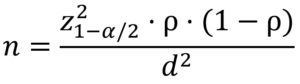ISSN: 0973-7510
E-ISSN: 2581-690X
One of the most significant pathogens involved in urinary tract infections is Uropathogenic E. coli (UPEC). In addition to assessing biofilm production and the effect of gold and silver nanoparticles on the isolated strains’ ability to form biofilms, the study intended to determine the prevalence of multidrug-resistant (MDR) UPEC in patients who were admitted to Menoufia University Hospitals with both hospital-acquired (HA) and community-acquired (CA) urinary tract infections (UTIs). E. coli strains were isolated from 312 urine samples, and the antibiotic resistance profile of the isolated strains was evaluated using the Kirby-Bauer disc diffusion technique. Using the tissue culture plate method, biofilm production was identified, and certain virulence genes were found. Lastly, biofilm development following incubation with different concentrations of both nanoparticles was measured to assess how well gold and silver nanoparticles inhibited biofilm formation. From 312 urine samples used, 100 E. coli were isolated. Of these isolates, 58 (58%) were isolated from HA-UTI and 42 (42%) from CA-UTI patients. Biofilm was produced by 89.5% of catheterized and 80% of non-catheterized HA E. coli, compared to 66.7% of CA isolates. MDR rates were not 44.7% for catheterized, 45% for non-catheterized hospital-acquired and 33.3% for community-acquired E. coli isolates. About 96% produced FimH, 24% produced Sfa and 68% produced IutA. Antibofilm effect of silver was much better than gold nanoparticles. FimH, Sfa and IutA were more predominant among HA isolates than community. Biofilm formation is effectively inhibited by silver nanoparticles (AgNPs). Therefore, AgNPs can be used in medical devices to stop biofilms from forming, whereas gold had a much less effective antibiofilm effect.
Biofilm, UPEC, MDR, Silver-gold Nanoparticles
Each year, more than 150-250 million people worldwide are affected by urinary tract infections (UTIs), one of the most prevalent infections in both community and hospital settings.1 These infections are most often caused by bacteria such as coagulase-negative Staphylococci, Klebsiella species, Staphylococcus aureus, and uropathogenic Escherichia coli (UPEC), with Pseudomonas aeruginosa, Enterobacter species, Proteus mirabilis, and Enterococcus species also being significant contributors.2
Forty percent to fifty percent of all hospital-acquired infections are caused by catheter-associated urinary tract infections (CAUTIs), and between 12% and 16% of hospitalized adults have an indwelling urinary catheter at some point. Every day the catheter is in place raises the risk of CAUTI by 3%-7%. Treatment for UTIs is now more difficult due to the emergence of multidrug-resistant (MDR) pathogens, which also raises healthcare expenses and increases morbidity and mortality from antibiotic resistance.3
Communities of microorganisms known as biofilms stick to surfaces and are encased in extracellular matrix that they create on their own. Biofilm-bound cells differ from free-floating (planktonic) cells in that they have longer doubling times and lower metabolic activity. The prevalence of biofilm in (UPEC) varies between 60% and 70%. Antimicrobial biofilm resistance has drawn attention, particularly because biofilms on medical devices can cause treatment failures and chronic infections, making their removal extremely difficult.4
To detect biofilm production, methods such as Congo red agar (CRA), the tissue culture plate (TCP), and the biofilm tube method (TM) are commonly used. The TCP assay, described by Christensen et al., is the most widely adopted and is considered the gold standard for biofilm detection.5
Numerous virulence factors, including flagella, toxins, and fimbriae, enable uropathogenic E. coli strains to evade the host’s immune system. FimH is a key gene with a high affinity for urinary tract receptors; consequently, FimH adhesion is essential for E. coli colonization of different niches and iron-acquisition systems. Additionally, Cnf1, HlyA, and IutA are essential for dissemination and for survival in iron-limited environments. The production of lipopolysaccharides by them also encourages the formation of biofilms, which raises the incidence of UTIs. Multidrug-resistance can make treating these infections challenging. The onset of a UTI depends on bacterial attachment to uroepithelial cells, which is facilitated by adhesion genes such as P fimbriae (Pap), fimbrial-adhesin1 (Afa), and S-fimbriae (Sfa).6
Many current antibiotics are losing effectiveness against MDR microorganisms in biofilms, necessitating alternative solutions. Recent studies on organic and inorganic nanoparticles show promise for biofilm inhibition. These nanoparticles, widely used in biomedicine, cosmetics, and environmental management, exhibit unique properties that enhance their bactericidal effects. They can potentially overcome exopolysaccharide barriers and enhance infection control strategies because of their small size, which enables them to penetrate biofilm layers.7
E. coli, S. aureus, P. aeruginosa, Proteus vulgaris, Proteus mirabilis, Enterobacter cloacae, and Staphylococcus epidermidis are just a few of the pathogens that are significantly inhibited by silver nanoparticles (Ag NPs) and gold nanoparticles (Au NPs). By interfering with the formation of biofilms and/or targeting the microbes directly, these nanoparticles stop the growth of bacteria. The study’s objectives were to determine the frequency of multidrug-resistant UPEC in patients at Menoufia University Hospital who had both community-acquired (CA) and hospital-acquired (HA) UTIs, evaluate biofilm formation, and determine how gold and silver nanoparticles affected the isolated strains’ ability to form biofilms.8
Study eligibility and design
From March 2023 to December 2023, this cross-sectional study was conducted at Menoufia University Department of Medical Microbiology and Immunology, Faculty of Medicine. A total of 312 clinically suspected hospital-acquired and community-acquired UTI cases were selected from inpatient and outpatient departments of Menoufia University Hospital with urinary tract infection (UTI) clinical symptoms. Patients were classified as hospital-acquired UTI if infection became evident 48 hr or more after hospital admission. Complete clinical and demographic history were taken from each patient. Every patient provided written informed consent. The Committee of Ethics, Faculty of Medicine, Menoufia University provided its approval to the study’s protocol (9/2022MICRO 23). The sample size for the current study was calculated to achieve significant results with a p-value of <0.05 and calculated according to this formula:
Where:
Z1-α/2 = 1.96 (for 95% confidence level)
p = estimated proportion (from a previous study) = 0.226
d = absolute precision (margin of error) = 0.05
A total of 312 unique urine samples were processed by culturing on CLED agar, followed by biochemical testing for the identification of E. coli. The isolates were then subjected to antibiotic susceptibility testing with antibiotics sourced from Oxoid (UK), in compliance with the Clinical and Laboratory Standards Institute (CLSI 2023) guidelines.9 The antibiotic panel used included Ampicillin (AMP) (10 µg), Piperacillin (PRL) (100 µg), Amoxicillin/Clavulanate (AMC) (20/10 µg), Ampicillin/Sulbactam (A/S) (10/10 µg), Ceftazidim/Avibactam (CZA) (30/20 µg), Ceftolozan/Tazobactam (CZA) (30/10 µg), Ceftriaxone (CTR) (30 µg), Cefoxitin (FOX) (30 µg), Ceftazidime (CAZ) (30 µg), Cefoperazone (CFP) (75 µg), Aztreonam (ATM) (30 µg), Meropenem (MEM) (10 µg), Imipenem (IMP) (10 µg), Gentamicin (CN) (10 µg), Amikacin (AK) (30 µg), Levofloxacin (LEV) (5 µg), Piperacillin/Tazobactam (TPZ) (100/10 µg), Azithromycin (AZM) (15 µg), Doxycycline (DOX) (30 µg), Trimethoprim/Sulfamethoxazole (SXT) (1.25/23.75 µg), Fosfomycin (FOS) (200 µg), and Nitrofurantoin (F) (300 µg).
Detection of biofilm formation by tissue culture plate method
Ten milliliters of TSB broth containing 1% glucose were used to inoculate uropathogenic E. coli isolates, which were then incubated for twenty-four hours at 37 °C. After that, the cultures were diluted (1:100), and 0.2 mL of the diluted culture-sterile broth serving as the negative control-was added to each well of a tissue culture plate (TCP). After another 24 hours of incubation at 37 °C, the plates were cleaned with PBS to get rid of any floating bacteria and allowed to dry at room temperature.
After being fixed with 2% sodium acetate and stained for 10 minutes with 0.1% crystal violet, the biofilms produced by the E. coli isolates were rinsed with PBS. After dissolving the stained biofilms in 95% ethanol, they were incubated for 15 minutes. A plate reader was used to measure the optical density (OD) at 590 nm. Each assay had three replicates, and the average absorbance values were noted.10,11
Evaluation of anti-biofilm effect of gold nanoparticles
Uropathogenic E. coli isolates were inoculated in TSB broth with 1% glucose and incubated for 24 hours at 37 °C to evaluate biofilm formation following exposure to gold nanoparticles. Each well of a Tissue Culture Plate (TCP) received 0.1 mL of the diluted cultures (1:100). With an untreated biofilm serving as a negative control column, different concentrations of gold nanoparticles (200, 100, 50, 25, and 12.5 µg/ml) were added. Following an overnight incubation period, the wells were cleaned, stained with crystal violet, and a micro-ELISA reader was used to measure the optical density (OD) of the biofilms at 590 nm.12
The following formula was used to determine the percentage inhibition of biofilm activity13:
Biofilm inhibition percentage (%) = 1 – (absorbance of cells treated with Au NPs / absorbance of non-treated wells) × 100
The data are expressed as mean ± SD
Molecular characterization of E. coli target genes (FimH, Sfa, IutA and Cnf)
DNA extraction
Bacterial DNA from 50 uropathogenic E. coli strains was extracted and purified using the gene JETTM genomic DNA purification kit (Thermo Fisher Scientific, UK).
PCR amplification
Primers from Invitrogen (Thermo Fisher, UK) with particular sequences and amplicon sizes were used for PCR, as shown in Table 1. The first cycle of the program involved denaturation at 94 °C, followed by 30 cycles of 94 °C for 1 minute, 60 °C annealing for 30 seconds, 72 °C extension for 1 minute, and final extension at 72 °C for 5 minutes for the FimH and Cnf3 genes. Denaturation at 94 °C for 5 minutes, 40 cycles of 94 °C for 30 seconds, annealing at 53 °C for 30 seconds, extension at 72 °C for 5 minutes, and final extension at 72 °C for 5 minutes comprised the second cycle for the Sfa and IutA genes. Ethidium bromide electrophoresis was performed on a 1.5% agarose gel, and the results were viewed using a 100-1000 bp ladder and a UV transilluminator.
Table (1):
Primer sequence and amplicon sizes
| Primer name | Primer sequence | Product size(bp) | Reference | |
|---|---|---|---|---|
| FimH | FimH-F | GTGCCAATTCCTCTTACCGTT | 164 | Moubayed et al.14 |
| FimH-R | TGGAATAATCGTACCGTTGCG | |||
| Sfa | Sfa-F | CCGTAAAGATGTCTGCGAG | 100 | Elkenany et al.15 |
| Sfa-R | AGCAAGTCTGGCAACGAG | |||
| IutA | IutA-F | ATGAGCATATCTCCGGACG | 587 | Deku et al.16 |
| IutA-R | CAGGTCGAAGAACATCTGG | |||
| Cnf3 | Cnf3-F | TAACGTAATTAGCAAAGA | 757 | Onlen et al.17 |
| Cnf3-R | GTCTTCATTACTTACAGT | |||
Statistical analysis
SPSS version 26 was used to analyze the data on an IBM-compatible computer. Two different types of statistics were employed. For qualitative data, descriptive statistics were shown as numbers and percentages, and for quantitative data, as mean and standard deviation (SD). Analytical Data: Using the chi-squared test (Χ2), the relationship between two qualitative variables was assessed. Using the Z test, two proportions were compared. Using the student t-test, the relationship between two quantitative variables was evaluated.
In this study, 100 E. coli strains were isolated from 312 urine samples taken from suspected cases of CA and HA-UTIs at Menoufia University Hospital. These were from 42% of CA-UTI patients and 58% of HA-UTI patients. With ages ranging from 8 to 76 years (mean age 35), 55% of the isolates were from females and 45% from males. Infections were most common in patients 60 years of age and older. Furthermore, 11% had renal insufficiency, 22% had diabetes, 12% had prostatic hypertrophy, and 42% had a history of antibiotic use as shown in Table 2.
Table (2):
Hundred E. coli isolates were distributed based on the clinical and demographic information of the patients under study
| Demographic data | Hospital-acquired UTI | Community-acquired UTI (n = 42) | Test of significance | P-value | |||
|---|---|---|---|---|---|---|---|
| Catheterized (n = 38) | Non catheterized (n = 20) | Total (n = 58) | |||||
| Age | ≤20 (n = 7) | 2 (28.6%) | 0 | 2 (100%) | 5 (71.4%) | Χ2 = 5.49 | 0.704 |
| 20-40 (n = 16) | 7 (43.8%) | 3 (18.8%) | 10 (62.5%) | 6 (37.5%) | |||
| 40-60 (n = 35) | 12 (35.3%) | 7 (20.6%) | 19 (55.9%) | 16 (44.2%) | |||
| ≥60 (n = 42) | 17 (40.5%) | 10 (23.8%) | 27 (64.3%) | 15 (35.7%) | |||
| Gender | Male (n = 45) | 22 (48.9%) | 13 (26.6%) | 35 (75.6%) | 11 (24.4%) | Χ2 = 11.44 | 0.001* |
| Female (n = 55) | 15 (27.3%) | 8 (14.5%) | 23 (42.8%) | 32 (58.2%) | |||
| Patient risk factor | No risk factor | – | – | – | 4 (4%) | Χ2 = 139 | 0.038* |
| Catheter | 10 (10%) | – | 10 (10%) | – | 0.004* | ||
| Immuno-compromised | 8 (8%) | – | 8 (8%) | – | 0.012* | ||
| DM | 5 (5%) | 5 (5%) | 10 (10%) | 12 (12%) | 0.008* | ||
| Renal insufficiency | 6 (6%) | – | 6 (6%) | 5 (5%) | 0.802 | ||
| Surgery | 5 (5%) | – | 5 (5%) | – | 0.031* | ||
| Menopause | – | – | – | 5 (5%) | 0.006* | ||
| Prostatic | – | 6 (6%) | 6 (6%) | 6 (6%) | 0.548 | ||
| hypertrophy | – | – | – | 8 (8%) | <0.001* | ||
| Pregnancy | – | 9 (9%) | 9 (9%) | – | <0.001* | ||
| Renal stone | 3 (3%) | – | 3 (3%) | 3 (3%) | 0.681 | ||
| Antibiotic regimen the patient on | Yes (n = 42) | 22 (52.4%) | 6 (14.3%) | 28 (66.7%) | 14 (33.3%) | Χ2 = 2.76 | 0.097 |
| No (n = 58) | 15 (25.9%) | 14 (24.1%) | 29 (50%) | 29 (50.0%) | |||
*: Statistically significant, FE: Fisher exact test, Χ2: Chi-squared test
In order to identify MDR UPEC, antibiotic susceptibility is tested using the disk diffusion method. The majority of uropathogenic Escherichia coli isolates displayed sensitivity to fosfomycin and nitrofurantoin representing 99% and 95% respectively, followed by doxycycline 84%, meropenem 72% and imipenem 63%. Ampicillin and pipracillin were the least effective antibiotics. Amoxicillin/clavulanate, Levofloxacin and Ceftriaxone were also found to be only effective in lesser than 50% of cases.
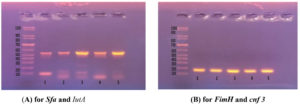
Comparing between uropathogenic E. coli isolates that cause HA and that causing CA-UTI regarding biofilm production, antibiotic resistance and gene production. Regarding biofilm production, about 89.5% (34/38) and 80% (16/20)of both catheterized and non-catheterized HA uropathogenic E. coli respectively were biofilm producer this was in contrast to 66.7% (28/42) of CA isolates which were biofilm producer with significant statistical difference (P <0.05).Regarding antibiotic resistance, 44.7% (17/38), 45% (9/20) and 33.3% (14/20) of catheterized, non-catheterized HA and CA uropathogenic E. coli which were MDR isolates. On the other hand, distribution of virulence among uropathogenic E. coli isolates showed that 96% (48/50), 24% (12/50) and 68% (34/50) were FimH, Sfa and Iut A genes producer respectively (Table 3, Figure 1 and Figure 2).
Table (3):
Comparison on biofilm production, antibiotic resistance and gene production between uropathogenic E. coli among hospital and community-acquired UTI
| Variable | Total | Hospital-acquired UTI | Community-acquired UTI | Test of significance (n = 42) | P-value | ||
|---|---|---|---|---|---|---|---|
| Catheterized (n = 38) | Non catheterized (n = 20) | Total (n = 58) | |||||
| Biofilm producing E. coli | |||||||
| Yes | 34 (89.5%) | 16 (80%) | 50 (86.3%) | 28 (66.7%) | Χ2 = 5.42 | 0.019* | |
| No | 4 (10.5%) | 4 (20%) | 8 (13.7%) | 14 (33.3%) | |||
| Antibiotic resistance | |||||||
| Non MDR/XDR | 58 | 19 (50%) | 11 (55%) | 30 (51.7) | 28 (66.7%) | Χ2 = 3.19 | 0.202 |
| 40 | 17 (44.7%) | 9 (45%) | 26 (44.8) | 14 (33.3%) | |||
| 2 | 2 (5.3%) | 0 | 2 (3.5) | 0 | |||
| Gene (28 hospital +22 community) = 50 | |||||||
| FimH +ve (n = 48) | (96%) | 18 (36 %) | 10 (20 %) | 28 (56%) | 20 (40%) | FE = 2.65 | 0.103 |
| -ve (n = 2) | (4%) | – | – | – | 2 | ||
| Sfa +ve (n = 12) | (24%) | 5 (10 %) | 3 (6%) | 8 (16%) | 4 (8%) | FE = 0.73 | 0.393 |
| -ve (n = 38) | (76%) | 13 (26 %) | 7 (14%) | 20 (40%) | 18 (36%) | ||
| IutA +ve (n = 34) | (68%) | 15 (30 %) | 8 (16 %) | 23 (46%) | 11 (22%) | Χ2 = 5.85 | 0.016* |
| -ve (n = 16) | (32%) | 3 (6 %) | 2 (4 %) | 5 (10%) | 11 (22%) | ||
| Cnf3 +ve-ve(n = 50) | (100%) | -18 (36%) | -10 (20%) | -28 (56%) | -22 (44%) | – | – |
Figure 1. Crystal violet In a 96-well microtiter plate with a flat bottom, adsorbed Biofilm to detect the formation of biofilms from isolated uropathogenic Escherichia coli
Figure 2. Agarose gel electrophoresis for amplified products of FimH, Sfa and IutA; (A). Multiplex PCR-amplified products of E. coli isolates. Lane M (ladder): DNA molecular size marker (100-1000 bp). Lane 1,4 and 5 were positive for Sfa (bp 100) and IutA (bp 587). Lane 2 and 3 were positive only for IutA (bp 587); (B). Multiplex PCR-amplified products of E. coli isolates. Lane 1,2,3,4 and 5 were positive for FimH (bp164). Lane 1,2,3,4 and 5 were negative for Cnf 3 (bp 757)
The concentration of 200 µg/ml of gold nanoparticles had the highest anti-biofilm effect (20.42%), followed by concentrations of 100, 50, 25, and 12.5 µg/ml, which were able to remove the UPEC biofilm from the plate surface by 16.95%, 15.98%, 9.43%, and 7.26%, respectively (Table 4 and Figure 3).
Table (4):
Biofilm inhibition percentage with gold nanoparticles
| Concen. (µg/ml) | Hospital-acquired UTI (n = 50) | Community-acquired UTI (n = 28) | Test of significance | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Catheterized (n = 34) | Non catheterized (n = 16) | Test of significance | P-value | Total | ||||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||
| 200 | 20.42 ± 8.64 | 21.57 ± 8.79 | t = 0.43 | 0.663 | 20.79 ± 8.61 | 20.42 ± 8.13 | t = 0.18 | 0.853 |
| 100 | 16.95 ± 7.78 | 18.5 ± 7.1 | t = 0.74 | 0.461 | 17.45 ± 6.85 | 18.66 ± 6.85 | t = 0.74 | 0.457 |
| 50 | 15.98 ± 6.08 | 17.12 ± 6.65 | t = 0.59 | 0.55 | 16.35 ± 6.22 | 16.60 ± 6.63 | t = 0.17 | 0.866 |
| 25 | 9.43 ± 4.51 | 11.12 ± 4.71 | t = 1.19 | 0.239 | 9.95 ± 4.59 | 10.37 ± 5.50 | t = 0.36 | 0.718 |
| 12.5 | 7.26 ± 3.39 | 9.77 ± 5.1 | t = 1.78 | 0.089 | 8.06 ± 4.14 | 8.45 ± 4.50 | t = 0.38 | 0.704 |
t: Student t test
Figure 3. Antibiofilm effect of gold nanoparticle using different concentrations and its effect on biofilm forming uropathogenic E. coli
200 µg/ml of silver nanoparticles had the strongest anti-biofilm effect (66.81%), followed by concentrations of 100, 50, 25, and 12.5 µg/ml, which were able to remove the UPEC biofilm from the plate surface by 66.51%, 56.52%, 42.3%, and 15.48%, respectively (Table 5 and Figure 4).
Table (5):
Biofilm inhibition percentage with silver nanoparticles
| Concen. (µg/ml) | Hospital-acquired UTI | Community-acquired UTI (n = 28) Mean ± SD | Test of significance | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Catheterized (n = 34) Mean ± SD | Non catheterized (n = 16) Mean ± SD | Test of significance | P-value | Total (n = 50) Mean ± SD | ||||
| 200 | 66.81 ± 5.43 | 66.73 ± 6.28 | t = 0.04 | 0.965 | 66.79 ± 5.65 | 66.72 ± 3.80 | t = 0.050 | 0.958 |
| 100 | 66.51 ± 5.44 | 66.66 ± 6.6 | t = 0.8 | 0.933 | 66.56 ± 5.77 | 66.33 ± 4.16 | t = 0.20 | 0.840 |
| 50 | 56.52 ± 3.63 | 56.9 ± 3.54 | t = 0.35 | 0.726 | 56.64 ± 3.57 | 55.79 ± 3.89 | t = 0.97 | 0.331 |
| 25 | 42.3 ± 2.58 | 34.79 ± 4.34 | t = 7.66 | <0.001* | 39.91 ± 4.77 | 42.37 ± 2.69 | t = 2.92 | 0.005* |
| 12.5 | 15.48 ± 4.68 | 15.85 ± 4.75 | t = 0.25 | 0.80 | 15.60 ± 4.66 | 16.28 ± 4.71 | t = 0.61 | 0.541 |
*: Statistically significant, t: Student t test
Figure 4. Antibiofilm effect of silver nanoparticle using different concentrations and its effect on biofilm forming uropathogenic E. coli
By comparison of the inhibition percentage of biofilm development of gold and silver nanoparticles. Results showed 20.66% versus 66.76%, 17.88 % vs 66.48%, 16.44% vs 56.33% and 10.10% vs 40.79% with concentration of 200 µg/ml which is recorded maximum anti biofilm effect for both followed by concentrations of 100, 50, and 25 µg/ml respectively. There was highly statistical difference between silver nanoparticles and gold nanoparticle regarding antibiofilm effect on uropathogenic E. coli at all concentrations (200 µg/ml, 100 µg/ml, 50 µg/ml, 25 µg/ml and 12.5 µg/ml) (p <0.001) (Table 6).
Table (6):
Comparative study between gold nanoparticle and silver nanoparticle on biofilm formation by uropathogenic E. coli
Concen. |
Silver nanoparticle |
Gold nanoparticle |
Test of significance |
P-value |
|---|---|---|---|---|
200 |
66.76 ± 5.03 |
20.66 ± 8.39 |
t = 48.17 |
|
100 |
66.48 ± 5.22 |
17.88 ± 6.83 |
t = 62.79 |
|
50 |
56.33 ± 3.69 |
16.44 ± 6.33 |
t = 55.62 |
|
25 |
40.79 ± 4.29 |
10.10 ± 4.91 |
t = 54.78 |
|
12.5 |
15.84 ± 4.66 |
8.20 ± 4.24 |
t = 15.86 |
*: Statistically significant, t: Student t test
The multidrug-resistant uropathogenic bacteria cause the spread of UTIs, and hence public health is greatly undermined by it. In order to know these viruses’ virulence and resistance properties, their virulence genetic profiles have to be determined.18 The research aims to discover the genetic factors for virulence of MDR uropathogens. To fight against such resistant strains, it also explores the potential of nanoparticles to serve as an alternate drug of therapy.
This study included 312 patients who were suspected of having a UTI. Urine samples were used to isolate one hundred strains of E. coli. 42 (42%) of these isolates were from CA-UTI patients, and 58 (58%) were from HA-UTI patients. Mancini et al.19 had different findings and discovered that (66.3%) for community-acquired UTIs and (33.7%) for hospital-acquired UTIs. Our study also showed that Fifty-five isolates (55%) were isolated from female and (45%) from male patients. Which was similar to Mancini et al.,19 at this point as (59.7%) of urine samples were from women and (40.0%) were from men the same finding was conducted by Prakash et al.20 This can be explained by the fact that urethra of woman is smaller in length than urethra of man, which gives bacteria easier access to the bladder. Additionally, the urethral opening is located near anus and vagina which are sources of bacteria. Among the study participants with an E. coli infection, the age of ≥60 years was the most frequently infected age this was similar to Doua et al.21 This can be explained by that age-related risk factors such as malnourishment, poorly managed diabetes mellitus, constipation, vaginal atrophy, prostate hyperplasia, prolonged hospital stays, urine retention or incontinence because of poor bladder control, unsanitary living conditions, and altered mental state.22 Regarding patients risk factors, 42 (42%), 22(22%), 12 (12%) and 11 (11%) had a history of antibiotics intake, DM, prostatic hypertrophy and renal insufficiency, respectively. While other research by Campos et al.23 observed that neurologic and neoplastic diseases followed by diabetes, were the most prevalent causes of UTI risk. In our study, comparison between uropathogenic E. coli isolates that caused HA and those that caused CA-UTI regarding biofilm production, antibiotic resistance and gene production was done. Biofilm production was about 89.5% (34/38) and 80% (16/20) of both catheterized and non-catheterized hospital-acquired uropathogenic E. coli respectively, this was in contrast to 66.7% (28/42) of community-acquired isolate which were biofilm producer with significant statistical difference (P <0.05). Because it gives the microorganisms a survival advantage, biofilm is extremely common on urinary catheters and is also challenging to remove.
Our results aligned with those of Alshaikh et al.24 in Egypt, who found that only 8% of UPEC isolates from patients with HA-UTI did not form biofilms. Similar findings were reported by Karigoudar et al.25 in India, who found that biofilms were produced by 89.7% of UPEC isolates from catheterized patients. They discovered that, in contrast to the 80% biofilm formation seen in non-catheterized patients in our study, only 49% of isolates from non-catheterized patients generated biofilms. However, Naziri et al.26 in Iran reported that 99% of UPEC isolates from both inpatients and outpatients demonstrated in vitro biofilm formation. Variations in patient or regional factors, or methodological variances, may be the cause of this disparity.
In this study, 44.7% (17/38), 45% (9/20), and 33.3% (14/20) of catheterized, non-catheterized HA, and CA uropathogenic E. coli isolates were identified as multidrug-resistant (MDR) being resistant to at least one antibiotic from three or more antimicrobial classes, which aligns with the findings of Radera et al.27 and Solyman et al.28 These studies also indicated that HA isolates tend to be more resistant than CA isolates, similar trend seen in numerous earlier investigations.29,30 However, Ramirez et al.31 observed even higher resistance, with over half of the strains in both hospital- and community-acquired infections exhibiting multidrug resistance (60.9% and 64.7%, respectively). Similarly, El-Baz et al.32 found MDR rates of 68% in inpatients and 61% in outpatients. These higher rates could be attributed to local factors and excessive antibiotic use in Egypt, which has contributed to the rise of MDR E. coli strains. In our study, the distribution of virulence genes among HA and CA E. coli isolates was as follows., 96% (48/50) of the isolates produced the FimH gene, 24% (12/50) produced the Sfa gene, and 68% (34/50) produced the IutA gene. Notably, the Cnf3 gene was not detected in any isolates similarly Katongole et al.33 reported that FimH was the most prevalent Urovirulence gene followed by Pap (21%), Sfa (13%), Afa (8%) and Cnf (5.5%). Our results indicated that these virulence genes were more prevalent among HA isolates compared to CA isolates. Specifically, 56% (28/50) of FimH positive E. coli were HA isolates, while 40% (20/50) were CA isolates. 16% (8/50) of Sfa positive E. coli were HA isolates and 8% (4/50) were CA isolates. Additionally, 46% (23/50) of Iut A positive E. coli were HA isolates compared to 22% (11/50) were CA isolates. Our findings were nearly similar to previous studies conducted by Hasanli et al.,34 who discovered that the fimH gene had the highest detection rate among the virulence genes (92%), followed by the IutA gene (91.3%) and the Sfa gene (20%). In a similar vein, numerous earlier studies.35,36
Understanding the mechanisms of biofilm formation is critical to the development of new anti-infective strategies.37 Over the past few years, a variety of approaches have been explored, and nanotechnology has emerged as a very promising tool for managing biofilms.38 Nanoparticles, owing to their very small size can penetrate more easily into the dense EPS matrix and directly interact with bacterial cells. They exhibit several modes of action, such as disrupting bacterial membranes, generating reactive oxygen species (ROS), inhibiting bacterial communication (quorum sensing), and may be used as carriers for targeted delivery of antimicrobial agents. These multi-mode actions reduce the chances of bacterial resistance. Various nanoparticles like metal and metal oxide nanoparticles (e.g., silver, zinc oxide, and titanium dioxide), polymeric nanoparticles, and lipid-based systems have come forth with promising potential in the prevention and eradication of biofilms.39
Using a microtiter plate assay with different concentrations of gold and silver nanoparticles, the study examined the inhibition of biofilm formation in UPEC. Inhibition percentages were used to compare the effects of gold and silver nanoparticles on biofilm development, and the anti-biofilm effect was dose-dependent. 20.66% versus 66.76%, 17.88 % vs 66.48%, 16.44% vs 56.33% and 10.10% vs 40.79 % with concentration of 200 µg/ml which is recorded maximum anti biofilm effect for both followed by concentrations of 100, 50, and 25 µg/ml, respectively.
In agreement with our finding, Mendez et al.40 demonstrated that Chitosan-Coated Silver Nanoparticles strongly suppressed biofilm formation in UPEC clinical isolates and the antibiofilm activity was concentration-dependent, with significant reductions at concentrations as low as 12.5 µg/ml. Similarly, A research by Fahmy et al.41 compared silver and selenium nanoparticles’ antibiofilm activity and thier results showed significant inhibition of biofilm formation, where AgNPs inhibited up to 94.36% at 15.6 µg/ml, highlighting the potent antibiofilm activity of silver nanoparticles. Earlier studies have determined that AgNPs (silver nanoparticles) can inhibit 60%-80% of E. coli biofilms at dosing concentrations ranging from 0.5 to 64 µg/ml.42,43 Water channels of the biofilm play a crucial role in this function as they facilitate AgNPs to diffuse and exhibit antibacterial activity. The channels, normally employed for the transport of nutrients, are disrupted by AgNPs, and they disrupt the development of the biofilm. Besides, AgNPs have been believed to inactivate sticky molecules required to construct biofilm, thereby disrupting the capacity of the bacteria to quorum-sense.44
In our recent study, there was highly statistical difference between silver nanoparticles and gold nanoparticle regarding antibiofilm effect on uropathogenic E. coli at all concentrations (200 µg/ml, 100 µg/ml, 50 µg/ml, 25 µg/ml and 12.5 µg/ml) (p <0.001) our result were similar to Singh et al.,45 who showed that, at varying concentrations, both kinds of nanoparticles prevented P. aeruginosa and E. coli from developing biofilms. They also demonstrated that AgNPs significantly reduced the formation of biofilms in both E. coli and P. aeruginosa compared to AuNPs. Additionally, Kang et al.46 found that gold nanoparticles exhibited biofilm inhibition effects against Pseudomonas aeruginosa and Staphylococcus aureus. However, the inhibition was less pronounced compared to silver nanoparticles, with maximum inhibition observed at higher concentrations, suggesting a lower efficacy of gold nanoparticles in biofilm inhibition.
In contrast to our result, Soliman et al.47 conducted study on P. aeruginosa and Staphylococcus aureus, they found that nanoparticles (Ag-NPs) exhibited a modest inhibitory effect on Pseudomonas aeruginosa and Staphylococcus aureus. On the other hand, when applied at concentrations lower than the minimum inhibitory concentration (MIC), gold nanoparticles (Au-NPs) showed a noticeably powerful effect against the biofilm formation of both Pseudomonas aeruginosa and Staphylococcus aureus, without affecting bacterial growth.
FimH, Sfa, and IutA were more prevalent in hospital-acquired isolates than in community isolates. Silver nanoparticles are very good at preventing biofilms from forming in MDR UPEC, which increases the efficiency of antibiotic therapy. Adding silver nanoparticles to medical device materials may have a bactericidal effect on pre-existing biofilms and aid in inhibiting bacterial adhesion, colonization, and biofilm formation. Further research and innovation are required to turn this into a workable preventive and therapeutic solution.
ACKNOWLEDGMENTS
The authors would like to express their deep gratitude to the clinical staff and fellow interns for their invaluable efforts in maintaining bacterial strains. Their commitment and careful attention to detail were crucial in completing our experiments successfully. This collaboration not only propelled our research forward but also exemplified the teamwork and dedication essential in scientific pursuits.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SAMA and RGM conceptualized the study. SAMA, RGM, KS, MSS and AFL applied methodology. AET performed experiments and investigated the data. SAMA, RGM performed formal analysis. SAMA performed visualization. SAMA, RGM, KS and MSS performed supervision. SAMA wrote the original draft. KS, RGM and MSS wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Committee of Ethics, Faculty of Medicine, Menoufia University, Egypt, vide protocol no. 9/2022MICRO23.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Zhu H, Chen Y, Hang Y, et al. Impact of inappropriate empirical antibiotic treatment on clinical outcomes of urinary tract infections caused by Escherichia coli: a retrospective cohort study. J Glob Antimicrob Resist. 2021;26:148-153.
Crossref - Bonten M, Johnson DR, van dan Biggelaar AHJ, et al. Epidemiology of Escherichia coli Bacteremia: A Systematic Literature Review. Clin Infect Dis. 2021;72(7):1211-1219.
Crossref - Reham R, Nesrene O, Mohamed D, Dalia M. Bacterial biofilm dependent catheter associated urinary tract infections: Characterization, antibiotic resistance pattern and risk factors. Egypt J Basic Appl Sci. 2021;8(1):64-74.
Crossref - Mathur T, Singhal S, Khan S, Upadhyay DJ, Fatma T, Rattan A. Detection of biofilm formation among the clinical isolates of staphylococci: an evaluation of three different screening methods. Indian J Med Microbiol. 2006;24(1):25-29.
Crossref - Sharma S, Mohler J, Mahajan SD, Schwartz SA, Bruggemann L, Aalinkeel R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms. 2023;11(6):1614.
Crossref - Bunduki GK, Heinz E, Phiri VS, Noah P, Feasey N, Musaya J. Virulence factors and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from urinary tract infections: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):753.
Crossref - Thambirajoo M, Maarof M, Lokanathan Y, et al. Potential of Nanoparticles Integrated with Antibacterial Properties in Preventing Biofilm and Antibiotic Resistance. Antibiotics. 2021;10(11):1338.
Crossref - Ruiz YPM, de Almeida CLA, Agreles MAA, Galembeck A, Cavalcanti IMF. Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance. Antibiotics. 2023;12(1):104.
Crossref - Clinical and Laboratory Standards Institute (CLSI): Performance standards for antimicrobial susceptibility testing. 2023 33rd Ed. CLSI supplement M100. Wayne, PA.
- Mohamed SAA, Al-Ahmadey ZZ. Biofilm formation and antifungal susceptibility of Candida isolates from various clinical specimens. 2013;3(4):590-601.
Crossref - Harika K, Shenoy VP, Narasimhaswamy N, Chawla K. Detection of Biofilm Production and Its Impact on Antibiotic Resistance Profile of Bacterial Isolates from Chronic Wound Infections. J Glob Infect Dis. 2020;12(3):129-134.
Crossref - Rahim KAA, Mohamed AMA. Bactericidal and antibiotic synergistic effect of nanosilver against methicillin resistant Staphylococcus aureus. Jundishapur J Microbiol. 2015;8(11):e25867.
Crossref - Elbehairy EN, El Nagdy MM, Weefky GF, Nabiel Y. Effect of Silver Nanoparticles on Biofilm Producing Multi Drug Resistant Uro-pathogenic E. coli isolated from Catheterized Patients in Mansoura University Hospital. Egypt J Med Microbiol. 2022;31(1):63-68.
Crossref - Moubayed S, Ghazzawi J, Mitri R, Khalife S. Recent Data Characterizing the Prevalence and Resistance Patterns of FimH-producing Uropathogenic Escherichia coli Isolated from Patients with Urinary Tract Infections in North Lebanon. Arch Clin Infect Dis. 2023;18(4):135782.
Crossref - El refaey, Elkenany R, Younis G. Virulotyping and Antibiograms of Pathogenic Escherichia coli Isolated from Calves Suffering from Diarrhea. J Adv Vet Res. 2023;13(9):1901-1906.
- Deku JG, Duedu KO, Kinanyok S, Kpene GE, Feglo PK. Phylogenicity and virulence profiles of clinical Escherichia coli isolates in the Ho teaching hospital of Ghana. BioMed Res Int. 2022;2022(1):1-8.
Crossref - Guneri CO, Koksal F, Kizilyildirim S, Bedir B, Nagiyev T. The distribution of cytotoxic necrotizing factors (CNF-1, CNF-2, CNF-3) and cytolethal distending toxins (CDT-1, CDT-2, CDT-3, CDT-4) in Escherichia coli isolates isolated from extraintestinal infections and the determination of their phylogenetic relationship by PFGE. Int J Clin Pract. 2022;2022(1):7200635.
Crossref - Khan A, Saraf VS, Siddiqui F, et al. Multidrug resistance among uropathogenic clonal group A E. coli isolates from Pakistani women with uncomplicated urinary tract infections. BMC Microbiol. 2024;24(1):74.
Crossref - Mancini A, Pucciarelli S, Lombardi FE, Barocci S, Pauri P, Lodolini S. Differences between community-and hospital-acquired urinary tract infections in a tertiary care hospital. New Microbiol. 2020;43(1):17-21.
- Devanand P, Saxena RS. Prevalence and antimicrobial susceptibility pattern of Escherichia coli in hospital acquired and community acquired patients related to urinary tract infection in India. J Appl Pharm Sci. 2020;2013:3(8):124-132.
- Doua J, Geurtsen J, Rodriguez-Bano J, et al. Epidemiology, Clinical Features, and Antimicrobial Resistance of Invasive Escherichia Coli Disease in Patients Admitted in Tertiary Care Hospitals. Open Forum Infect Dis. 2023;10(2):ofad026.
Crossref - Dutta C, Pasha K, Paul S, et al. Urinary Tract Infection Induced Delirium in Elderly Patients: A Systematic Review. Cureus. 2022;14(12):e32321.
Crossref - Campos ACDC, Andrade N, Correal JC, et al. Resistance and virulence properties of extraintestinal pathogenic E. coli causing nosocomial-and community-acquired urinary tract infections in hospitalized patients in Rio de Janeiro, Brazil. 2020.
Crossref - Alshaikh SA, El-Banna T, Sonbol F, Farghali MH. Correlation between antimicrobial resistance, biofilm formation, and virulence determinants in uropathogenic Escherichia coli from Egyptian hospital. Ann Clin Microbiol Antimicrob. 2024;23(1):20.
Crossref - Karigoudar RM, Karigoudar MH, Wavare SM, Mangalgi SS. Detection of biofilm among uropathogenic Escherichia coli and its correlation with antibiotic resistance pattern. J Lab Physicians. 2019;11(1):17-22.
Crossref - Naziri Z, Kilegolan JA, Moezzi MS, Derakhshandeh A. Biofilm formation by uropathogenic Escherichia coli: a complicating factor for treatment and recurrence of urinary tract infections. J Hosp Infect. 2021;117:9-16.
Crossref - Radera S, Srivastava S, Agarwal J. Virulence Genotyping and Multidrug Resistance Pattern of Escherichia coli Isolated From Community-Acquired and Hospital-Acquired Urinary Tract Infections. Cureus. 2022;14(9):e29404.
Crossref - Solyman, Awatif A, et al. Comparative study between community acquired and hospital acquired UTI caused by E. coli. Egypt J Microbiol. 38.5781 (2017): 1-10.
- Neamati F, Firoozeh F, Saffari M, Zibaei M. Virulence Genes and Antimicrobial Resistance Pattern in Uropathogenic Escherichia coli Isolated From Hospitalized Patients in Kashan, Iran. Jundishapur J Microbiol. 2015;8(2):e17514.
Crossref - Matta R, Hallit S, Hallit R, Bawab W, Rogues AM, Salameh P. Epidemiology and microbiological profile comparison between community and hospital acquired infections: A multicenter retrospective study in Lebanon. J Infect Public Health. 2018;11(3):405-411.
Crossref - Castillo FYR, Barrera ALG, Harel J, et al. Biofilm Formation by Escherichia coli Isolated from Urinary Tract Infections from Aguascalientes, Mexico. Microorganisms. 2023;11(12):2858.
Crossref - El-Baz R, Said HS, Abdelmegeed ES, Barwa R. Characterization of virulence determinants and phylogenetic background of multiple and extensively drug resistant Escherichia coli isolated from different clinical sources in Egypt. Appl Microbiol Biotechnol. 2022;106(3):1279-1298.
Crossref - Katongole P, Nalubega F, Florence NC, Asiimwe B, Andia I. Biofilm formation, antimicrobial susceptibility and virulence genes of Uropathogenic Escherichia coli isolated from clinical isolates in Uganda. BMC Infect Dis. 2020;20(1):1-6.
Crossref - Hasanli L, Dagi HT, Arslan U. Investigation of antibiotic susceptibility and virulence genes in Escherichia coli strains isolated from blood and urine samples. Journal of Pediatric Infectious Diseases. 2022;17(11):098-105.
Crossref - Dadi BR, Abebe T, Zhang L, Mihret A, Abebe W, Amogne W. Distribution of virulence genes and phylogenetics of uropathogenic Escherichia coli among urinary tract infection patients in Addis Ababa, Ethiopia. BMC Infect Dis. 2020;20(1):108.
Crossref - Fonseca-Martinez SA, Martinez-Vega RA, Farfan-Garcia AE, Gonzalez Rugeles CI, Criado-Guerrero LY. Association Between Uropathogenic Escherichia coli Virulence Genes and Severity of Infection and Resistance to Antibiotics. Infect Drug Resist. 2023;16:3707-3718.
Crossref - Pompilio A, Crocetta V, Savini V, et al. Phylogenetic relationships, biofilm formation, motility, antibiotic resistance and extended virulence genotypes among Escherichia coli strains from women with community-onset primitive acute pyelonephritis. PLoS One. 2018;13(5):e0196260.
Crossref - Sahli C, Moya SE, Lomas JS, Gravier-Pelletier C, Briandet R, Hemadi M. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics. 2022;12(5):2383-2405.
Crossref - Purbowati R, Listyawati AF, Masfufatun, Tjandra L, Indahsari NK. Antibacterial and antibiofilm effect of silver and gold nanoparticles in Uropathogenic Escherichia coli. Berkala Penelitian Hayati. 2022;27(2):67-72.
Crossref - Mendez-Pfeiffer P, Ballesteros-Monrreal MG, et al. Chitosan-Coated Silver Nanoparticles Inhibit Adherence and Biofilm Formation of Uropathogenic Escherichia coli. ACS Infect Dis. 2024;10(4):1126-1136.
Crossref - Fahmy NF, Abdel-Kareem MM, Ahmed HA, Helmy MZ, Mahmoud EA. Evaluation of the antibacterial and antibiofilm effect of mycosynthesized silver and selenium nanoparticles and their synergistic effect with antibiotics on nosocomial bacteria. Microb Cell Fact. 2025;24(1):6.
Crossref - Barapatre A, Aadil KR, Jha H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Biosour Bioprocess. 2016;3:8(3).
Crossref - Akkther T, Ranjani S, Hemlatha S. Nanoparticles engineered from endophytic fungi (Botryosphaeria rhodina) against ESBL producing pathogenic multidrug resistant E. coli. Environ Sci Eur. 2021;33(1):83.
Crossref - Kalishwaralal K, Barathmaniknath S, Pandian SRK, Deepak V, Gurunathan S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf B Biointerfaces. 2010;79(2):340-344.
Crossref - Singh P, Pandit S, Beshay M, et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif Cells Nanomed Biotech. 2018;46(sup3):886-899.
Crossref - Kang MG, Khan F, Tabassum N, Cho KJ, Jo DM, Kim YM. Inhibition of Biofilm and Virulence Properties of Pathogenic Bacteria by Silver and Gold Nanoparticles Synthesized from Lactiplantibacillus sp. Strain C1. ACS Omega. 2023;8(11):9873-9888.
Crossref - Soliman MKV, Salem SS, Abu-Elghait M, Azab MS. Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Appl Biochem Biotechnol. 2023;195(2):1158-1183.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.