive overview of bacterial resistance mechanisms, including efflux pumps, enzymatic detoxification, and biofilm formation, reinforcing the ecological and biotechnological significance of using native microbes for remediation. In addition to detoxification mechanisms, microbes also play a crucial role in supporting phytoremediation in metal-contaminated soils,5 provided detailed insights into the role of rhizospheric and endophytic bacteria in improving heavy metal uptake and tolerance in plants. They emphasized that genera such as Pseudomonas, Bacillus, Azospirillum, and Enterobacter assist in phytoremediation by mobilizing metals, producing plant growth-promoting substances, and inducing stress resistance mechanisms. These findings expand the relevance of microbial applications from direct bioremediation to integrated plant-microbe remediation strategies, especially under field conditions.
Chemical precipitation, ion exchange, membrane filtration, and adsorption are examples of conventional heavy metal cleanup procedures that are often associated with high operational costs, the generation of secondary pollutants, and reduced efficiency in low-concentration scenarios.6 In contrast, bioremediation, which exploits the natural detoxification capabilities of microorganisms, has emerged as a sustainable, cost-effective, and environmentally friendly alternative for chromium removal.2,7
Several bacteria have evolved efficient mechanisms to detoxify Cr(VI), including biosorption, bioaccumulation, and enzymatic reduction to the less toxic trivalent form [Cr(III)].8 These mechanisms may operate individually or synergistically, depending on the bacterial species and environmental conditions. Recent studies between 2015 and 2024 have reported multiple bacterial strains with remarkable chromium-resistance and detoxification capabilities. For instance, Enterobacter cloacae B2-DHA, isolated from contaminated soil, demonstrated 81% Cr(VI) reduction and could accumulate chromium intracellularly at concentrations of 320 µ/g biomass after 120 hours.9 Similarly, Sporosarcina saromensis M52, isolated from offshore sediment, showed the ability to tolerate up to 500 mg/L Cr(VI), with rapid removal efficiency of 50-200 mg/L within 24 hours.10
Understanding the molecular and physiological basis of Cr(VI) resistance and transformation is key to optimizing bacterial performance under contaminated conditions. A comprehensive review by Fernendez et al.11 emphasized that bacterial response to chromium includes chromate efflux pumps, enzymatic detoxification systems, antioxidant responses, and stress-regulatory pathways, all of which contribute to metal resistance and adaptation. However, the real-world application of microbial bioremediation faces challenges such as varying pH, temperature, heavy metal concentration, and microbial competition in natural environments.12 In this context, indigenous bacteria-organisms isolated from the contaminated sites-have shown promise due to their inherent adaptability to the native physicochemical conditions. A study by Bhattacharjee et al.13 illustrated that bacteria isolated from tannery wastewater significantly reduced Cr(VI) levels under laboratory conditions, validating the potential of site-specific strains.
Several studies have shown that organisms such as Pseudomonas, Bacillus, and Enterobacter have the capacity to remediate chromium. However, most research focuses on single isolates in the laboratory, with little attention paid to mixed microbial consortia or real-world applications. This work aims to bridge the gap by identifying naturally Cr-tolerant bacteria, enhancing their removal capacity, and evaluating the performance of a synthetic microbial consortium. This research focuses on the isolation, screening, and characterisation of chromium-tolerant bacteria from industrially polluted sites. By identifying promising strains that thrive in chromium-rich environments and characterising their morphological, biochemical, and molecular features, we aim to lay the groundwork for the future development of bacterial consortia with an enhanced potential for bioremediation of chromium-contaminated ecosystems.
Sample collection
Environmental samples were collected from industrially impacted sites known for heavy metal contamination. These included sewage treatment plants, electroplating industries, dye industry discharges, sludge, and pond water from regions such as Panipat, Sonepat (STP 1 with a 30 MLD), Guru gram, Faridabad (STP 1 with a 30 MLD capacity), Yamunanagar (Haryana) (STP 1 with 7 MLD capacity), Chandigarh, and Baddi (Himachal Pradesh). Sampling bottles were acid-washed, rinsed with deionized water, and pre-rinsed with source water before collection. Samples were stored at 4 °C and processed within 24 hours.
Preparation of chromium stock solution
A stock solution of Cr(VI) was prepared by dissolving 2.84 g of potassium dichromate (K2Cr2O7) in 1 litre of double-distilled water, yielding a concentration of 1000 mg/L. Working solutions were prepared via serial dilution with sterile distilled water as required.
Enrichment and isolation of chromium-tolerant bacteria
To isolate chromium-tolerant bacteria from environmental sources, enrichment culture techniques were employed to selectively promote the growth of resistant strains. For this, 5 mL of each collected wastewater sample was introduced into 100 mL of sterile nutrient broth supplemented with 100 mg/L of Cr(VI) in the form of potassium dichromate. The flasks were incubated at 37 ± 2 °C with constant agitation at 120 rpm for 48 hours to facilitate optimal aeration and bacterial proliferation under metal stress. After incubation, the enriched cultures were serially diluted from 100 to 106 to reduce microbial density and isolate individual colonies. A 1 mL aliquot from each dilution was plated onto nutrient agar medium also containing 100 mg/L Cr(VI) to maintain selection pressure. These plates were then incubated under the same temperature conditions for 24-48 hours. Distinct colonies that appeared on the plates were picked based on differences in morphology and further purified through repeated streaking on fresh agar plates. Pure isolates were preserved on nutrient agar slants and stored at 4 °C for subsequent analysis.
Primary screening and Minimum Inhibitory Concentration (MIC) determination
For MIC determination, nutrient agar (NA) was prepared by dissolving 28 g/L of commercially available NA powder (HI Media Laboratories Pvt. Ltd., India) in distilled water and sterilized by autoclaving at 121 °C for 15 minutes. The plates were supplemented with graded concentrations of potassium dichromate ranging from 100 to 1000 mg/L. Each bacterial isolate was streaked on the surface of the plates and incubated at
37 ± 2 °C for 48 hours under static conditions. Control plates without chromium were also included to assess normal growth behaviour. The MIC was determined visually based on the lowest Cr(VI) concentration that inhibited visible bacterial growth as shown in Table 1.
Table (1):
Universal primers used for the amplification of bacteria’s rDNA regions
| Organism | Universal Primer | Sequence | Bases |
|---|---|---|---|
| Bacteria | 27F | (5′-AGAGTTTGATCCTGGCTCAG-3′) | 20 |
| (5′-TACGGYTACCTTGTTACGACTT-3′) | 20 |
Secondary screening for chromium removal efficiency
To evaluate the chromium-removal capability of the most tolerant bacterial isolates, a secondary screening was performed under controlled conditions. The selected strains were inoculated into the nutrient broth supplemented with 100 mg/L of Cr(VI) and cultured in 250 mL Erlenmeyer flasks. Each flask received 1 mL of bacterial inoculum, standardized to 2.1 × 107 CFU/mL using a hemocytometer to maintain uniform cell density across experiments. Cultures were incubated at 37 ± 2 °C with continuous shaking at 120 rpm for 48 hours to ensure proper aeration and nutrient availability. After incubation, the bacterial cells were separated by centrifugation at 6000 rpm for 10 minutes. The collected supernatant was then analyzed for remaining hexavalent chromium concentration using Atomic Absorption Spectroscopy (AAS), which provided precise quantification of chromium reduction by each isolate.
Biochemical characterization
Biochemical characterization of the isolates was carried out using standard procedures to support their phenotypic identification. Tests included catalase and oxidase to assess respiratory enzyme activity, urease for nitrogen metabolism, and nitrate reduction to detect anaerobic respiration capability. Indole production was tested to evaluate tryptophan degradation, while the methyl red and Voges-Proskauer (MR-VP) tests determined the type of glucose fermentation pathway. Colony morphology, Gram staining, and cell shape were also recorded. Results for both isolates, MBCR26 and MBCR44, were documented in tabular form for comparative analysis.
Molecular characterization and phylogenetic analysis
Genomic DNA from the selected chromium-tolerant bacterial isolates was extracted using the ZR Fungal/Bacterial DNA MiniPrep Kit, following the manufacturer’s instructions. The quality and quantity of the extracted DNA were checked using agarose gel electrophoresis. For molecular identification, the 16S rRNA gene region was amplified through polymerase chain reaction (PCR) using universal bacterial primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) as the forward primer and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) as the reverse primer as shown in Table 2.
Table (2):
Growth pattern of bacterial isolates on nutrient broth supplemented with 100, 200, 300, 400, and 500 mg/L of Cr following a 24 h incubation period
| No. | Bacterial isolate | Chromium dose (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| Control | 100 | 200 | 300 | 400 | 500 | ||
| 26 | MBCR26 | ++++ | ++++ | ++++ | ++++ | ++++ | +++ |
| 44 | MBCR44 | ++++ | ++++ | ++++ | ++++ | ++++ | +++ |
The PCR products (amplicons) were visualized on a gel to confirm the presence of the expected fragment size and were then purified for sequencing.
The sequencing was carried out in a direction to ensure accurate base calling. The resulting sequences were subjected to a similarity search using the BLAST tool available on the NCBI website to identify the closest known relatives. For multiple sequence alignment, ClustalW software was employed to align the 16S rRNA sequences with reference sequences. To determine the evolutionary relationships and taxonomic positioning of the isolates, phylogenetic trees were generated using the neighbour-joining method in MEGA version 11. Bootstrap analysis with 1000 replicates was performed to assess the reliability of the tree branches. The finalized sequences were submitted to NCBI GenBank, and unique accession numbers were obtained for each isolate.
Standardization of bacterial inoculum
For uniform experimental conditions, cultures were grown on NA slants for 48 hours. Cells were suspended in 10 mL sterile distilled water, homogenized, and cell density adjusted to 2.1 × 107 CFU/mL using a hemocytometer. 1 mL of standardized inoculum was used in all test flasks.
Isolation and preliminary screening of Chromium-Resistant Bacteria
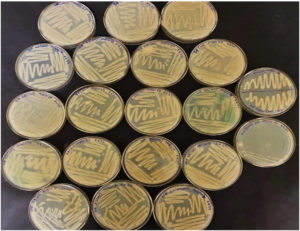
62 morphologically distinct bacterial isolates were obtained from heavy metal-contaminated water samples collected from tannery and electroplating industrial sites. The selected isolates were initially tested for chromium resistance by cultivating them on the nutrient agar enriched with 100 mg/L of potassium dichromate (K₂Cr₂O₇). Of these, 24 isolates demonstrated visible growth, suggesting chromium tolerance, as shown in Figure 1, represents the growth pattern for the two selected isolates, i.e. MBCR26 and MBCR44 on the NA plates enriched with Cr. In comparison, two isolates, MBCR26 and MBCR44, exhibited robust growth and were selected for further studies based on colony morphology and tolerance level.
Secondary evaluation of bacterial isolates in nutrient broth supplemented with chromium
To further evaluate the chromium-removal potential of the selected bacterial isolates, they were subjected to a broader concentration gradient of potassium dichromate ranging from 100 ppm to 1000 ppm in nutrient agar. The cultures were incubated under standard conditions, and growth responses were monitored visually. It was observed that the bacterial isolates were able to withstand chromium concentrations up to 400 ppm, with pronounced growth recorded at both 300 ppm and 400 ppm. However, a noticeable decline in cellular proliferation occurred as concentrations increased beyond this range. Only marginal growth was detected at 500 ppm and 700 ppm, suggesting a stress-induced reduction in metabolic activity, as shown in Figure 2 and tabulated in Table 3.
Table (3):
MIC Values of Chromium-Tolerant Bacterial Isolates
Isolate Code |
Maximum Cr(VI) Tolerance (mg/L) |
Growth Intensity at 400 ppm |
MIC (mg/L) |
|---|---|---|---|
MBCR26 |
500 |
++++ |
700 |
MBCR44 |
500 |
+++ |
700 |
Figure 2. Bacterial isolates showing growth at different Chromium concentrations as: (A) Control without any chromium; (B) Growth of bacteria at 100 ppm of Chromium; (C) Growth of bacteria at 200 ppm of Chromium; (D) Growth of bacteria at 300 ppm of chromium; (E) Growth of bacteria at 400 ppm of chromium; (F) Growth of bacteria at 500 ppm; (G) Growth of bacteria at 700 ppm; (H) Growth of bacteria at 1000 ppm
No observable growth occurred at 1000 ppm, indicating that this concentration exceeded the physiological tolerance limits of the tested strains. These results helped define the upper inhibitory threshold of chromium for the isolates and guided the concentration selected for subsequent biosorption and optimization experiments.
For the most promising bacterial isolates, secondary screening was conducted using a batch culture approach. The isolates that demonstrated robust growth at elevated chromium concentrations (300 and 400 mg/L) during preliminary trials were subjected to this assessment, as shown in Figure 3. Each isolate was independently cultivated in nutrient broth containing 100 m/L of potassium dichromate (K₂Cr₂O₇), as shown in Figure 4. Following a 24-hour incubation period at optimal growth conditions, cultures were centrifuged to separate the bacterial biomass. The supernatant was then analysed using Atomic Absorption Spectroscopy (AAS) to quantify the residual concentration of chromium in the medium. Among the tested strains, isolates MRCB26 and MRCB44 (as shown in Figures 3, 4 and Table 4) exhibited the highest metal-removal efficiencies, achieving 68.72 % and 72.36% chromium reduction, respectively, indicating their substantial biosorptive capabilities.
Table (4):
Removal efficiency of Cr from the liquid medium by efficient bacterial isolates on nutrient broth containing 100 mg/L of Cr
No. |
Bacterial Isolates |
% removal of Cr |
|---|---|---|
26 |
MBCR26 |
68.72 ± 0.98 |
44 |
MBCR44 |
72.36 ± 0.43 |
Figure 3. Bacterial Isolates (A) MBCR26 and (B) MBCR44 grow on an NA plate. These figures represent the growth of bacteria on Nutrient agar in the presence of Chromium
Figure 4. Cultures of selected bacterial isolates on Liquid Nutrient Broth Medium as: (A) First flask on the left represents the Control (without any turbidity) and flask on the right represents MBCR26 growth in Liquid Nutrient Broth with Chromium. (B) First flask on the left represents Control without any turbidity and flask on the right represents MBCR44 growth in the presence of Chromium
Characterization and identification of desired isolates
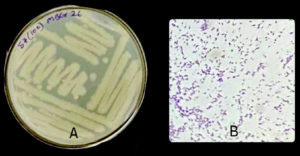
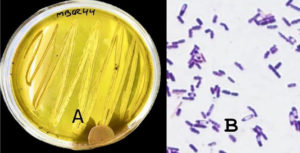
The two potential isolates were characterized morphologically based on colony morphology and cell morphology. The two bacterial isolates, i.e., MBCR26 and MBCR44, were cream in color. All the isolates were rod-shaped and gram-positive. The microscopic and morphological characteristics of bacterial isolates MBCR26 and MBCR44 are shown in Figures 5 and 6.
Figure 5. Microscopic and cultural characteristics of isolate MBCR26; (A) Colony features on Nutrient Agar; (B) Gram-positive, Rod-shaped
Figure 6. Microscopic and cultural characteristics of isolate MBCR44; (A) Colony features on Nutrient Agar; (B) Gram-positive, Rod-shaped
Biochemical characterization
The biochemical and morphological characteristics of the bacterial isolates MBCR26 and MBCR44 provide essential information for their preliminary identification and differentiation. Both isolates produced cream-colored colonies on nutrient agar, suggesting similar pigmentation and colony texture. Upon Gram staining, they were found to be Gram-positive, which confirms the presence of a thick peptidoglycan layer in their cell wall structure. Microscopically, both strains were observed to be rod-shaped, a common feature in many soil and environmental bacteria, including species of Bacillus and Lysinibacillus (Table 5).
Table (5):
Biochemical characterization of chromium-degrading bacterial isolates
Characteristics |
MBCR26 |
MBCR44 |
|---|---|---|
Colony morphology |
Cream |
Cream |
Gram staining |
+ |
+ |
Morphology |
Rods |
Rods |
Catalase |
+ |
+ |
Urease |
– |
– |
Oxidase |
– |
+ |
Indole |
– |
– |
Nitrate reduction |
+ |
– |
MR |
+ |
– |
VP |
– |
+ |
In terms of enzymatic activity, both isolates tested positive for the catalase enzyme, indicating their ability to decompose hydrogen peroxide into water and oxygen, a common trait in aerobic or facultative anaerobic bacteria. However, both strains were urease-negative, showing they do not hydrolyze urea, which helps distinguish them from certain urease-producing species. The oxidase test results showed variation: MBCR26 was oxidase-negative, whereas MBCR44 was oxidase-positive, pointing toward a difference in their respiratory enzyme systems.
Furthermore, both isolates were negative for indole production, suggesting the absence of tryptophanase activity. In the nitrate reduction test, MBCR26 was positive, demonstrating its ability to reduce nitrate to nitrite or other nitrogenous compounds, while MBCR44 was negative, indicating it lacks this capability. The methyl red (MR) and Voges–Proskauer (VP) tests, which are used to differentiate fermentation pathways, also showed contrasting results. MBCR26 was MR-positive and VP-negative, indicating it utilizes mixed acid fermentation, whereas MBCR44 was MR-negative and VP-positive, suggesting a butanediol fermentation pathway, as shown in Table 5.
These differences in biochemical behaviour, along with the observed morphology, support the identification of MBCR26 as Bacillus subtilis and MBCR44 as Lysinibacillus macroides, which was further confirmed through molecular techniques.
Molecular characterization of the two chromium-degrading bacterial isolates
The 16S rRNA gene regions of two chromium-remediating bacterial isolates, MBCR26 and MBCR44, were amplified using universal primer sets to enable molecular identification. Following successful PCR amplification, the resulting amplicons were subjected to sequencing. To establish the taxonomic identity of the isolates, a BLAST analysis was conducted using the obtained sequences. The results confirmed the isolates as Bacillus subtilis (MBCR26) and Lysinibacillus macroides (MBCR44), respectively. The gene sequences of the chromium-degrading bacterial isolates were submitted to NCBI under the following accession numbers given in Table 6.
Table (6):
Molecular characterization of two chromium-degrading efficient bacterial isolates with accession numbers
No. |
Isolate name |
Name of bacteria |
Accession number |
|---|---|---|---|
1 |
MBCR26 |
Bacillus subtilis |
PP837960 |
2 |
MBCR44 |
Lysinibacillus macroides |
PP837951 |
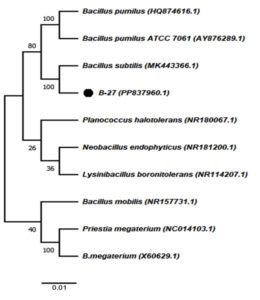
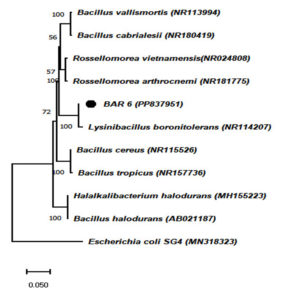
Partial 16S rRNA gene sequences obtained from the chromium-tolerant isolates MBCR26 and MBCR44 were subjected to sequence alignment and comparative analysis against entries in the NCBI nucleotide database. The BLAST analysis revealed the highest sequence similarity of these isolates with Bacillus subtilis and Lysinibacillus macroides, respectively. The phylogenetic affiliations and evolutionary relationships of these isolates with closely related taxa are illustrated in Figures 7 and 8, which were constructed using the neighbour-joining method.
Figure 7. The phylogenetic tree illustrating the evolutionary relationships among the identified bacterial isolates (Note: MBCR26 refers to B27)
This study focused on isolating and characterizing indigenous chromium-resistant bacterial strains from wastewater-contaminated sites to assess their biosorptive potential for bioremediation applications. Among the 64 bacterial isolates obtained from sewage, sludge, and industrial effluent, two strains-designated MBCR26 and MBCR44-exhibited strong tolerance to hexavalent chromium, maintaining active growth up to 400 ppm. These isolates also showed significant chromium removal efficiency in nutrient broth containing 100 ppm potassium dichromate, achieving 54.6% and 50.8% removal, respectively. These results suggest that native microbial populations exposed to prolonged environmental stress develop adaptive mechanisms that enable survival and detoxification of toxic heavy metals like Cr(VI), consistent with findings from earlier studies on metal-tolerant Bacillus and Lysinibacillus spp.8,14 The MIC values observed in this study (700 ppm for both MBCR26 and MBCR44) are within a higher range compared to several previously reported chromium-resistant bacteria. For instance, Huang et al.10 reported that Sporosarcina saromensis tolerated Cr(VI) concentrations up to 500 mg/L, while Enterobacter cloacae described by Paul et al.9 showed growth at concentrations up to 320 ppm. Similarly, Bhattacharjee et al.13 isolated a microbial consortium from tannery effluents that tolerated a maximum of 600 ppm Cr(VI) under laboratory conditions. In contrast, the isolates characterized in the present work not only withstood up to 700 ppm but also showed active growth at 400-500 ppm, indicating strong chromium tolerance.
Biochemical and molecular characterization confirmed the identity of MBCR26 as Bacillus subtilis and MBCR44 as Lysinibacillus macroides, both of which are Gram-positive, endospore-forming bacilli previously documented for their resistance to toxic metals and oxidative stress.15,16 The notable biosorption ability of B. subtilis has been attributed to surface functional groups such as carboxyl, phosphate, and amino groups that actively bind metal ions, while L. macroides are known to produce extracellular polymeric substances (EPS) that facilitate chromium sequestration.17 The relatively high chromium removal observed under batch conditions supports the likelihood of combined biosorption and enzymatic reduction mechanisms at play. These findings reinforce the suitability of these strains for integration into biological treatment systems targeting Cr(VI)-contaminated effluents.
The observed tolerance limits and removal capacities of the identified isolates provide valuable insight for future scale-up and application in continuous treatment systems. Moreover, their natural origin from contaminated sites may give them a physiological edge in maintaining stability and functionality under environmental stress. Taken together, this study not only contributes to the growing database of chromium-remediating bacteria but also offers a practical basis for designing microbial consortia tailored to site-specific bioremediation.
The isolation and identification of Bacillus subtilis (MBCR26) and Lysinibacillus macroides (MBCR44) from chromium-contaminated environments highlight the potential of indigenous bacterial strains in bioremediation strategies. Both isolates demonstrated considerable chromium tolerance and removal efficiency under laboratory conditions, supporting their applicability in eco-friendly treatment of industrial effluents. The combination of biochemical adaptability and genetic robustness suggests their utility as standalone agents or as part of synergistic microbial consortia.
Future work will focus on formulating a stable microbial consortium using MBCR26 and MBCR44 in combination with other compatible chromium-resistant strains. Compatibility tests will be carried out through co-culture assays to evaluate mutual growth support and chromium reduction efficiency. Once a suitable consortium is developed, it will be tested under controlled conditions using synthetic wastewater to optimize parameters such as pH, contact time, and inoculum size. Based on these optimizations, a pilot-scale field trial will be conducted using actual industrial effluent from electroplating or tanning units. The hypothesis is that a well-adapted microbial consortium derived from native strains will demonstrate enhanced chromium removal efficiency and resilience under fluctuating environmental conditions.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Kota J, Stasicka Z. Chromium occurrence in the environment and methods of its speciation. Environ Pollut. 2000;107(3):263-283.
Crossref - Sharma S, Sharma M, Kumar R, et al. Recent advances and mechanisms of microbial bioremediation of nickel from wastewater. Environ Sci Pollut Res. 2024;31(28):40224-40244.
Crossref - Zhao L, Islam R, Wang Y, Zhang X, Liu LZ. Epigenetic regulation in chromium-, nickel- and cadmium-induced carcinogenesis. Cancers. 2022;14(23):5768.
Crossref - Patil A, Chakraborty S, Yadav Y, Sharma B, Singh S, Arya M. Bioremediation strategies and mechanisms of bacteria for resistance against heavy metals: a review. Environ Pollut Bioremediat J. 2024:1-33.
Crossref - Khatoon Z, Orozco-Mosqueda Ma del C, Santoyo G. Microbial contributions to heavy metal phytoremediation in agricultural soils: a review. Microorganisms. 2023;12(10):1945.
Crossref - Barakat MA. New trends in removing heavy metals from industrial wastewater. Arab J Chem. 2011;4(4):361-377.
Crossref - Joutey NT, Sayel H, Bahafid W, El Ghachtouli N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. In: Whitacre D, ed. Reviews of Environmental Contamination and Toxicology. Vol 233. Springer, Cham; 2014:45-69.
Crossref - Cheung KH, Gu JD. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeterior Biodegrad. 2007;59(1):8-15.
Crossref - Paul D, Pandey G, Pandey J, Jain RK. Accessing microbial diversity for bioremediation and environmental restoration. Trends Biotechnol. 2005;23(3):135-142.
Crossref - Huang Y, Zeng Q, Hu L, Zhong H, He Z. Bioreduction performances and mechanisms of Cr(VI) by Sporosarcina saromensis W5, a novel Cr(VI)-reducing facultative anaerobic bacterium. J Hazard Mater. 2021;413:125411.
Crossref - Fernandez PM, Vinarta SC, Bernal AR, Cruz EL, Figueroa LIC. Bioremediation strategies for chromium removal: current research, scale-up approach, and future perspectives. Chemosphere. 2018;208:139-148.
Crossref - Megharaj M, Avudainayagam S, Naidu R. Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol. 2003;47(1):51-54.
Crossref - Bhattacharjee A, Chaudhuri R, Pandey P, Mitra AK. Bioremediation of chromium (VI) by a microbial consortium isolated from tannery effluents and their potential industrial application. J Environ Eng Landsc Manag. 2021;29(4):418-429.
Crossref - Tamindzija D. Isolation and characterization of Cr(VI) tolerant soil bacteria [dissertation]. University of Novi Sad (Serbia); 2019
- Ahsan N, Shimizu M. Lysinibacillus species: their potential as effective bioremediation, biostimulant, and biocontrol agents. Rev Agric Sci. 2021;9:103-116.
Crossref - Seragadam P, Rai A, Ghanta KC, Srinivas B, Lahiri SK, Dutta S. Bioremediation of hexavalent chromium from wastewater using green bacteria technology. Biodegradation. 2021;32(4):449-466.
Crossref - Ayangbenro AS, Babalola OO. A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health. 2017;14(1):94.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.