ISSN: 0973-7510
E-ISSN: 2581-690X
Secondary metabolites produced from marine fungi showed promising roles in Biomedical applications. This study aimed to exhibit the Anti-Microbial efficacy of the potent fungal strain Aspergillus terreus JMDL-20. Aspergillus terreus JMDL-20 was isolated from Velankanni Beach, South Coast of Tamil Nadu, India and subjected to Anti-Microbial, Myco-chemical and nanoparticle screening. Morphological and Molecular identification was done for the potent fungal strain. Antioxidant activity and growth curve were studied. Characterisation of Aspergillus terreus JMDL-20 was done by using HPLC and FTIR analysis. A total of twenty-five fungal cultures were isolated. Based on Antimicrobial and Myco-chemical screening of bioactive metabolites, potent fungal strain, JMDL-20 is selected and molecularly identified as Aspergillus terreus. Extracellular Cadmium Biogenic Nanoparticle was synthesized. The Antioxidant activity (%) was found to be 42.8357. Maximum production of secondary metabolites was observed at 16 days. In HPLC analysis, the extracellular compound Lovastatin was identified. FTIR analysis exhibited 31 absorption peaks. Fungal crude metabolite extract and cell-free filtrate of Aspergillus terreus JMDL-20 exhibited a wide range of Anti-Microbial activity.
Antioxidant Activity, HPLC, Lovastatin, Marine Fungi, Scanning Electron Microscope
From the beginning of mankind, nature has played a major role in drug manufacturing by humans to provide remedial treatments. Marine biotopes are one of nature’s treasures which occupy nearly three parts of the earth’s surface. In biomedical research and drug development, natural products from marine play an important role as direct drugs or core structures for biologically inspired chemical drug synthesis. Hence, the chemists showed interest in investigating drug discovery from marine bacteria and fungi which leads to vast untapped reservoirs of metabolic diversity.1 In the production of antimicrobial drugs, marine fungi are the potential source and showed unique applications.2 Many diseases that are pathogenic to humans are caused by bacteria, fungi, viruses and parasites reduced by these antimicrobial substances.3 Fungi show attractive biomass production and it is very easy to extract antimicrobial metabolites is the main advantage over bacteria. Due to the simple and inexpensive nature of fungi, it is easy to cultivate on both industrial and laboratory scales.4 Micro-organisms like bacteria, fungi, actinomycetes, and plants also play a crucial role in the biogenic synthesis of nanoparticles and they are widely used in medical fields, drug delivery, cosmetics, agriculture, cancer treatment as well as diagnosis and purifying the contaminated water from pathogenic microorganisms.5 Antimicrobial metabolites produced from marine fungi have been used as antioxidant, antibacterial and anticancer agents.6 The present study aimed to exhibit the anti-microbial activity of the Potent fungi Aspergillus terreus JMDL-20. Biosynthesis of Nanoparticles and determination of their antimicrobial efficacy.
Sample collection
At a depth of 10 to 20 centimetres, sterile containers were used to collect marine soil samples from various areas of Velankanni Beach, which is situated on the southern coast of Tamil Nadu, India. Soil samples were safely transferred to the laboratory. The soil samples underwent drying at ambient temperature and were used for further studies.7
Isolation of marine fungi
Marine fungi were isolated using Sabouraud Dextrose Agar (SDA), Czapek Dox Agar (CDA), and Potato Dextrose Agar (PDA) medium. All of the media listed above were autoclaved separately at 121 °C for 15 lbs pressure and transferred into sterile petri plates. Streptomycin (50 µg/ml) was mixed into the medium to inhibit bacterial growth. A tenfold serial dilution was performed, with dilutions ranging from 10-1 to 10-9. Each dilution was inoculated with 0.1 ml of sample by spread plate technique. Inoculated Petri dishes were incubated at 30 + 2 °C and monitored regularly for 5-14 days. Visually distinct fungal colonies were observed and they were sub-cultured on SDA slants and preserved for future study.8,9
Screening of fungi for antimicrobial metabolites
A preliminary examination was performed for all the isolated fungal strains, with potent strain JMDL-20 showing the highest zone of inhibition. The fungal strain JMDL-20 was cultivated in 100 ml of Sabouraud dextrose broth and incubated for 14 days. Post-incubation, the broth was filtered by Whatman No. 1 filter paper. The filtrate was separated by solvent extraction with ethyl acetate to isolate the compounds. To extract the crude compounds, ethyl acetate was mixed with filtrate in a ratio of 1:1 and vigorously shaken. Following evaporation of the Ethyl acetate extract, the obtained residue was used for anti-microbial activity against clinical pathogens such as Bacillus megaterium (NCIM 2187), Bacillus subtilis (MTCC 441), Escherichia coli (MTCC 443), Pseudomonas aeruginosa (MTCC 424), Klebsiella pneumoniae (MTCC-452), Aspergillus niger (MTCC 282), Aspergillus fumigatus (ATCC 46645) and Candida albicans (MTCC 227) on nutrient agar plates. Ethyl acetate as a negative control. Standard antibiotic, such as streptomycin (25 mg/ml), is employed for positive control. Anti-bacterial activity was evaluated by determining the zone of inhibition (mm) at 48 hours post-incubation. The standard antifungal drug fluconazole was used as a positive control for antifungal activity and the incubation of the plates was carried out at 32 °C for 7 days. To screen for potent marine fungi, inhibition zone diameters were measured. Potent fungi were chosen for further Myco-chemical screening.10
Preliminary myco-chemical screening of the strain JMDL-20
Following anti-microbial screening, the strain JMDL-20 was selected for preliminary Myco-chemical investigation. The fungal crude metabolite extract was analyzed for the existence of several Myco-chemicals including glycosides, phenols, flavones, flavonoids, alkaloids, tannins, steroids, terpenoids and saponins.
The Presence of glycosides was determined in the solvent-extracted broth, to the solvent-extracted broth 3 ml (5%) ferric chloride and three ml of distilled H2O were added. Later, heating the above solution for 15 minutes, subjected to cooling, and then 1.5 ml of benzene was supplemented to this mixture and mixed thoroughly for 15 sec. 5 to 6 drops of concentration. Ammonia was supplemented with this mixture and examined for the appearance of pink colour, indicating the existence of glycosides. For phenols, 6 ml of lead acetate (10%) was supplemented with 4 ml of fungal metabolite, the white precipitate formation confirms the phenolic compounds.
Similarly, the presence or absence of flavones was determined by 4 ml of diluted NaOH mixed with 2 ml of concentrated HCl to 6 mL of metabolite extract. The formation of a yellow-to-orange colour change confirms the flavones existence, thus a small volume of ferric chloride (10%) was supplemented with the 3 ml of fungal metabolite extract. Green precipitate formation indicating the conformation of flavonoids and detection of alkaloids in the sample was done by adding 3 ml of HCL (2%) along with 2-4 drops of Mayer’s reagent was amended with 2 ml of fungal metabolite extract with the formation of white or yellowish precipitate.
Adding a small volume of concentrated ferric chloride to the one ml of metabolite extract revealed the presence of tannins in the crude metabolites. The appearance of greenish-black or blue indicates the presence of catechol and gallic tannins. Saponins detection in the fungal metabolite extract by adding the one ml sample with 20 ml of distilled water resulted in the formation of one centimetre of the foam layer.
Identification of steroids and terpenoids to the crude extract 4 ml of chloroform was added to the 4 ml of acetic acid to this 3 to 5 drops of concentration H2SO4 was also mixed. When this mixture was added to the crude extract, a greenish-blue colour was produced, indicating the presence of steroids and terpenoids.11
Screening of fungi for biosynthesis of cadmium nanoparticle
All the strains were subjected to screening for the biosynthesis of Cadmium nanoparticles. All the fungal strains were cultivated in 250 ml flasks with 100 ml of Sabouraud dextrose broth and incubated at 30 ± 2 °C for seven days. Later incubation, filter paper Whatman No. 1 was used to collect the fungal mycelial mat from the fungal broth. The obtained fungal mycelial mat was cleaned with distilled H2O. Then, 100 ml of distilled H2O was used to resuspend the fungal mycelial mat for 2 days in a rotatory Shaker at 100 rpm. After completing the 2 days of incubation period, the above solution was filtered to remove cell debris. From the obtained cell-free filtrate a volumetric ratio of 1:1 was used for biogenic synthesis of nanoparticles. 20 ml of cell-free filtrate of all the fungal strains were added to the 20 ml of 1 mM, 8 mM and 10 mM concentrations of cadmium metal oxide. They were subjected to incubation for 48 h in the dark to avoid photochemical reaction at 28 °C until the colour changed. After colour change, at 300 nm-700 nm optical density of the solution was measured.12 Cadmium nanoparticles were tested for their antibiotic potential upon clinical pathogens such as Streptococcus mutants (MTCC 497), Bacillus subtilis (MTCC 441), Klebsiella pneumoniae (MTCC 452), Pseudomonas aeruginosa (MTCC 424), Bacillus megaterium NCIM 2187.13
Identification of the potent fungal strain
Cultural characteristics of the strain JMDL-20
To determine the influence of different growth media, Sabouraud-dextrose agar (SDA), Czapek-Dox agar (CDA), Malt extract agar (MEA), Yeast extract agar (YEA), and Potato dextrose agar (PDA) media were used to yield bioactive compounds by the fungal strain JMDL-20. The potent fungal strain JMDL-20 was spot inoculated on all selective media and kept for incubation at 30 + 2 °C for 7-14 days. After completion of the incubation period, colony Size, colour and pigmentation were observed.14
Morphological Identification of JMDL-20
Slide culture technique
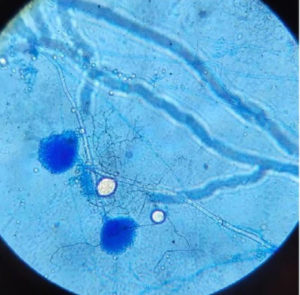
The strain JMDL-20 was Microscopically characterized using the slide culture technique. Sabouraud-Dextrose agar petri plates were prepared. After solidification, square-shaped agar blocks were prepared and placed on a sterile glass slide. Further over the edges of each block culture was inoculated and covered with coverslips. The culture slides were kept for an incubation period for 3 to 7 days at 28 °C. Mycelia and spores were transferred from the coverslip after the incubation period to a clean glass slide and stained with lactophenol cotton blue stain. Microscopic characteristics including the shape of mycelia, conidiophores, and conidia were observed under a motic microscope.15
Scanning Electron Microscopic analysis
Scanning Electron Microscope (SEM) is employed to analyse the characteristics of conidia of the strain JMDL-20. 2.5% glutaraldehyde with 0.1 M phosphate buffer (pH 7.2) was applied to fix the strain for 4 h at 4 °C. Then 2% aqueous osmium tetroxide was used for post-fixing for 4 h. Different alcohol concentrations were used to dehydrate the sample. By using the CPD (EMS 850) unit the sample was subjected to dryness to a critical point. The Processed sample was mounted and to the samples, a thin layer of gold coat was applied using an automated sputter coater before being scanned under SEM (Model: JOEL-JSM 5600) at the desired magnifications for 3 minutes according to standard procedure.16
Molecular Identification
The fungal strain JMDL-20 was identified by molecular methods such as 18S rRNA sequencing. The strain was analysed by its internal transcribed spacer (ITS) sequences. The extraction of fungal genomic DNA from mycelia was performed using cetyl trimethyl ammonium bromide (CTAB). ITS1 and ITS4 are the Primers used to amplify the ITS region of rDNA. At an oligonucleotide synthesis facility, the primers were made and diluted to 20 mM in sterilized water. Based on the manufacturer’s instructions, a QIA quick PCR purification kit was used to purify the PCR products. Denaturation was done at 95 °C for five minutes then 50 °C for 5 seconds as primer annealing, primer extension ran for 18 seconds at 68 °C, and for one minute, elongation was done at 72 °C. Using a purification kit QIA quick, primers and dNTPs were eliminated from the mixture after the amplification of the ITS templates. The cycle sequencing reaction mixture contained 2.5 µl of template DNA (10-15 ng/µl), 2 µl of Big Dye terminator premix, 4 µl of ultrapure sterile water, and 0.5 µl of each primer (5 pmol/l) in its total reaction volume. Ethanol/ethylene diamine tetra acetic acid (EDTA) was used to purify the sequencing products, and an ABI PRISM 3130 Genetic Analyzer from Applied Biosystems was used to analyse the samples.
Pair-wise sequence alignment
BLAST was used to align the gene sequence of strain JMDL-20 with the Aspergillus species gene library in NCBI and GenBank. MEGA-11 software was used to calculate pairwise evolutionary distances.
Multiple sequence alignment
The neighbor-joining method with BLAST and CLUSTAL W was used for the phylogenetic analysis. CLUSTAL W was used with the MEGA 11 version to identify, retrieve, and compare the sequence. The obtained 18S rRNA gene sequence was added to the GenBank database.17
Antioxidant activity of Aspergillus terreus JMDL-20
2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging method was used to determine the antioxidant activity of fungal crude extracts of Aspergillus terreus (JMDL-20). DPPH solution was prepared by 0.1 mM DPPH in ethanol, at 517 nm its absorbance was recorded. Then 3 ml of DPPH solution was supplemented to the 50 µl of fungal metabolite extract and kept for 15 minutes in the dark. At 515 nm again the absorbance was recorded. By using the formula, the inhibition of DPPH percentage was calculated.18
% scavenging rate = [1- (A1 – A2) / A0] x 100
Growth curve and antimicrobial activity of the strain Aspergillus terreus JMDL-20
On 100 ml Sabouraud-dextrose broth, the fungal strain Aspergillus terreus JMDL-20 was inoculated to determine the growth pattern. The fungal broth was kept incubated for 24 days at 30 + 2 °C. For every two to twenty-four days the flasks were recovered. The culture broth was filtered through Whatman No. 1 filter paper and the growth of the strain was analysed by the dry weight of the mycelium. From the filtrate, antimicrobial compounds were extracted through the solvent extraction method. The filtrate was vigorously shaken with ethyl acetate at 1:1. Ethyl acetate extract was evaporated and the residue was used for antimicrobial activity against clinical pathogens such as Bacillus subtilis (MTCC 441), Klebsiella pneumoniae (MTCC-452), Pseudomonas aeruginosa (MTCC 424), Bacillus megaterium (NCIM2187) and Escherichia coli (MTCC 443). The standard antibiotic streptomycin at 25 mg/ml and ethyl acetate were used as positive control and negative control and 80 µl of crude extract were filled in the separate wells. The Plates were incubated for 2-4 days at 37 °C and the zone of inhibition (mm) was measured.19
High-Performance Liquid Chromatography Analysis (HPLC)
Cadmium nanoparticle synthesized cell-free filtrate of Aspergillus terreus JMDL-20 was subjected to High-Performance liquid chromatography analysis. It was analysed on a Shimadzu LC-10AT VP HPLC system, it contains a 20-ll loop Rheodyne injector, S908 an auto-injector SIL-10AT, LC-10AT pump, UV-VIS detector SPD-10AT. A Hypersil BDS C-18 column (4.6 × 250 mm, 5 mm size) and a C-18 guard column were employed. 0.2 L membrane filter was used to filter mobile phase components and the fluid flowed at a rate of 1 ml/min the solvent reservoir was pumped, then the column relief pressure was maintained at 260-270 kg/cm and the temperature at 27 °C was maintained for the column. A Rheodyne syringe (Model 7202, Hamilton) was used to inject the twenty microliters of the sample. At a temperature of 25-28 °C with gradient solvent systems elutes were obtained at 1 ml/min at flow rate. The mobile phase was prepared, filtered and sonicated before use. The US-VIS detector wavelength maintained at 254 nm and 20 ml was the sample injection volume.20
FTIR analysis
Cadmium Nanoparticles synthesized cell-free filtrate of Aspergillus terreus JMDL-20 were studied for its FTIR analysis by using a Shimadzu spectrometer. On 300 mg of spectroscopic grade, KBr 3.0 mg of sample was dispersed and pressed for 3 min into the disk at 10 MPa. An average of 25 scans of spectral analysis was recorded at a 4 cm-1 resolution in the range of 4000-400 cm-1.21
Isolation of marine fungi
From marine soil samples of Velankanni Beach, South coast of Tamil Nadu, India, a total 25 fungal strains were isolated. After seven days of incubation, morphologically white, fluffy, smooth, and flowery colonies, as well as cream-coloured fungal colonies, were observed. All the strains were initially screened for their antimicrobial properties and they were designated as JMDL-1 to JMDL-25 and maintained on SDA slants for further studies.
Screening of fungi for anti-microbial metabolites
All the isolated fungal strains were subjected to screening for their anti-microbial potential among them the strain JMDL-20 was proved to have a potentiality exhibited in the height zone of inhibition and hence selected for further studies. One potent strain, JMDL-20 exhibits the highest zone of inhibition against bacterial and fungal pathogens out of 25 fungal isolates (Table 1). The fungal metabolite extract of JMDL-20 had a maximum zone of inhibition such as 40 mm against Bacillus megaterium, 34 mm against Pseudomonas aeruginosa, and a minimum zone of inhibition 25 mm against Escherichia coli. For fungal pathogens, Aspergillus niger 15 mm was reported as the minimum inhibition zone, while Candida albicans had a maximum inhibition zone at 29 mm. Our present study was supported by previous work22 reported that the fungal crude extract showed a broad range of anti-microbial activity against clinical pathogens. Present investigation showed significant variations and retard the growth of clinical pathogens when compared with previous reports22 supported our present work.
Table (1):
Antibacterial activity of JMDL-20 to screen potent fungi
No. |
Test Pathogen |
Zone of inhibition (mm) |
|---|---|---|
1 |
Bacillus megaterium |
40 mm |
2 |
Pseudomonas aeruginosa |
34 mm |
3 |
Klebsiella pneumoniae |
29 mm |
4 |
Escherichia coli |
25 mm |
5 |
Bacillus subtilis |
32 mm |
6 |
Candida albicans |
29 mm |
7 |
Aspergillus niger |
15 mm |
8 |
Aspergillus fumigatus |
18 mm |
Preliminary Myco-chemical screening of JMDL-20
To determine the production of various bioactive compounds, the fungal isolate JMDL-20 was subject to preliminary Myco-chemical screening. seven days old fungal broth was used for the Myco-chemical screening. To filter the broth, Whatman No. 1 filter paper was used and a solvent separation technique was used to separate the filtrate from the broth by using ethyl acetate. Myco-chemical screening is carried out with the obtained crude metabolite extract. Metabolite Extract of JMDL-20 was reacted with various reagents. When it came to the production of different types of myco-chemicals, the fungal crude metabolite produced both positive and negative outcomes as shown in Table 2. The fungal metabolite extract of JMDL-20 exhibited a positive response for Mayer’s reagent test and picric analysis by changing the colour of the metabolite into yellowish revealing the presence of alkaloids. In the lead acetate test, the fungal metabolite extract produced a white precipitate, which indicates a positive report for the production of phenolic compounds. In the glycosides test, the transformation of the fungal metabolite extract into a pink solution indicates the presence of glycosides. The colour change from yellow to orange shows the existence of flavones in the fungal metabolite extract of JMDL-20. The potent fungal strain JMDL-20 was subjected to different Myco chemical screening, among them it was proved to be positive for Glycosides, alkaloids, flavones and phenols production and negative results for flavonoids, steroids and terpenoid production. The same type of phytochemical screening of secondary metabolites was reported earlier by previous workers23 using fungal metabolite extract supports our present research work. The present investigation showed significant variation in the contents like Glycosides, alkaloids, flavones and phenols when compared to above-mentioned reports. Then, the potent fungal strain JMDL-20 showed a promising role in the production of antimicrobial metabolites.
Table (2):
Myco-chemical screening of the strain JMDL-20
No. |
Test Name |
Result |
|---|---|---|
1 |
Glycosides test |
+ |
2 |
Alkaloids test |
+ |
3 |
Flavonoids test |
– |
4 |
Flavones test |
+ |
5 |
Phenols test |
+ |
6 |
Steroids test |
– |
7 |
Terpenoids Test |
– |
8 |
Saponins test |
– |
9 |
Tannins test |
– |
“+” indicates positive result, “-” indicates negative result
Screening of fungi for biosynthesis of Cadmium Nanoparticle
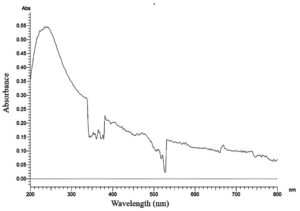
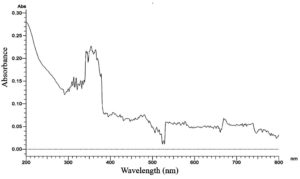
In our present study, the obtained Cell-free filtrate of all the fungal mycelia was mixed with a precursor compound of 8 mM, 10 mM of cadmium sulphate (CdSO4) metal oxide the sample was kept for 48 h at dark. After 48 h, out of 25 fungal isolates the colour change in the CFF of JMDL-20 at 10 mM solution was observed as colourless to light yellow. Maximum uv absorption (lmax) was analysed at the different wavelengths from 300 nm-700 nm by spectrophotometer. The absorption peak of maximum was observed at a wavelength of 370 nm when compared with the control (Figure 1), without adding the cadmium precursor. The maximum absorption observed in Figure 2 with cadmium precursor it indicates the synthesis of cadmium nanoparticles by the fungal strain Aspergillus terreus (JMDL-20). Early studies reported that,24 the cell-free filtrate derived from Aspergillus species supported our present work. Similarly, previous workers reported that, at 410 nm the CdS NPs were absorbed and synthesized using K. pneumoniae. In our present study, the anti-microbial activity of the synthesized cadmium nanoparticle solution showed a maximum inhibition zone against Streptococcus mutants-16 mm, followed by Bacillus subtilis and Klebsiella pneumoniae -15 mm, Pseudomonas aeruginosa and Bacillus megaterium 14 mm zones were observed (Table 3). Anti-microbial activity results of previous workers12 supported our present work.
Table (3):
Anti-microbial activity of synthesized Cadmium Nanoparticle
No. |
Test Pathogen |
Zone of inhibition (mm) |
|---|---|---|
1 |
Bacillus megaterium |
14 mm |
2 |
Pseudomonas aeruginosa |
14 mm |
3 |
Klebsiella pneumoniae |
15 mm |
4 |
Streptococcus mutants |
16 mm |
5 |
Bacillus subtilis |
15 mm |
Identification of the Potent Fungal Strain
Cultural and Morphological Characteristics of the Strain JMDL-20
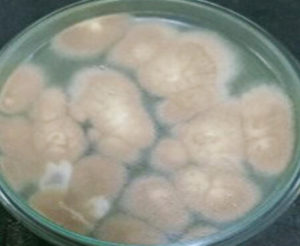
Cultural Morphology of JMDL-20 observed on six different media. The outcomes for SDA, CDA, MEA, YEA, and PDA are presented in Table 4. On various media, JMDL-20 displayed excellent growth. The colonies are compact and white. The spore colour changed to cream colour on SDA and a 46 mm sized colony was observed after 3 days of incubation period. Lemon yellow-coloured colonies were present on YEA with a colony size of 35 mm. Brown colour colonies were observed on CDA with 45 mm size. In PDA 40 mm size colony with brown colour was observed. Cream coloured with fluffy textured colonies were observed on MEA with a colony size of 35 mm. The size of each colony ranged from 35-45 mm in diameter on incubation at 30 °C for 7 days.
Table (4):
Cultural and morphological characteristics of JMDL-20
No. |
Media |
Colony colour |
Colony size (mm) |
Texture |
Growth |
|---|---|---|---|---|---|
1 |
SDA |
Lite greenish white |
46 |
Fluffy |
Excellent |
2 |
CDA |
Lite brown |
45 |
Fluffy |
Good |
3 |
PDA |
Lite brown |
40 |
Fluffy |
Good |
4 |
YEA |
Lite lemon yellow |
35 |
Fluffy |
Moderate |
5 |
MEA |
Cream colour |
35 |
Fluffy |
Moderate |
(SDA – Sabouraud-Dextrose agar, CDA – Czapek-Dox agar, NAM – Nutrient agar, MEA – Malt extract agar, YEA – Yeast extract agar, PDA – Potato dextrose agar)
Fungal colonies were velvety and cinnamon-buff to sand-brown in colour (Figure 3). The phialides are as long as the metulae and columnar, biseriate and compact Conidial heads. The wall of conidiophore stipes is hyaline and smooth. Conidia are globose to ellipsoidal (Figures 4 and 5).
Molecular identification
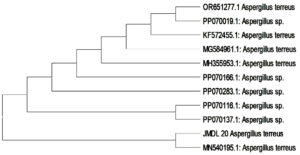
Molecular identification of Aspergillus terreus JMDL-20 done by 18S rRNA sequencing. By utilising the multisequence advanced BLAST comparison tool, alignment of the partial sequence is done and these sequences are compared with all the available 18S rRNA gene sequences in the GenBank. The CLUSTALW program from the MEGA 11 Version was used to align the 18S rRNA gene sequence for phylogenetic analysis. Phylogenetic tree Aspergillus terreus JMDL-20 constructed by MEGA software Version 11 using the Neighbor-joining method (Figure 6). Accession number PQ650931 provided by GenBank database for Partial 18S rRNA sequence of Aspergillus terreus JMDL-20.
Antioxidant activity
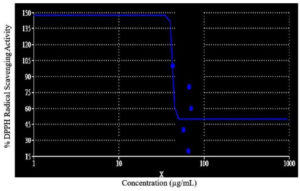
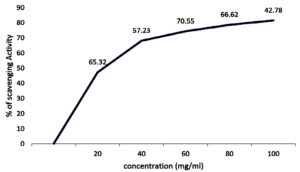
Antioxidant activity of the strain Aspergillus terreus JMDL-20 was evaluated by using DPPH. By utilising the UV-Vis spectrophotometer, the optical density was measured at 515 nm. A strong yellow colour indicates the high capability of extracts to scavenge free DPPH radicals and strong antioxidant potential. Free radical scavenging activity of ethyl acetate extract of Aspergillus terreus JMDL-20 was explored in a dose-dependent (100-500 µg/ml) manner. When the concentration of extract increases, increased DPPH scavenging ability was also observed. The results showed dose-dependent scavenging activity, it was expressed as IC50 as 42.8357 (Figure 7). The radical scavenging activity (%) was found to be 65.32, 57.23, 70.55, 66.62, 42.78 at concentrations of 20, 40, 60, 80, 100 mg/ml
(Figure 8).
Growth curve and anti-microbial activity of the strain Aspergillus terreus JMDL-20
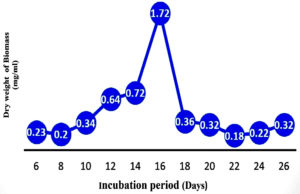
The growth of the strain Aspergillus terreus JMDL-20 (Figure 9) and antimicrobial activity were observed in a batch culture for 26 days. On the fifth day, the strain enters into the log phase which lasts to sixteen days. The stationary phase lasts from the 16th to the 18th day and later enters into the death phase. The secondary metabolites collected from the sixteen-day-old culture broth showed the highest anti-antibiotic activity against the test pathogens. The metabolites extracted from the sixteen-day-old culture exhibited a broad spectrum of activity. Sixteen days-old metabolites showed a broad spectrum of activity as shown in Table 5 similarly these results were interpreted with Previous reports25 noticed that 21 days of fungal isolate showed maximum activity for the production of bioactive metabolites.26
Table (5):
Anti-Microbial spectrum of Aspergillus terreus (JMDL-20) for growth curve
No. |
Test Pathogen |
Zone of inhibition (mm) |
|---|---|---|
1 |
Bacillus megaterium |
29 |
2 |
Pseudomonas aeruginosa |
32 |
3 |
Klebsiella pneumoniae |
30 |
4 |
Escherichia coli |
25 |
5 |
Baccillus subtilis |
27 |
HPLC analysis
Cell-free filtrate of Aspergillus terreus JMDL-20 was subjected to HPLC analysis. The HPLC fingerprint profile of Aspergillus terreus JMDL-20 reveals the presence of Lovastatin (Figure 10) is an extracellular compound responsible for the biogenic production of cadmium nanoparticles from the cell-free filtrate of Aspergillus terreus JMDL-20 at 5.36667 retention time showed the highest peak value and the area percentage was 100. Based on these values compared with control, it confirms the production of lovastatin by Aspergillus terreus JMDL-20.
FTIR analysis
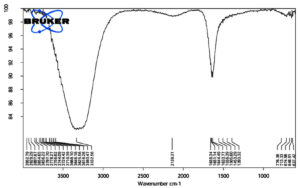
FTIR analysis of Cadmium Nitrate from a frequency of 600-4000 wavelength cm-1 reveals that the cell-free filtrate of Aspergillus terreus JMDL-20 exhibited 31 absorption peaks as shown in Figure 11. Major absorption peaks were reported at 1659 cm-1 and 3332 cm-1 with open-chain imino (-C=N-) and imino compounds (=N-H) stretch. Among 31 absorption peaks 1505 cm-1, 1556 cm-1 and 1633 cm-1 have aromatic nitro compound, aliphatic nitro compound and organic nitrates. These peaks reveal that the cell-free filtrate of Aspergillus terreus JMDL-20 contains Cadmium Nitrate. As earlier studies, it was reported that Cadmium nanoparticles synthesised from Aspergillus niger is having the same absorption peak as our cell-free filtrate supports our present work.27
In our present study, Fungal crude metabolite extract of Aspergillus terreus JMDL-20 showed a broad spectrum of anti-microbial activity against clinical pathogens. The fungal cell-free filtrate of Aspergillus terreus JMDL-20 was utilized for the biogenic production of cadmium nanoparticles. This cell-free filtrate also showed a broad range of antagonistic activity upon five distinct pathogenic bacteria. HPLC analysis of the synthesized cadmium nanoparticle solution reveals the conformation of Lovastatin in the cell-free filtrate of Aspergillus terreus JMDL-20 and FTIR Analysis revealed the functional group. Antioxidant efficacy was exhibited by the fungal crude metabolite extract of Aspergillus terreus JMDL-20 at different concentrations by using DPPH. These results indicate that the cadmium nanoparticles have anti-microbial activity upon many microorganisms and have a significant role in biomedical applications. Thus cadmium nanoparticles may help in exploiting the broad spectrum of bioactive compounds. The Potential of antimicrobial metabolites exhibits a wide range of applications in the areas of health and agriculture.
ACKNOWLEDGMENTS
The Authors acknowledge the Department of Botany and Microbiology, Acharya Nagarjuna University, for providing the research facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MJ conceptualised the study and performed literature review. LPD performed experiments and wrote the manuscript. MD reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analysed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Debbab A, Aly AH, Lin WH, Proksch P. Bioactive compounds from marine bacteria and fungi. Microb biotechnol. 2010;3(5):544-563.
Crossref - Wang P, Huang X, Jiang C, et al. Antibacterial properties of natural products from marine fungi reported between 2012 and 2023: a review. Arch Pharm Res. 2024;47(6):505-537.
Crossref - Mohamed EAA, Muddathir AM, Osman MA. Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan. Sci Rep. 2020;10(1):17148.
Crossref - Gade AK, Bonde P, Ingle AP, Marcato PD, Duran N, Rai MK. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy. 2008;2(3):243-247.
Crossref - Iranmanesh S, Bonjar GHS, Baghizadeh A. Study of the biosynthesis of gold nanoparticles by using several saprophytic fungi. SN Appl Sci. 2020;2(11):1851.
Crossref - Ghasempour A, Dehghan H, Ataee M, et al. Cadmium sulfide nanoparticles: preparation, characterization, and biomedical applications. Molecules. 2023;28(9):3857.
Crossref - Aarthi G, Sarath A, Harikrishnan S, Sudarshan S, Muthezhilan R, Jayalakshmi S. Bioactive potential of Aspergillus sp. Isolated from uppanar estuarine sediment samples against plant pathogens. Biochem Cell Arch. 2022;22(2):4165.
Crossref - Suthindhiran K, Kannabiran K. Diversity and exploration of bioactive marine actinomycetes in the Bay of Bengal of the Puducherry coast of India. Indian J Microbi. 2010;50(1):76-82.
Crossref - Khalid M, Yang WJ, Kishwar N, Rajput ZI, Arijo AG. Study of cellulolytic soil fungi and two nova species and new medium. J Zhejiang Univ Sc B. 2006;7(6):459-466.
Crossref - Kareru PG, Gachanja AN, Keriko JM, Kenji GM. Antimicrobial activity of some medicinal plants used by herbalists in eastern province, Kenya. Afr J Tradit Complement Altern Med. 2008;5(1):51-55.
Crossref - Singh PK, Singh J, Medhi T, Kumar A. Phytochemical screening, quantification, FT-IR analysis, and in silico characterization of potential bio-active compounds identified in HR-LC/MS analysis of the polyherbal formulation from Northeast India. ACS Omega. 2022;7(37):33067-33078.
Crossref - Basheer MA, Abutaleb K, Abed NN, Mekawey AA. Mycosynthesis of silver nanoparticles using marine fungi and their antimicrobial activity against pathogenic microorganisms. J Genet Eng Biotechnol. 2023;21(1):127.
Crossref - Rajeshkumar S, Ponnanikajamideen M, Malarkodi C, Malini M, Annadurai G. Microbe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. J Nanostruct Chem. 2014;4(96):1-7.
Crossref - Sharma KK, Singh US. Cultural and morphological characterization of rhizospheric isolates of fungal antagonist Trichoderma. J Appl & Nat Sci. 2014;6(2):451-456.
Crossref - Kalyani P, Lakshmi BK, Hemalatakha KP. Isolation, identification of marine fungi with antibacterial activity. Int J Curr Adv Res. 2017;6(12):8085-8091.
Crossref - Pirali-Kheirabadi KH, Razzaghi-Abyaneh M, Eslamifar A, Halajian A, Nabian S. Scanning Electron Microscopy (SEM) analysis and biological control of Ixodes ricinus using entomopathogenic fungi. Mycologia Iranica. 2016;3(1):39-46.
Crossref - Chin JMW, Puchooa D, Bahorun T, Jeewon R. Molecular characterization of marine fungi associated with Haliclona sp. (sponge) and Turbinaria conoides and Sargassum portierianum (brown algae). Proc Nat Acad Sci India Sect B: Biol Sci. 2021;91(3):643-56.
Crossref - Arora DS, Chandra P. Assay of antioxidant potential of two Aspergillus isolates by different methods under various physio-chemical conditions. Braz J Microbiol. 2010;41(3):765-777.
Crossref - Cruz RC, Werneck SMC, Oliveira CS, et al. Influence of different media, incubation times, and temperatures for determining the MICs of seven antifungal agents against Paracoccidioides brasiliensis by microdilution. J Clin Microbiol. 2013;51(2):436-443.
Crossref - Raubbin RS, Laju RL, Ambika P, Pushparaj A. HPLC, FTIR and GC-MS analysis of ethyl acetate extract of red seaweed Hypnea flagelliformis Graville ex J. Agardh 1851. Int J Pharm Sci Res. 2020;11(8):3953-3959.
Crossref - Ramya S, Chandran M, King IJ, et al. Phytochemical Screening, GCMS and FTIR Profile of Bioactive Compounds in Solanum lycopersicum Wild Fruits collected from Palani Hill Ranges of the Western Ghats. J Drug Delivery Ther. 2022;12(6):56-64.
Crossref - Witasari LD, Wahyu KW, Anugrahani BJ, et al. Antimicrobial activities of fungus comb extracts isolated from Indomalayan termite (Macrotermes gilvus Hagen) mound. AMB Expr. 2022;12(1):14.
Crossref - Sikandar A, Zhang M, Wang Y, et al. Mycochemical screening and analysis, antioxidant activity, and biochemical composition of fermentation strain Snef1216 (Penicillium chrysogenum). J Anal Methods Chem. 2020;2020(1):3073906.
Crossref - Raliya R, Tarafdar JC. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric Res. 2013;2:48-57.
Crossref - Taritla S, Kumari M, Kamat S, Bhat SG, Jayabaskaran C. Optimization of PhysicoChemical Parameters for Production of Cytotoxic Secondary Metabolites and Apoptosis Induction Activities in the Culture Extract of a Marine Algal-Derived Endophytic Fungus Aspergillus sp. Front Pharmacol. 2021;12:542891.
Crossref - Navarri M, Jegou C, Bondon A, et al. Bioactive metabolites from the deep subseafloor fungus Oidiodendron griseum UBOCC-A-114129. Mar Drugs. 2017;15(4):111.
Crossref - Shakibaie M, Riahi-Madvar S, Ameri A, Amiri-Moghadam P, Adeli-Sardou M, Forootanfar H. Microwave assisted biosynthesis of cadmium nanoparticles: characterization, antioxidant and cytotoxicity studies. J Clust Sci. 2022;33(5):1877-1887.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.