Antibiotic resistance is expeditiously reducing the effectiveness of standard therapies, playing a significant role in the increase in drug-resistant microbes and causing a global health emergency. Increasing alarm calls for innovative and better safety features, a critical priority. Medicinal plants, specifically members of the Lamiaceae family, have gained attention due to the presence of diverse essential oils, flavonoids and phenolic acids which exhibit antioxidant, and anti-inflammatory activity and antibacterial potential. The antibacterial properties of Lamiaceae species are reviewed in this paper, with particular attention paid to their bioactive components, modes of action against bacterial pathogens, and synergistic effects with conventional antibiotics. These bioactive components work in a variety of ways, disrupting microbial membranes, inhibiting enzyme activity, and preventing the formation of biofilms. Thymol, carvacrol, and rosmarinic acid have been found to exhibit significant synergism with antibiotics like chloramphenicol, gentamicin, ciprofloxacin, and vancomycin, against multidrug-resistant pathogens like Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Salmonella spp. Although these results are encouraging, clinical verification and additional research are needed to maximize the therapeutic potential of Lamiaceae-based combination therapies in the battle against antibiotic resistance.
Alkaloids, Antibiotics, Antibacterial Activity, Essential Oils, Lamiaceae, Medicinal Plants, Phenolics, Phytochemicals, Combination Therapy
Conventional therapies for bacterial infections are in danger of failing due to the growing number of antibiotic-resistant organisms. A decline in mortality and morbidity rates has resulted in a sharp decline in therapeutic options for treating common illnesses caused by the rapid evolution of antibiotic-resistant strains, especially multidrug-resistant (MDR) bacteria.1 Several nations have implemented laws restricting or outlawing the use of synthetic antibiotics in an attempt to slow the emergence of resistant strains in response to the escalating public health emergency.2 Antibiotic-resistant bacteria, however, are still spreading at a startling rate in spite of these attempts. Initially, the development of drug-resistant bacteria was predominantly confined to hospital settings, where the frequent use of antibiotics created a breeding ground for resistant strains. But now these resistant strains are no longer restricted to hospitals and are found in diverse environments.3 The ability of resistant pathogens to spread across borders has made antibiotic resistance a global issue that requires international collaboration and innovative approaches to restrict the spread effectively. Besides, it is utmost needed to search for alternative antibacterial agents as the conventional antibiotics are losing their efficacy. This global issue has prompted researchers to explore the natural sources of antibacterial compounds.
Plants are among the best alternative with the least side effects.4 They have evolved over billions of years, developing effective defense mechanisms to survive hostile environments, including microbial threats. India is often referred to as the “botanical garden of the world” due to its wealth of over 2,200 identified species of medicinal and aromatic plants.5 The use of plants in treating diseases dates back to early human history, with traditional knowledge contributing significantly to their therapeutic applications, even when their chemical constituents was not fully understood. Phytotherapy, or plant-based treatment, is a traditional approach to manage the simple diseases, though it often lacks scientific validation. They can be a source of natural drugs.6 The medicinal plants, used for centuries in traditional medicine, offer a promising reservoir of bioactive compounds such as alkaloids, flavonoids, terpenoids and phenolic acids with potential antibacterial properties. The chemical constituents present in plants are a part of the physiological functions of the living flora and hence they are believed to have better compatibility with the human body. Numerous studies have already determined the efficacy of plant extracts and essential oils against drug-resistant bacteria, including multidrug-resistant strains of Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus.7-9 Various plant species have been employed to treat bacterial, viral, and fungal infections in traditional medicine systems across the world.10 Thus medicinal plants could serve as an alternative or complementary option to conventional antibiotics in the treatment of infectious diseases.
Among various known medicinal plants, the plants of Lamiaceae family are gaining importance due to their medicinal uses, culinary and ornamental effects.11 It is also called as mint family and is well known for their flowering nature, comprising 236 genera and about 7200 known species.12-14 The plants belonging Lamiaceae have long been utilized in Asian and other folk medicine due to the antibacterial properties thereby making them useful tools in the fight against antibiotic-resistant pathogens.15,16 The present review summarises the bioactive components of medicinal plants in the Lamiaceae family and focusing the mechanisms of action and potential to counteract microbial resistance. Besides challenges, limitations and future prospects will also be covered.
Bioactive compounds from Lamiaceae
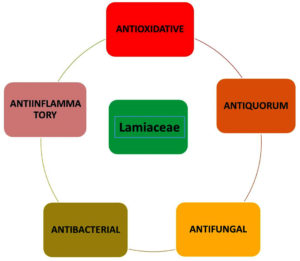
The Lamiaceae family represent a varied group of plants that are rich in bioactive compounds with great antioxidant, anti-inflammatory, antibacterial and therapeutic activities (Figure 1). Genus within the family Lamiaceae e.g. Mentha, Ocimum, Origanum, Rosmarinus and Thymus yield a range of secondary metabolites including alkaloids (which include flavonoids, tannins, and quinones) and terpenes that contribute to their pharmacological effects. Table provides an overview of some representative bioactive chemicals that are present in these taxa.
Table:
Overview of antibacterial activities of selected plants, their active compounds along corresponding mechanisms of action against pathogens (preferably bacteria)
Plant |
Part Used |
Compound |
Mechanism of Action |
Target Pathogens |
Ref. |
|---|---|---|---|---|---|
Ajuga reptans |
Flowers extract |
Bractic acid, Sphingolipids, Bractin |
Alters membrane; possible DNA interaction |
E. coli, L. monocytogenes, P. aeruginosa |
19 |
Hyssopus officinalis |
Essential oil, Leaf extract |
Thymol, β-pinene |
Membrane disruption and possible DNA damage |
E. coli, S. aureus |
77 |
Lavandula angustifolia Mill. |
Essential oil |
Linalool |
Membrane disruption and possible DNA damage |
S. hominis, S. haemolyticus, S. epidermidis, S. aureus, |
78 |
Mentha spp. |
Peppermint oil |
Menthol, Rosmarinic acid |
Disrupts bacterial membranes |
B. subtilis, E. coli, P. aeruginosa, S. marcescens, S. aureus and Vibrio spp. |
79 |
Melissa officinalis |
Essential oil |
Rosmarinic acid, Caffeic acid, Citronellal |
ROS generation, DNA damage, viral replication inhibition |
B. subtilis, P. aeruginosa, S. marcescens, V. parahaemolyticus |
80 |
Ocimum sanctum; Ocimum basilicum |
Leaves |
Eugenol |
Disrupts bacterial and membranes, Efflux pump inhibition |
E. coli, S. aureus |
81 |
Ocimum vulgare |
Essential oil |
Carvacrol, Thymol, p-Cymene, γ-Terpinene. |
Disrupts bacterial membranes, inhibits enzyme synthesis, antiquorum |
S. aureus, Candida spp. |
82,83 |
Perilla frutescens |
Seed, Stems, Leaves, Essential oil |
Apigenin, Caffeic acid Luteolin, Rosmarinic acid |
Interrupt amino acid, carbohydrate, lipid and Nucleotide Metabolism, Obstruct Biosynthesis of cell wall and disrupt the integrity of cell membrane |
S. pneumoniae and Salmonella spp. Aspergillus spp., F. solani, and R. solani |
84,85 |
Rosmarinus officinalis (rosemary) |
Essential oil and Methanolic extracts |
Camphor, 1,8-cineole, α-Pinene and Rosmarinic acid |
Increase in membrane permeability and leakage of cellular contents Modulates immune response and induce oxidative stress |
C. perfringens, M. leutus, S. sonnei, S. typhimurium, C. albicans |
86 |
Satureja hortensis |
Essential oil and Extracts |
Thymol, Carvacrol, p-Cymene, γ-Terpinene |
Disrupts cell membrane; inhibit quorum sensing |
Escherichia coli, C. albicans, Staphylococcus typhimurium |
87 |
Salvia officinalis |
Essential oil and Extracts |
Thujone, Camphor, Apigenin, Cineole |
DNA interaction; inhibits topoisomerase; oxidative stress |
E. faecalis and Staphylococcus spp. |
88 |
Alkaloids
A number of species in the family Lamiaceae are characterized by its scented plants yielding bioactive alkaloids that could be of therapeutic value e.g. Rosmarinus officinalis or rosemary comprises the alkaloid rosmarinine.17 Salvia officinalis, commonly known as sage, contain labiatines. Mentha species, such as Mentha piperita primarily feature menthol, which, while mainly classified as a terpenoid, also exhibits some alkaloid-like effects in certain forms.18 The Ajuga species have the alkaloid aconitine, which is linked with toxic effects, particularly at high concentrations.19 Ocimum basilum contains eugenol and linalool.20 Phenolic compounds includes flavones, flavanols, flavonoids, quinones, and tannins.21 One significant phenolic acid is rosmarinic acid, which is found in large amounts in Melissa officinalis (lemon balm) and Rosmarinus officinalis (rosemary) and many Salvia species (sage).22 Most of the phenolic acids in Salvia plants originate come from caffeic acid. Caffeic acid is crucial because it is precursor for the synthesis of more complex compound in these plants e.g. it combines with another compound to make rosmarinic acid, which is widespread in Lamiaceae plants.15 Carvacrol is very common in Origanum vulgare (oregano), Thymus capitatus (thyme) and Satureja thymbra (savory). These examples show that the Lamiaceae family is rich in valuable chemicals, and its phenolic acids are particularly significant for their health and healing properties. Flavonoids, mostly in conjugated forms, are known to exist in the genus Leucas. Baicalein, a free flavonoid in the ethereal fraction of a hydro-methanolic extract was reported from the flowers of Leucas aspera23 A large no of quinones are reported in Salvia officinalis24 and Thymus vulgaris.25 This bioactive compound works in conjunction with flavonoids and terpenoids and boosts the overall antibacterial action of plant extracts.26
Terpenes
Essential oils (EOs) are mostly composed of low molecular weight and volatile phytochemicals primarly terpenes such as monoterpenes, sesquiterpenes and their oxygenated derivatives, known as terpenoids. Besides, these also contain other chemical classes, such as alcohols, aldehydes, ketones, phenolics and esters.27 The concentration and type of these compounds decides the biological potential of these Eos. Terpenes are hydrocarbons that are made up of isoprene units (C5H8), while terpenoids are their oxygenated derivatives, often bearing functional groups such as alcohols, ketones, or aldehydes. The members of Lamiaceae family e.g. Mentha longifolia28 and Tetradenia riparia contain numerous monoterpenoids and sesquiterpenoids.29 The most common monoterpenes are α-Pinene and β-Pinene, 1,8-cineole, menthol, γ-terpinene, and limonene.30,31 Caryophyllene germacrene D, and spathulenol are the main sesquiterpenes. These compounds are often found in nature and are known for their fragrant properties. Some Lamiaceae species also produce higher terpenes, such as diterpenes and triterpenes, although these are not components of essential oils due to their non-volatile nature. The roots of Leucas aspera contains leucasperones A and B; leucasperols A and B, and diterpenes.32 Three diterpenes (Leucasdins A, B, and C) and two protostane-type triterpenes (Leucastrins A and B) were reported together with oleanolic acid from the roots of Leucas cephalotes.33
Mechanisms of antibacterial action of Lamiaceae
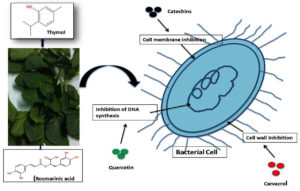
The antibacterial action of medicinal plant (Lamiaceae) is multifaceted, often involving multiple mechanisms (Table and Figure 2). However, the antibacterial action of Lamiaceae can be broadly categorized into three main mechanisms discussed below:
Cell membrane disruption
Phenolic compounds found abundantly in the Lamiaceae family exhibit a wide range of antibacterial mechanisms such as inhibit efflux pumps, alter membrane permeability and interact with enzymes. Phenolics especially flavones are particularly noted for their ability to disrupt microbial envelopes.34,35 Flavanols e.g. catechins tend to form complexes with microbial cell walls and inactivate key enzymes, likely through interactions with sulfhydryl groups or nonspecific protein binding.36 Flavonoids have the ability to complex with extracellular proteins as well as with bacterial cell walls, rendering them inactive.37 As noted by Cushnie et al., catechins can destroy the bacterial membrane, which results in the leakage of potassium in methicillin-resistant Staphylococcus aureus (MRSA). This is one of the earliest indications that the bacterial membrane is compromised.38 Research has indicated that catechins are less potent against Gram-negative bacteria since Gram-negative bacteria possess an outer membrane containing negatively charged LPS (lipopolysaccharides), which renders it more difficult for catechins to function.39 This is consistent with other studies indicating that catechins exert weaker antibacterial activity against Gram-negative bacteria than against Gram-positive bacteria.40 Quinones, present in O. basilicum,41 Salvia officinalis24,42 and Thymus vulgaris25 disrupt the integrity of microbial cell walls and membranes, inhibiting oxidative phosphorylation, and binding to nucleophilic amino acids in microbial proteins, which disrupts normal cellular functions. affecting the function of microbial proteins by forming complexes with them and producing persistent free radicals.43 Besides, quinones also inhibit virulence factors such as quorum sensing receptors and enzymes i.e. disrupting microbial communication thereby inhibiting biofilm formation.44 Tannins interfere with cellular metabolism, breaking down the oxidative phosphorylation mechanisms, and weaken the cellular oxidative phosphorylation. Furthermore, they interfere with the metabolism of microbial enzymes, intercalating with DNA to prevent transcription, protein synthesis and ultimately causing cell death.45 Carvacrol, menthol and thymol present in the essential oils of plants Origanum vulgare (Oregano), Metha piperita (peppermint) and Thymus vulgaris (Thyme)46 disrupt microbial cell membranes, leading to the cell lysis and death. These lipophilic compounds cause permeability in the cell wall by integrating into the lipid bilayer of cell surroundings causing leakage and cell death.47
Enzyme inhibition
Plants possess large number of compounds which can alter the growth several microbes by causing disturbance in their essential enzymatic activities, metabolic processes, availability of binding sites etc.48 Flavonoid compounds of Lamiaceae are also known for their inhibitory activity of protein synthesis in bacteria, i.e. Quercetin a type of flavonoid is responsible for the inactivation of ATP synthase resulting in the loss of energy for microbial cells.49 Terpenoids including carvacrols, thymols found in oregano are good inhibitors of ATPase impairing metabolisms in the cell for energy.50 Alkaloids causes an interference in the DNA gyrase activity and causes loss of DNA replication which results death of microbial cells.51
Antioxidant activity
Plant-derived antioxidants perform a vital part in neutralizing free radicals, thereby mitigating oxidative stress and enhancing the immune response against pathogenic challenges. Members of the Lamiaceae family, including Thymus vulgaris (thyme), Mentha spp. (peppermint), O. basilicum (basil), S. officinalis (sage), and Lavandula angustifolia (lavender), are well-documented sources of bioactive compounds with strong antioxidant properties.52 The antioxidants derived from plant extracts plays a role in neutralizing free radicals thereby reducing oxidative stress and enhancing the immune response against pathogens. Rosmarinic acid and its derivatives are one such example that is reported to be responsible for antioxidant activity.53 Carnosic acid, quercetin and luteolin are present abundantly in O. basilicum54 and Mentha spp.55 and exhibits antioxidant properties by neutralizing free radicals thereby protecting the lipid layers of cell from peroxidation. Similarly, the component of essential oils such as thymol, carvacrol, and menthol also exhibits oxidative damage and scavenge the free radicals and show anti-inflammatory properties.56 In a broad study, Fernandez et al. investigated seventy distinct Lamiaceae species and reported the significant antioxidant activity across many of them, as confirmed through DPPH assay results.53
Role of medicinal plants (Lamiaceae) in combating antibiotic resistance
The aqueous leaf extract and essential oils of O. sanctum disrupts cell membrane and interferes with the cellular enzymes of inhibits some of the pathogenic bacteria e.g. Candida albicans, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Mycobacterium tuberculosis.57 The rich content of carvacrol, eugenol, linalool, ursolic acid and other phenolics are responsible for exhibiting antibacterial activities in O. sanctum.58 Compounds such as menthol and rosmarinic acid played a vital role in the inhibition of infectious organisms.52 The essentials oil of rosemary can inhibit the growth of B. subtilis, E. coli, P. vulgaris, P. aeruginosa, S. aureus and S. epidermidis.59 The essential oil of Mentha exhibited strongest antibacterial activity against multidrug-resistant bacteria strains both in vivo and in vitro.60 The antibacterial activity of O. sanctum was reported against multidrug-resistant bacterial strains i.e. Enterococcus spp., Pseudomonas spp., Staphylococcus spp.61 The plants of Lamiaceae e.g. carvacrol and thymol of O. vulgare have the potential of inhibiting the growth and development of biofilms formed by C. albicans.62 The essential oil from oregano was highly effective against drug-resistant microbes and used as disinfectant.63 T. vulgaris and L. angustifolia are the common European Mediterranean plants used in the treatment of many infections and general care of wounds.64 The leaf extracts of R. officinalis inhibit the biofilm formation by P. aeruginosa. The ethanolic extracts of the flowers of L. aspera performed better in terms of antibacterial activity than the aqueous extract against B. subtilis and E. coli.65
Synergy between medicinal Lamiaceae and traditional antibiotics
Synergy is basically reported when the combinational therapy of drugs works more efficiently than the sum of individual drugs.66 The combinational use of species of Lamiaceae and antibiotics is reported to have less resistance in comparison to use of antibiotics alone.9,67 The combination of the leaf extract of O. sanctum (Lamiaceae) with chlorophenicol and trimethoprim actively killed Salmonella enterica serovar typhi.68 The essential oil of Ocimum sanctum Linn. with Gentamycin or streptomycin killed E. coli and Salmonella typhi.69 The combination of thymol and carvacrol with penicillin and ciprofloxacin showed activity against S. aureus and P. aeruginosa.70,71 The combination of oregano oil, vancomycin and ampicillin was effective against methicillin-resistant S. aureus. Rosmarinic acid reduced the resistance to ciprofloxacin and tetracycline against E. coli and B. cereus.72,73 The synergistic effects of S. officinalis (sage) and Cichorium intybus (chicory) extracts in combination with antibiotics (amoxicillin and chloramphenicol) showed activity against several bacterial strains. Combinations of amoxicillin or chloramphenicol with sage extracts (acetone or ethyl acetate) exhibited synergistic effects, reducing antibiotic MIC values by 2- to 10-fold, except against E. coli.74 The essential oils (EOs) from four Calamintha species exhibited selective action against Staphylococcus aureus when combined with antibiotics (gentamicin or ciprofloxacin).75 The essential oils (EOs) from L. angustifolia, R. officinalis, M. piperita and T. vulgaris both individually as well in combination with antibiotics were used against four pathogenic bacteria B. cereus, E. coli, P. aeruginosa and S. aureus and resulted in synergistic effects offering potential applications in the medical industry.17 The essential oils (EOs) from four Calamintha species exhibited selective action against S. aureus when combined with antibiotics (gentamicin or ciprofloxacin).75 The antibacterial effectiveness of four plant-derived compounds thymol, gallic acid, gentisic and salicylic acid were evaluated against fourteen pathogenic bacteria. Thymol showed the maximum activity, with minimum inhibitory concentrations (MICs) ranging from 125 to 250 µg/mL for various bacterial species.76 Additionally, thymol was tested in combination with eight antibiotics, revealing synergistic effects with chloramphenicol against A. baumannii; gentamicin and streptomycin against S. aureus and streptomycin against S. agalactiae thereby reducing the antibiotic MICs by 75%-87.5%. The combination was bactericidal, with thymol enhancing the antibiotic’s effectiveness by inhibiting bacterial growth.76 Hence, the synergistic effect of bioactive compounds from Lamiaceae with antibiotics holds a great effect against different multidrug-resistant bacteria.
Challenges and future prospects
The plants belonging to Lamiaceae family has attracted a lot of attention due to its efficiency as antibacterial agents. However, it has a few challenges also. Perhaps the most important limitation is phytochemical heterogeneity, which occurs due to genetic variability, environmental factors, geographical differences and results in the erratic bioactivity.16 Additionally, very few studies examine the safety profiles and toxicity of these plant components in terms of dosage and extended exposure. The excessive dependency on in vitro experiments also diminishes their appreciation of their real therapeutic significance in physiological and clinical settings. Lack of standardized methods for extraction and formulation, as well as a lack of adequate in vivo and clinical studies, continues to restrict their translational value. It is necessary to overcome these shortcomings to fully unlock the pharmacological potential of the Lamiaceae family. Novel biotechnological techniques such as genetic manipulation, optimization of metabolic pathways and tissue culture of plants must be included. Omics based techniques must to be explored to have an improved understanding of the biosynthetic processes and regulatory patterns underlying antibacterial metabolite biosynthesis. Widespread exploration of various genera and species of Lamiaceae family is necessary to reveal the immense and relatively untapped source of bioactive compounds concealed within this vast and diversified plant family.
Lamiaceae or the mint family is renowned for its bioactive constituents of diverse variability including essential oils, flavonoids, terpenoids, and phenolic acids. Bioactive components present in them have antibacterial, antioxidant, anti-inflammatory, and therapeutic effects through diverse mechanisms including distort cell membrane of microbes, impede enzymatic activity, disturbs the biosynthesis of nucleic acid, quorum sensing and inhibits biofilm. The synergistic potential of the Lamiaceae family medicinal plants with standard antibiotics offers a promising approach to control multidrug-resistant (MDR) bacterial pathogens. Compounds like thymol, carvacrol, and rosmarinic acid have shown intense synergistic effects with a host of antibiotics against the pathogens like E. coli, P. aeruginosa, S. aureus and Salmonella species. Therefore, the combination of Lamiaceae-based phytochemicals with standard antibiotics may provide an effective, sustainable resolution to the growing worldwide problem of antibiotic resistance.
ACKNOWLEDGMENTS
The authors are thankful to RIMT University, Punjab, India, for providing infrastructure support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PG conceptualized the manuscript and supervised the work. GS drafted the manuscript.
PG and SG revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Salam MA, Al-Amin MY, Salam MT, et al. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare. 2023;11(13):1946.
Crossref - Csiko G. Residues relating to the veterinary therapeutic or growth-promoting use and abuse of medicines. Present Knowledge in Food Safety. 2023:96-113.
Crossref - Larsson DGJ, Flach CF. Antibiotic resistance in the environment. Nat Rev Microbiol. 2022;20(5):257-269.
Crossref - Zubko MK. Towards sustainable antimicrobials from plants: Some ways to abridge current methodological approaches. Sustain Mater Technol. 2024;39:e00801.
Crossref - Singh S. A review on some medicinal plant species with the most traditional medicinal usage in India. Int J Biol Innov. 2023;05(01):52-62.
Crossref - Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites. 2019;9(11):258.
Crossref - Kon KV, Rai MK. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev Anti Infect Ther. 2012;10(7):775-790.
Crossref - Melo RS, Albuquerque Azevedo AMA, Gomes PAM, et al. Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Molecules. 2019;24(21):3864.
Crossref - Alam M, Bano N, Ahmad T, et al. Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum b-Lactamases. Antibiotics. 2022;11(7):855.
Crossref - Suntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev. 2020;19(5):1199-1209.
Crossref - Ortiz-Mendoza N, Martinez-Gordillo MJ, Martinez-Ambriz E, Basurto-Pena FA, Gonzalez-Trujano ME, Aguirre-Hernandez E. Ethnobotanical, Phytochemical, and Pharmacological Properties of the Subfamily Nepetoideae (Lamiaceae) in Inflammatory Diseases. Plants. 2023;12(21):3752.
Crossref - Karpinski TM. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules. 2020;10(1):103.
Crossref - Marchioni I, Najar B, Ruffoni B, Copetta A, Pistelli L, Pistelli L. Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers. Plants. 2020;9(6):691.
Crossref - Das S, Sultana KW, Chandra I. In vitro propagation, phytochemistry and pharmacology of Basilicum polystachyon (L.) Moench (Lamiaceae): A short review. South African J Bot. 2023;155:178-186.
Crossref - Sprea RM, Caleja C, Pinela J, et al. Comparative study on the phenolic composition and in vitro bioactivity of medicinal and aromatic plants from the Lamiaceae family. Food Res Int. 2022;161:111875.
Crossref - Uritu CM, Mihai CT, Stanciu GD, et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res Manag. 2018;2018(1):1-44.
Crossref - Fahimi S, Hajimehdipoor H, Shabanpoor H, Bagheri F, Shekarchi M. Synergic Antibacterial Activity of Some Essential Oils from Lamiaceae. 2015;2(3):23-29.
- Gholamipourfard K, Salehi M, Banchio E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. South Afr J Bot. 2021;141:183-195.
Crossref - Toiu A, Mocan A, Vlase L, et al. Comparative Phytochemical Profile, Antioxidant, Antimicrobial and In Vivo Anti-Inflammatory Activity of Different Extracts of Traditionally Used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules. 2019;24(8):1597.
Crossref - Badescu B, Buda V, Romanescu M, et al. Current State of Knowledge Regarding WHO Critical Priority Pathogens: Mechanisms of Resistance and Proposed Solutions through Candidates Such as Essential Oils. Plants. 2022;11(14):1789.
Crossref - Kurek A, Grudniak AM, Kraczkiewicz-Dowjat A, Wolska KI. New Antibacterial Therapeutics and Strategies. Polish J Microbiol. 2011;60(1):3-12.
Crossref - Sik B, Kapcsandi V, Szekelyhidi R, Hanczne EL, Ajtony Z. Recent Advances in the Analysis of Rosmarinic Acid From Herbs in the Lamiaceae Family. Nat Prod Commun. 2019;14(7).
Crossref - Das SN, Patro VJ, Dinda SC. A review: Ethnobotanical survey of genus Leucas. Pharmacogn Rev. 2012;6(12):100.
Crossref - Bisio A, Pedrelli F, D’Ambola M, et al. Quinone diterpenes from Salvia species: chemistry, botany, and biological activity. Phytochem Rev. 2019;18(3):665-842.
Crossref - Aldosary SK, El-Rahman SNA, Al-Jameel SS, Alromihi NM. Antioxidant and antimicrobial activities of Thymus vulgaris essential oil contained and synthesis thymus (Vulgaris) silver nanoparticles. Braz J Biol. 2023;83:e244675.
Crossref - Wronska N, Szlaur M, Zawadzka K, Lisowska K. The Synergistic Effect of Triterpenoids and Flavonoids-New Approaches for Treating Bacterial Infections? Molecules. 2022;27(3):847.
Crossref - Rattray RD, Van Wyk BE. The Botanical, Chemical and Ethnobotanical Diversity of Southern African Lamiaceae. Molecules. 2021;26(12):3712.
Crossref - Yousefian S, Esmaeili F, Lohrasebi T. A Comprehensive Review of the Key Characteristics of the Genus Mentha, Natural Compounds and Biotechnological Approaches for the Production of Secondary Metabolites. Iran J Biotechnol. 2023;21(4).
Crossref - Panda SK, Gazim ZC, Swain SS, et al. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Tetradenia riparia (Hochst.) Codd (Lamiaceae). Front Pharmacol. 2022;13:896078.
Crossref - Shutava HG, Kavalenka NA, Supichenka NhN, Leontiev NvN, Shutava NtG. Essential Oils of Lamiaceae with High Content of a-, b-Pinene and Limonene Enantiomers. J Essent Oil Bear Plants. 2014;17(1):18-25.
Crossref - Verma RS, Pandey V, Chauhan A, Tiwari R. Essential Oil Composition of Mentha longifolia (L.) L. Collected from Garhwal Region of Western-Himalaya. J Essent Oil Bear Plants. 2015;18(4):957-966.
Crossref - Kundu S, Salma U, Sutradhar M, Mandal N. An Update on the Medicinal uses, Phytochemistry and Pharmacology of Leucas Aspera, A Medicinally Important Species. Int J Agric Innov Res. 2018;6(4):39-44.
- Chouhan HS, Singh SK. A review of plants of genus Leucas. J Pharmacogn Phyther. 2011;3(3):13-26.
- Cazarolli L, Zanatta L, Alberton E, et al. Flavonoids: Prospective Drug Candidates. Mini-Reviews Med Chem. 2008;8(13):1429-1440.
Crossref - Tofighi Z, Molazem M, Doostdar B, et al. Antimicrobial activities of three medicinal plants and investigation of flavonoids of Tripleurospermum disciforme. Iran J Pharm Res. 2015;14(1):225-231.
- Farhadi F, Khameneh B, Iranshahi M, Iranshahy M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phyther Res. 2019;33(1):13-40.
Crossref - Rodriguez B, Pacheco L, Bernal I, Pina M. Mechanisms of Action of Flavonoids: Antioxidant, Antibacterial and Antifungal Properties. Ciencia, Ambient y Clima. 2023;6(2):33-66.
Crossref - Lambert PA, Hammond SM. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem Biophys Res Commun. 1973;54(2):796-799.
Crossref - Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta – Biomembr. 1993;1147(1):132-136.
Crossref - Cushnie TPT, Taylor PW, Nagaoka Y, Uesato S, Hara Y, Lamb AJ. Investigation of the antibacterial activity of 3-O-octanoyl-(-)-epicatechin. J Appl Microbiol. 008;105(5):1461-1469.
Crossref - Backiam ADS, Duraisamy S, Karuppaiya P, et al. Analysis of the main bioactive compounds from Ocimum basilicum for their antimicrobial and antioxidant activity. Biotechnol Appl Biochem. 2023;70(6):2038-2051.
Crossref - Bisio A, Romussi G, Russo E, et al. Antimicrobial Activity of the Ornamental Species Salvia corrugata, a Potential New Crop for Extractive Purposes. J Agric Food Chem. 2008;56(22):10468-10472.
Crossref - Garcia A, Bocanegra-Garcia V, Palma-Nicolas JP, Rivera G. Recent advances in antitubercular natural products. Eur J Med Chem. 2012;49:1-23.
Crossref - Bouarab-Chibane L, Forquet V, Lanteri P, et al. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front Microbiol. 2019;10:829.
Crossref - Alvarez-Martinez FJ, Barrajon-Catalan E, Encinar JA, Rodriguez-Diaz JC, Micol V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr Med Chem. 2020;27(15):2576-2606.
Crossref - Diniz do Nascimento L, Moraes AAB de, Costa KS da, et al. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules. 2020;10(7):988.
Crossref - Yap PSX, Yusoff K, Lim SHE, Chong CM, Lai KS. Membrane Disruption Properties of Essential Oils-A Double-Edged Sword? Processes. 2021;9(4):595.
Crossref - Radulovic NS, Blagojevic PD, Stojanovic-Radic ZZ, Stojanovic NM. Antimicrobial Plant Metabolites: Structural Diversity and Mechanism of Action. Curr Med Chem. 2013;20(7):932-952.
Crossref - Ravera S, Tancreda G, Vezzulli L, Schito AM, Panfoli I. Cirsiliol and Quercetin Inhibit ATP Synthesis and Decrease the Energy Balance in Methicillin-Resistant Staphylococcus aureus (MRSA) and Methicillin-Resistant Staphylococcus epidermidis (MRSE) Strains Isolated from Patients. Molecules. 2023;28(17):6183.
Crossref - Nourbakhsh F, Lotfalizadeh M, Badpeyma M, Shakeri A, Soheili V. From plants to antimicrobials: Natural products against bacterial membranes. Phyther Res. 2022;36(1):33-52.
Crossref - Sharif SA, Ismaeil AS, Ahmad AA. Synergistic Effect of Different Plant Extracts and Antibiotics on Some Pathogenic Bacteria. Sci J Univ Zakho. 2020;8(1):7-11.
Crossref - Böhme K, Barros-Velázquez J, Calo-Mata P, Aubourg SP. Antibacterial, Antiviral and Antifungal Activity of Essential Oils: Mechanisms and Applications. In: Villa T, Veiga-Crespo P. (eds) Antimicrobial Compounds. Springer, Berlin, Heidelberg. 2014:51-81.
Crossref - Armendariz-Fernandez KV, Herrera-Hernandez IM, Munoz-Marquez E, Sanchez E. Characterization of Bioactive Compounds, Mineral Content, and Antioxidant Activity in Bean Varieties Grown with Traditional Methods in Oaxaca, Mexico. Antioxidants. 2019;8(1):26.
Crossref - Zhakipbekov K, Turgumbayeva A, Akhelova S, et al. Antimicrobial and Other Pharmacological Properties of Ocimum basilicum, Lamiaceae. Molecules. 2024;29(2):388.
Crossref - Tafrihi M, Imran M, Tufail T, et al. The Wonderful Activities of the Genus Mentha: Not Only Antioxidant Properties. Molecules. 2021;26(4):1118.
Crossref - Khorsand GJ, Morshedloo MR, Mumivand H, Bistgani ZE, Maggi F, Khademi A. Natural diversity in phenolic components and antioxidant properties of oregano (Origanum vulgare L.) accessions, grown under the same conditions. Sci Rep. 2022;12(1):5813.
Crossref - Potente G, Bonvicini F, Gentilomi GA, Antognoni F. Anti-candida activity of essential oils from lamiaceae plants from the mediterranean area and the middle east. Antibiotics. 2020;9(7):9070395.
Crossref - Hussain AI, Chatha SAS, Kamal GM, Ali MA, Hanif MA, Lazhari MI. Chemical composition and biological activities of essential oil and extracts from Ocimum sanctum. Int J Food Prop. 2017;20(7):1569-1581.
Crossref - Jiang Y, Wu N, Fu YJ, et al. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ Toxicol Pharmacol. 2011;32(1):63-68.
Crossref - Muntean D, Licker M, Alexa E, et al. Evaluation of essential oil obtained from Mentha piperita L. against multidrug-resistant strains. Infect Drug Resist. 2019;12:2905-2914.
Crossref - Moore-Neibel K, Gerber C, Patel J, Friedman M, Jaroni D, Ravishankar S. Antimicrobial activity of oregano oil against antibiotic-resistant Salmonella enterica on organic leafy greens at varying exposure times and storage temperatures. Food Microbiol. 2013;34(1):123-129.
Crossref - Galovieova L, Borotova P, Valkova V, et al. Thymus vulgaris Essential Oil and Its Biological Activity. Plants. 2021;10(9):1959.
Crossref - Lemos MF, Lemos MF, Pacheco HP, Endringer DC, Scherer R. Seasonality modifies rosemary’s composition and biological activity. Ind Crops Prod. 2015;70:41-47.
Crossref - Petrakou K, Iatrou G, Lamari FN. Ethnopharmacological survey of medicinal plants traded in herbal markets in the Peloponnisos, Greece. J Herb Med. 2020;19:100305.
Crossref - Chew AL, Jessica JJA, Sasidharan S. Antioxidant and antibacterial activity of different parts of Leucas aspera. Asian Pac J Trop Biomed. 2012;2(3):176-180.
Crossref - Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol. 2006;2(9):458-466.
Crossref - Yakoubi R, Megateli S, Sadok TH, Bensouici C, Bagci E. A synergistic interactions of Algerian essential oils of Laurus nobilis L., Lavandula stoechas L. and Mentha pulegium L. on anticholinesterase and antioxidant activities. Biocatal Agric Biotechnol. 2021;31:101891.
Crossref - Mandal S, Mandal MD, Pal NK. Enhancing chloramphenicol and trimethoprim in vitro activity by Ocimum sanctum Linn. (Lamiaceae) leaf extract against Salmonella enterica serovar Typhi. Asian Pac J Trop Med. 2012;5(3):220-224.
Crossref - Dharsono HDA, Putri SA, Kurnia D, Dudi D, Satari MH. Ocimum Species: A Review on Chemical Constituents and Antibacterial Activity. Molecules. 2022;27(19):6350.
Crossref - Langeveld WT, Veldhuizen EJA, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol. 2014;40(1):76-94.
Crossref - Swetha TK, Vikraman A, Nithya C, Prasath NH, Pandian SK. Synergistic antimicrobial combination of carvacrol and thymol impairs single and mixed-species biofilms of Candida albicans and Staphylococcus epidermidis. Biofouling. 2021;36(10):1-16.
Crossref - Mittal RP, Rana A, Jaitak V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr Drug Targets. 2019;20(6):605-624.
Crossref - Ng WJ, Shit CS, Ee KY, Chai TT. Plant Natural Products for Mitigation of Antibiotic Resistance. 2021:57-91.
Crossref - Dias-Souza MV, Dias CG, Ferreira-Marcal PH. Interactions of natural products and antimicrobial drugs: Investigations of a dark matter in chemistry. Biointerface Res Appl Chem. 2018;8(3).
- Milenkovic M, Stosovic J, Slavkovska V. Synergy between Essential Oils of Calamintha Species (Lamiaceae) and Antibiotics. Nat Prod Commun. 2018;13(3):1934578X1801300.
Crossref - Gan C, Langa E, Valenzuela A, Ballestero D, Pino-Otin MR. Synergistic Activity of Thymol with Commercial Antibiotics against Critical and High WHO Priority Pathogenic Bacteria. Plants. 2023;12(9):1868.
Crossref - Micovic T, Usjak D, Milenkovic M, Samardzic S, Maksimoviז Z. Antimicrobial activity of Hyssopus officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae) essential oils from Montenegro and Serbia. Lek sirovine. 2023;43(1):1-6.
Crossref - Gismondi A, Di Marco G, Redi EL, Ferrucci L, Cantonetti M, Canini A. The antimicrobial activity of Lavandula angustifolia Mill. essential oil against Staphylococcus species in a hospital environment. J Herb Med. 2021;26:100426.
Crossref - Kapp K, Pussa T, Orav A, et al. Chemical Composition and Antibacterial Effect of Mentha spp. Grown in Estonia. Nat Prod Commun. 2020;15(12):1-14.
Crossref - Ehsani A, Alizadeh O, Hashemi M, Afshari A, Aminzare M. Phytochemical, antioxidant and antibacterial properties of Melissa officinalis and Dracocephalum moldavica essential oils. Vet Res forum an Int Q J. 2017;8(3).
- Mallikarjun S, Rao A, Rajesh G, Shenoy R, Pai M. Antimicrobial efficacy of Tulsi leaf (Ocimum sanctum) extract on periodontal pathogens: An in vitro study. J Indian Soc Periodontol. 2016;20(2):145-150.
Crossref - Jnaid Y, Yacoub R, Al-Biski F. Antioxidant and antimicrobial activities of Origanum vulgare essential oil. Int Food Res J. 2016;23(4).
- Stringaro A, Colone M, Cecchetti S, Zeppetella E, Spadaro F, Angiolella L. “In vivo” and “in vitro” antimicrobial activity of Origanum vulgare essential oil and its two phenolic compounds on clinical isolates of Candida spp. Arch Microbiol. 2023;205(1):15.
Crossref - Ahmed HM, Al-Zubaidy AMA. Exploring natural essential oil components and antibacterial activity of solvent extracts from twelve Perilla frutescens L. Genotypes. Arab J Chem. 2020;13(10):7390-7402.
Crossref - Tian J, Zeng X, Zhang S, et al. Regional variation in components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind Crops Prod. 2014;59:69-79.
Crossref - Soliman MM, Elsaba YM, Soliman MSA, Ahmed EZ. Composition and antimicrobial activity of Rosmarinus officinalis L. and Artemisia monosperma L. leaf essential oils and methanolic extracts from plants grown in normal and saline habitats in Egypt. Sci Rep. 2024;14(1):342.
Crossref - Haji Seyedtaghiya M, Nayeri Fasaei B, Peighambari SM. Antimicrobial and antibiofilm effects of Satureja hortensis essential oil against Escherichia coli and Salmonella isolated from poultry. Iran J Microbiol. 2021;13(1).
Crossref - Santos T da SA, Meccatti VM, Pereira TC, et al. Antibacterial Effect of Combinations of Salvia officinalis and Glycyrrhiza glabra Hydroalcoholic Extracts against Enterococcus spp. Coatings. 2023;13(9):1579.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.