ISSN: 0973-7510
E-ISSN: 2581-690X
Conventional agricultural practices rely on the usage of synthetic herbicides to control weed proliferation in the absence of mulching, potentially leading to soil dysbiosis. Conversely, the technique of straw mulching entails the application of a substantial layer of material over the soil. The effects of different mulch types on the bacterial and fungal community distributions vary. Additionally, there is a lack of information on how agricultural straw mulching impacts the makeup of soil microbial populations. Hence, it is crucial to identify the types of mulches that enhance soil quality by influencing different microbial populations. The study investigated the effects of straw mulching, various mulching methods, and absence of mulching on the soil microbial populations within three distinct agricultural systems. Fifteen soil samples were procured from gardens situated in Iowa, USA. For every individual specimen, soil specimens were procured from six distinct locations and amalgamated. DNA was extracted and analyzed the taxonomic assessment of both bacterial and fungal. Straw mulching showed considerably greater levels of Actinobacteria (p < 0.000001), Proteobacteria (p < 0.0001), Ascomycota (p < 0.000001), and Basidiomycota (p < 0.01) when compared with other mulching. The most elevated bacterial and fungal diversity were discovered in straw mulching, as evidenced by the elevated values of the Chao1 and Shannon indices (p < 0.001). Linear discriminant analysis showed significantly higher levels of beneficial microbes including nitrogen-fixing bacteria (p < 0.008) in straw mulching. To summarize, our results indicated that agricultural straw mulching induced alterations in bacterial composition and enhancements in fungal presence compared to the microbiota of other mulching methods and no mulching.
Soil Microbiota, Straw Mulching, Nitrogen-fixing Bacteria, Herbicides, Agriculture and Farming
The escalation in global food demand is primarily fueled by population growth, leading to a consequent expansion in the agricultural sector. The existence of weeds presents a challenge to crop cultivation by competing for crucial energy resources, leading to reduced crop yields and increased labor costs.1,2 Although herbicides play a crucial role in weed management, their use poses potential risks to both human health and ecosystems.3,4 Mulching, a practice that entails the application of straw, biodegradable paper, or plastic sheets on the soil surface, presents a viable approach for inhibiting the germination and proliferation of weeds. In spite of the abundance of research conducted on soil microbiota,1 only a limited number of researchers have explored the impact of mulching on the microbiota. The presence of bacteria and fungi within the soil is crucial for the preservation of soil quality, as they are responsible for the breakdown of organic materials and the production of essential minerals and nutrients necessary for the growth of plants.5 Moreover, the presence of beneficial soil microorganisms plays a pivotal role in enhancing plant resilience to environmental challenges, including abiotic factors like climate variability.6
Fluctuations in soil parameters, whether induced by seasonal fluctuations or the utilization of synthetic fertilizers and pesticides, possess the capacity to induce dysbiosis among the soil microbiome, consequently influencing the development of vegetation.6 Conversely, adopting healthy agricultural practices can promote a diverse microbial community, which supplies nutrients for plant growth and protects plants from pests and pathogens.7 Furthermore, fungi are involved in the sequestration of carbon in soil, which means that even slight variations in the fungus content of soil can have an impact on climate change. Optimizing agricultural techniques requires an understanding of how various practices affect the microbiome. In light of the growing threat posed by climate change, this adaptation is crucial for promoting sustainable ecosystems in addition to being advantageous for crop productivity.8 Hence, this study focused on determining the impact of different mulching methods on soil microbiota composition to increase our understanding on the effects of mulching and pave the way for adaptations to benefit agriculture and the environment.
Site description and sampling
Fifteen soil samples were procured from gardens situated in Iowa, USA, for the research investigation. Two consecutive days devoid of precipitation in September 2018 were designated for the acquisition to effectively manage the climatic conditions. To accommodate fluctuations in meteorological parameters, specimens were presently obtained from a singular season; however, two sampling sessions encompassing two seasons are planned for subsequent endeavors. Within the sampling process, Herbicides and pesticides were administered to the pastures, while plastic and paper mulches were employed for alternative mulching samples, and straw mulches were specifically utilized for the straw mulching sample. Table 1 contained a list of sampling specifics. According to USDA soil sampling guidelines, a stainless-steel soil sampling probe was used to collects soil with 6 inches deep. For every individual specimen, soil specimens were procured from six distinct locations (spaced 50 cm apart) and amalgamated. Soil specimens designated for straw mulching were acquired from five parcels (n = 5), comprising a mixture with a 50:50 ratio of desiccated grass and alfalfa straw. Specimens were amassed from two agricultural plots utilizing plastic mulches (n = 1) and two plots employing paper mulches (n = 2). Additionally, soil specimens were amassed from two agricultural plots lacking mulching, five specimens gathered from grazing areas (n = 5), and two specimens from regions subjected to herbicides and pesticides (n = 2).
Table (1):
Sampling Details
| Types of Sample | Sub-Sample Details | No. of Sample |
|---|---|---|
| Straw Mulching | Straw mulches | 5 |
| Other Mulching | Plastic mulches | 1 |
| Paper mulches | 2 | |
| No Mulching | Pastures | 5 |
| Herbicides & Pesticides | 2 |
DNA extraction
For DNA extraction, duplicates from each sample were used and carried out using the ZymoBIOMICS®-96 MagBead kit (Zymo Research, Irvine, CA) following the manufacturer’s instructions and subsequently analyzed through the ZymoBIOMICS® Service. Bacterial 16S primers were employed to amplify the V3-V4 regions of the 16S rRNA gene, as described previously.9 For fungal sequencing, primers targeting the Internal Transcribed Spacer 2 (ITS2) region between the small and large subunits of ribosomal RNA (rRNA) were used. For sequencing, Illumina® MiSeq™ instrument was utilized.
Bioinformatics analysis
Taxonomic assessment was performed using Qiime v.1.9.1 with the Zymo Research database, where the 16S database was designed and used. Composition visualization, alpha-diversity and beta-diversity analyses were conducted using Qiime v.1.9.1.10,11 ANOVA analysis was performed using GraphPad Prism. Identification of taxa exhibiting significant abundance differences between groups were performed by LEfSe using default settings.12,13
Effects of mulching on the soil microbiota
Sequencing Data produced 1,417,444 raw paired reads from the soil samples. Operational Taxonomic Unit of sequences was listed in Table 2. Similarly, on organic mulching, 576,090 exceptional reads of bacteria and 886,572 superior reads of fungi with average lengths of 418 bp and 234 bp, respectively, were still present in the dataset.14
Table (2):
Operational Taxonomic Unit (OTU)
Classification |
16S |
16S |
ITS2 |
ITS2 |
ITS2 |
ITS2 |
|---|---|---|---|---|---|---|
Kingdom |
Archaea |
Bacteria |
Protista |
Plantae |
Fungi |
Protozoa |
Phylum |
2 |
26 |
2 |
3 |
7 |
1 |
Genus |
3 |
563 |
2 |
3 |
390 |
1 |
Species |
9 |
3642 |
2 |
4 |
731 |
1 |
Effects of straw mulching and other mulching on soil microbiota
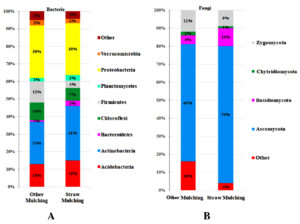
Straw mulching demonstrated a notable impact on soil microbial communities, revealing its effect in bacterial and fungal phyla composition. The study indicated a significant increase
(p < 0.000002) in Actinobacteria and Proteobacteria, and a significant decrease (p < 0.000001) in Acidobacteria and Firmicutes (Figure 1A), suggesting an enhanced variance in the microbial balance. Actinobacteria and Proteobacteria are known as the essential contributors as symbiotic and free-living nitrogen fixers crucial for crop nutrient enrichment. Taxonomic distributions of bacterial and fungal (Figure 1) phyla in soil using samples of straw and other mulching is displayed as a percentage on the bar. Upon comparison to other mulching, straw mulching showed a significant increase in Actinobacteria (p < 0.000002) in the bacterial phyla. Upon comparison to other mulching, the straw mulching showed a significant increase in Ascomycota (p < 0.000003) in the fungal phyla. Phylum-level bacterial and fungal sequence diversity was different in other mulching soil compared to straw mulching soil (Figure 1A and B). Actinobacteria were present at significantly higher levels in soil mulched with straw compared to soil mulched using plastic and paper sheets. This is likely because straw mulching itself is an organic material that can be easily decomposed by microbes.
These bacteria play a pivotal role in enhancing plant growth by elevating nutrient availability, supplying essential elements (iron, zinc, selenium, phosphorus), and inducing resistance against phytopathogens.15,16 Proteobacteria is known for decomposing organic matter, works synergistically with Actinobacteria in this process.13 Actinobacteria is recognized for their resilience to abiotic stress factors, that contribute to crop resilience under conditions such as extreme temperatures, drought, flooding, salinity, metal stress, and nutrient stress, potentially mitigating adverse impacts on yields.17 Fungi are primary decomposers because they can decompose complex substances such as lignin into organic matter. Both Ascomycota and Basidiomycota fungi increased significantly, suggesting that straw mulching increased organic matter decomposition activity in the soil (Figure 1B). These beneficial bacteria and fungi produce organic compounds called biofilms and glomalin, respectively. To which the mineral soil particles adhere, the organic litter, and roots cause further aggregation.18 In addition, it prevents nutrient loss and also stabilizes soil structure, as well as creating pores and water-stable aggregates that enhance water retention and drainage.19,20
Figure 1. Taxonomic distribution of bacterial and fungal phyla in soil using straw and other mulching methods. (A) Distribution of dominant bacterial phyla, showing significantly higher levels of Actinobacteria and Proteobacteria in straw-mulched soil compared to plastic and paper mulches. (B) Distribution of fungal phyla, highlighting increased abundance of Ascomycota and Basidiomycota in straw-mulched soil versus other mulching methods
Effects of straw mulching and no mulching on soil microbiota
Phylum-level bacterial and fungal sequence diversity differed between no mulched soil and straw mulched soil (Figure 2). Taxonomic distributions of bacterial (Figure 2A) and fungal (Figure 2B) phyla in soil using samples of straw and no mulching is displayed as a percentage on the bar. Upon comparison to no mulching, straw mulching showed a significant increase in Actinobacteria (p < 0.000001) in the bacterial phyla. Upon comparison to no mulching, the straw mulching showed a significant increase in Ascomycota (p < 0.000001) in the fungal phyla. It has been reported that the use of herbicides, pesticides, and grazing reduces the decomposition activity of soil microbiota in no mulched soil, consequently leading to an increased dependence on expensive fertilizers.21 Soil health-promoting actinomycetes increased in straw mulched soil compared to no mulched soil treated with herbicides and pesticides. Fungal activity decreased in no mulched soil, as reported in other studies.17 Therefore, straw mulching emerges as a superior alternative to no mulched soil treated with herbicides.
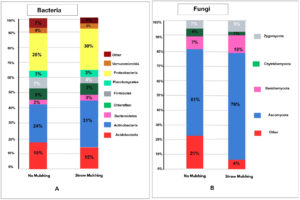 Figure 2. Taxonomic distribution of bacterial and fungal phyla in soil using straw and no mulching methods. (A) Bacterial phyla composition, indicating elevated Actinobacteria in straw-mulched soil compared to no mulching. (B) Fungal phyla composition, showing increased Ascomycota in straw-mulched soil over no mulching, supporting enhanced microbial activity
Figure 2. Taxonomic distribution of bacterial and fungal phyla in soil using straw and no mulching methods. (A) Bacterial phyla composition, indicating elevated Actinobacteria in straw-mulched soil compared to no mulching. (B) Fungal phyla composition, showing increased Ascomycota in straw-mulched soil over no mulching, supporting enhanced microbial activity
Biodiversity analysis
Alpha diversity
Alpha diversity of bacteria and fungi was illustrated in Tables 3 and 4 and the values are the average of samples (n). The Shannon diversity index estimates species richness and evenness with more weight on species richness. Simpson’s index estimates species richness and evenness with more weight on species evenness. Chao1 estimate species richness. A higher Shannon value means greater diversity. Lower Simpson value means high evenness and greater diversity. Higher Chao1 value means more rare species and more diversity count of different species (OTUs). The Tables 3 and 4 values are calculated by the mean, or average, which is calculated by adding all the sample scores within the data set and then dividing by the number of samples within the set. It reflects the richness and evenness of individual species (INS) within a habitat unit, and is a good indicator of soil and plant health.5,16 In this study, straw mulching shows maximum alpha diversity in soil microbiota which increases plant stability, productivity, and resistance to invasion and abiotic stress.22 In all the diversity analyses, like Phylogenetic Diversity (PD) shows higher values in straw mulching reflecting species from different branches and greater diversity.
Table (3):
Alpha diversity of bacteria
Name and No. of sample |
PD whole tree |
Chao1 (unique) |
Observed Species |
Shannon (richness) |
Simpson (evenness) |
|---|---|---|---|---|---|
Straw Mulching (n = 5) |
43 |
629 |
612 |
8.8 |
0.6 |
Other Mulching (n = 3) |
37 |
479 |
473 |
8.4 |
0.5 |
No Mulching (n = 7) |
33 |
354 |
352 |
8.1 |
0.9 |
Table (4):
Alpha diversity of fungi
Name and No. of sample |
Observed Species |
Shannon (richness) |
Simpson (evenness) |
|---|---|---|---|
Straw Mulching (n = 5) |
270 |
6.3 |
0.1 |
Other Mulching (n = 5) |
206 |
6 |
0.2 |
No Mulching (n = 5) |
230 |
6 |
0.1 |
Beta and gamma diversity
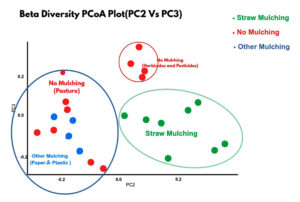
It expresses microbial diversity between habitats. Principal Coordinate Analysis (PCoA) is used for Beta diversity analysis to provide a measure of the distance or dissimilarity between each sample pair. More distance or dissimilarity indicates more diversity. In this analysis, the straw mulch samples tended to cluster together with high similarity (green dots group), whereas the other mulching and no mulching were more decentralized (mixed blue-red dots group). The no mulching farms are grouped separately as red dots group (Figure 3). It expresses diversity of INS from different habitats. Straw mulch samples from different locations behave similarly but are distinct from other mulched and no mulched soil samples (Beta Diversity).
Statistical analysis
Linear discriminant analysis (LDA) and effect size (LEfSe) statistical tool was used to find high-dimensional biomarker (features) that discriminate straw mulching soil from no mulching soil groups and other mulching soil groups (Figures 4 and 5). LDA scores and cladograms show taxa with higher than 3 to identify bacterial groups with statistically significant differences.21,23
 Figure 4. Linear Discriminant Analysis (LDA) and LEfSe analysis of bacteria in straw and other mulching soils. (A) Bacterial taxa enriched in straw mulching soil, with LDA scores >3 indicating significantly higher presence of beneficial taxa. (B) Bacterial taxa enriched in other mulching soils (plastic/paper), contrasting with the microbial profile in straw-mulched soils
Figure 4. Linear Discriminant Analysis (LDA) and LEfSe analysis of bacteria in straw and other mulching soils. (A) Bacterial taxa enriched in straw mulching soil, with LDA scores >3 indicating significantly higher presence of beneficial taxa. (B) Bacterial taxa enriched in other mulching soils (plastic/paper), contrasting with the microbial profile in straw-mulched soils
Figure 5. Linear Discriminant Analysis (LDA) and LEfSe analysis of bacteria in straw and no mulching soils. (A) Bacterial lineages significantly more abundant in straw mulching soil, suggesting higher nutrient cycling potential. (B) Bacterial lineages enriched in no mulching conditions, associated with lower microbial diversity and fertility
LDA and LEfSe analysis of soil bacteria
Linear discriminant analysis (LDA) and effect size (LEfSe) of bacteria in straw mulching (red) and other mulching soil (blue) soil were illustrated in Figure 4A. Figure 4B illustrates the Linear discriminant analysis (LDA) and effect size (LEfSe) of bacteria in straw mulching (blue) and other mulching soil (red). Linear discriminant analysis (LDA) effect size (LEfSe) of bacteria in straw mulching (red) and other mulching soil (blue) soil illustrated in Figure 5A. Figure 5B illustrates the Linear discriminant analysis (LDA) and effect size (LEfSe) of bacteria in straw mulching (red) and other mulching soil (blue). Functionally important soil microbes in straw mulching samples compared to no mulching and other mulching soil samples. Significant differences are indicated as LDA scores >2.5 and (p < 0.0004 to 0.05) (Table 5). Recent studies showed that use of pesticides affected soil bacteria and fungal populations.24 Verrucomicrobia had high LDA scores in no mulching soils and other mulching soils. It also had high statistical significant levels (p < 0.001) compared to no mulching soils. Verrucomicrobes are negatively related to soil factors linked to soil fertility, such as total nitrogen, phosphorus, potassium, calcium, magnesium and potassium contents (more Verrucomicrobe indicates less soil fertility).25 Gemmatimonadetes had high LDA scores and statistically significant levels (p < 0.001) in conventional soil and were inversely related to water content, which means low moisture content in these soil.26
Table (5):
Functionally important soil microbes in straw mulching samples
Functional roles |
Straw mulching soil |
LDA |
P-Value |
|---|---|---|---|
Nitrogen fixers |
Actinobacteria.c__Actinobacteria |
4.4 |
0.003 |
Abundant in increased carbon:nitrogen |
Proteobacteria |
4.3 |
0.01 |
Increases phosphate availability |
Proteobacteria.c__Alphaproteobacteria |
4.2 |
0.002 |
Tolerating cold stress |
Actinobacteria.c__Actinobacteria.o__Micrococcales |
4.1 |
0.0004 |
Nitrogen fixers |
Proteobacteria.c__Alphaproteobacteria.o__Rhizobiales |
4.0 |
0.01 |
Mineral solubilizers |
Actinobacteria.f__Micrococcaceae.g__Arthrobacter_Pseudarthrobacter |
3.8 |
0.0004 |
Nitrogen fixers and increases phosphorous |
Proteobacteria.c__Alphaproteobacteria.o__Rhizobiales. f__Bradyrhizobium |
3.2 |
0.03 |
Manganese transformers |
Actinobacteria.c__Actinobacteria.o__Corynebacteriales |
3.4 |
0.01 |
Sulphur solubilization |
Proteobacteria;c__o__Desulfurellales;f__Desulfurellaceae |
3.9 |
0.01 |
Increase potassium availability |
Ascomycota.c____Eurotiales.f__Trichocomaceae. g_Aspergillus_niger |
2.8 |
0.03 |
Phosphorous mobilization |
Zygomycota.c__Mortierellales.f__Mortierellaceae.g__NA |
4.2 |
0.03 |
Phosphorous mobilization |
Zygomycota.c___Mortierellales.f__Mortierellaceae.g__NA |
4.2 |
0.03 |
Key decomposers |
Ascomycota.c__Dothideomycetes.o__Capnodiales.f__Davidiellaceae |
3.9 |
0.02 |
Key decomposers |
Ascomycota.c__Sordariomycetes.o_Xylariales_fam_Incertae _sedis |
3.7 |
0.05 |
Key decomposers |
Ascomycota.c__Eurotiomycetes.o__NA.f__NA.g__NA.s__Eurotiomycetes_sp |
3.3 |
0.04 |
Phosphate solubilization |
Ascomycota.c__Dothideomycetes.Pleosporaceae. g__Alternaria.s__Alternaria_ |
2.9 |
0.04 |
Global carbon cycle |
Ascomycota.c_Eurotiales.f__Trichocomaceae. g__Aspergillus_piperis |
2.8 |
0.03 |
Phosphate solubilization |
Ascomycota.c__Eurotiales.f__Trichocomaceae.Penicillium_citrinum |
2.6 |
0.03 |
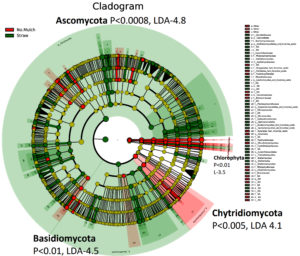
LEfSe analysis of soil fungi
Cladogram differences in the abundance of fungi taxa in straw mulching (green) no mulching and other mulching (red) soil samples, based on LEfSe. Ectomycorrhizal fungi (Basidiomycota and Ascomycota), which are associated with carbon sequestration, were increased in straw mulching soil (Figures 5 and 6). Thus, prevents global warming. Chytridiomycota (commonly found in ice-lands) are significantly increased in no mulching and other mulching soils because they can help to withstand low temperatures, but are also obligate or facultative pathogens.27 Ascomycota, Basidiomycota are high in straw mulching because straw mulching protects soil and its diverse beneficial microbiota from cold winter without the need to increase pathogenic Chytridiomycota for weather protection. Ectomycorrhizal fungi (EM) produce enzymes that allow them to absorb more nitrogen from organic matter, increasing 70 percent more carbon per unit than nitrogen in soil.28 This prevents several microbes from growing and decomposing dead organic matter and releasing carbon to the atmosphere. Hence, EM makes carbon to be locked up in plants, soil, fungal bodies, and less in the atmosphere. Therefore, EM helps plants to grow in higher carbon dioxide levels even when nitrogen is low. These results show that straw mulching results in more significant microbial activity than no mulching and other mulching. Straw mulching was significantly associated with changes in specific bacterial lineages involved in nutrient cycling and consequent increased soil fertility and moisture content. Moreover, Fungi do not fix Nitrogen but provide nutrients like carbon and Phosphorus to nitrogen-fixing bacteria.18
Determination of nitrogen fixing bacteria
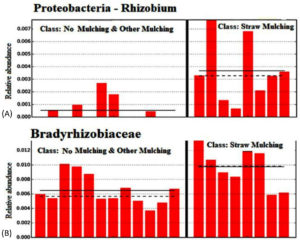
This study reveals a significant increase in nitrogen (N) fixing bacteria, such as Rhizobium and Bradyrhizobiaceae, in straw mulching soil, indicating a reduced reliance on artificial fertilizers (Figure 7). The findings suggest that straw-based mulching fosters a robust and diverse soil microbiota, including bacteria and fungi associated with organic matter decomposition and nutrient production. This research is particularly importance for assisting small-scale farmers and home gardeners in reducing fertilizer costs and obtaining the “organic” credentials necessary to market their product at local farmers’ markets. At the phylum level of bacteria, we observed a significant decrease in the relative abundance of Firmicutes from 3% to 0.87% when the straw mulch amount was increased in the soil of the maize field. This change may result in the production of urease and/or catalase, ultimately promoting the soil nitrogen cycle (nitrogen cycle).29
Figure 7. Relative abundance of important nitrogen-fixing bacteria in soils with no mulching, other mulching, and straw mulching. Bar graph comparison showing increased abundance of nitrogen-fixing bacteria such as Rhizobium (A) and Bradyrhizobium (B) in straw-mulched soils compared to other treatments, indicating reduced dependency on synthetic fertilizers
Though this study has limitations in the number of samples that was tested and in one single geographical region, the results concluded a positive impact of straw mulching on enhancing the richness and diversity of soil microbiota that leads to improved soil fertility. The protective barrier formed by straw mulching shields the soil and its beneficial bacteria from adverse weather conditions, and helps promote tolerance to abiotic stress. Additionally, the increased presence of EM fungus, facilitated by straw mulching, holds promise for mitigating climate change by aiding in carbon sequestration. The practice of straw mulching proves beneficial for farms of various sizes, contributing to sustainable agriculture practices. Integration into a mulching cycle, either with other techniques or without any other mulch, promotes environmentally friendly and cost-effective agriculture. This approach reduces reliance on expensive fertilizers; controls weed growth, enhance soil fertility, and avoid the use of harmful herbicides, addressing the escalating challenges associated with modern agriculture.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Ma D, Chen L, Qu H, Wang Y, Misselbrook T, Jiang R. Impacts of plastic film mulching on crop yields, soil water, nitrate, and organic carbon in Northwestern China: A meta-analysis. Agric Water Manag. 2018;202:166-173.
Crossref - Duppong LM, Delate K, Liebman M, et al. The Effect of Natural Mulches on Crop Performance, Weed Suppression and Biochemical Constituents of Catnip and St. John’s Wort. Crop Sci. 2004;44(3):861-869.
Crossref - Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003.
Crossref - Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front Public Health. 2016;4:148.
Crossref - Tipton L, Darcy JL, Hynson NA. A Developing Symbiosis: Enabling Cross-Talk Between Ecologists and Microbiome Scientists. Front Microbiol. 2019;10:292.
Crossref - Prathyusha AMVN, Bramhachari PV. Novel Perspectives of Biotic and Abiotic Stress Tolerance Mechanism in Actinobacteria. In: Gupta VK, eds. New and Future Developments in Microbial Biotechnology and Bioengineering. 2018:235-244.
Crossref - Saha D, Marble SC, Pearson BJ. Allelopathic effects of Common Landscape and Nursery Mulch Materials on Weed Control. Front Plant Sci. 2018;9:733.
Crossref - Averill C, Turner BL, Finzi AC. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature. 2014;505(7484):543-5.
Crossref - Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581-583.
Crossref - Kuramae EE, Hillekens RH, de Hollander M, et al. Structural and functional variation in soil fungal communities associated with litter bags containing maize leaf. FEMS Microbiol Ecol. 2013;84(3):519-31.
Crossref - López-García A, Pineda-Quiroga C, Atxaerandio R, et al. Comparison of Mothur and QIIME for the Analysis of Rumen Microbiota Composition Based on 16S rRNA Amplicon Sequences. Front Microbiol. 2018;9:3010.
Crossref - Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Bioinformatics. 2011;36(1),10.7.1-10.7.20.
Crossref - Dai Z, Su W, Chen H, et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob Chang Biol. 2018;24(8):3452-3461.
Crossref - Zhang S, Wang Y, Sun L, et al. Organic mulching positively regulates the soil microbial communities and ecosystem functions in tea plantation. BMC Microbiol. 2020;20(1):103.
Crossref - Dimkpa CO, Bindraban PS. Fortification of micronutrients for efficient agronomic production: A review. Agron Sustain Dev. 2016;36(7):1-26.
Crossref - Frac M, Hannula SE, Belka M, Jedryczka M. Fungal Biodiversity and Their Role in Soil Health. Front Microbiol. 2018;9:707.
Crossref - Wei Z, Hu X, Li X, et al. The rhizospheric microbial community structure and diversity of deciduous and evergreen forests in Taihu Lake area, China. PLoS One. 2017;12(4):e0174411
Crossref - Rashid MI, Mujawar LH, Shahzad T, Almeelbi T, Ismail MII, Oves M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res. 2016;183:26-41.
Crossref - Terrer C, Vicca S, Hungate BA, Phillips RP, Prentice IC. Mycorrhizal association as a primary control of the CO2 fertilization effect. Science. 2016;353(6294):72-74.
Crossref - Ma A, Zhuang X, Wu J, et al. Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. PLoS One. 2013;8(6):e66146.
Crossref - Wang W, Wang H, Feng Y, et al. Consistent responses of the microbial community structure to organic farming along the middle and lower reaches of the Yangtze River. Sci Rep. 2016;6:35046.
Crossref - Hacquard S, Garrido-Oter R, Gonzalez A, Spaepen S, Ackermann G, Lebeis S. Microbiota and Host Nutrition across Plant and Animal Kingdoms. Cell Host Microbe. 2015;17(5):603-616.
Crossref - Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60.
Crossref - Arora S, Arora S, Singh A, Sehgal M, Srivastava DS, Singh A. Pesticides use and its effect on soil bacteria and fungal populations, microbial biomass carbon and enzymatic activity. Curr Sci. 2019;116(4):643-649.
Crossref - Navarrete AA, Soares T, Rossetto R, van Veen JA, Tsai SM, Kuramae EE. Verrucomicrobial community structure and abundance as indicators for changes in chemical factors linked to soil fertility. Antonie Van Leeuwenhoek. 2015;108(3):741-752.
Crossref - DeBruyn JM, Nixon LT, Fawaz MN, Johnson AM, Radosevich M. Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. Appl Environ Microbiol. 2011;77(17):6295-6300.
Crossref - Rojas-Jimenez K, Wurzbacher C, Bourne EC, Chiuchiolo A, Priscu JC, Grossart HP. Early diverging lineages within Cryptomycota and Chytridiomycota dominate the fungal communities in ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Sci Rep. 2017;7(1):15348.
Crossref - Jansson JK, Hofmockel KS. The soil microbiome-from metagenomics to metaphenomics. Curr Opin Microbiol. 2018;43:162-8.
Crossref - Zhang J, Shi Q, Fan S, Zhang Y, Zhang M, Zhang J. Distinction between Cr and other heavy-metal-resistant bacteria involved in C/N cycling in contaminated soils of copper-producing sites. J Hazard Mater. 2021;402:123454.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.