ISSN: 0973-7510
E-ISSN: 2581-690X
The present study addresses the critical contamination issue of arsenic (As) in agricultural soils, which delimits crop productivity as well as food safety. This research explores the potential of the arsenic-tolerant plant growth-promoting bacteria (PGPB) Pseudomonas putida CKVF1 to alleviate As-induced negative effects in Vicia faba L. (also known as broad bean or faba bean) seeds when sown with and/or without As (50 mg/kg soil) and inoculated with P. putida CKVF1. Morphological parameters such as chlorophyll content, moisture retention, and nodulation were assessed alongside biochemical markers, including malondialdehyde (MDA), proline levels, antioxidant enzyme activities, and phenolic compound profiles. Microscopic analyses were conducted to evaluate cellular integrity. Results showed that arsenic exposure significantly impaired the growth of plants and increased MDA level which is an oxidative stress marker. However, PGPR treatment enhanced chlorophyll content, moisture retention, and nodulation by 35%, 28%, and 40%, respectively, while reducing oxidative damage through elevated antioxidant enzyme activities. Microscopic observations confirmed improved cellular structure in PGPR-treated plants. Additionally, PGPR inoculation increased total phenolic content and specific phenolic compounds, enhancing stress tolerance. The results highlight the effectiveness of P. putida CKVF1 in alleviating As-toxicity through physiological and biochemical improvements; and present a defensible approach to augmenting crop resilience in As-contaminated regions. This study emphasizes the PGPR potential as a bioremediation tool for promoting agricultural sustainability in the areas affected by heavy metal contamination.
V. faba, Arsenate, ROS, Microscopy, Phenolic Compounds, PGPR
One of the most prevalent naturally occurring metalloids in the crust of the Earth, human body, and seawater is arsenic (As).1 Although arsenic occurs naturally, its poisoning has emerged as a major global health issue, especially in South and East Asia, and is predominantly linked to groundwater sources.2 More than 100 million people in India are at risk because drinking water has dangerously high amounts of arsenic, which in some areas can surpass 3200 µg/L.3 The pollution is mostly caused by natural geological processes, such as the oxidation of arsenic-bearing sulfide minerals and the reductive dissolution of iron and aluminum oxyhydroxides in aquifers.4 In India, arsenic contamination is prevalent in states like West Bengal, Bihar, and Jharkhand, where irrigation with arsenic-laden groundwater has led to significant soil contamination, impacting agricultural productivity.4-6 Additionally, the reliance on untreated sewage for irrigation exacerbates the problem, contributing to the bioaccumulation of arsenic in crops, leading to chronic health issues such as skin lesions and various cancers.7
Numerous industries, including electronics, metallurgy, chemical engineering, agriculture, medicines, and livestock management, may find use for arsenic.8 The four oxidation states of arsenic-arsine (As(-III)), arsenite (As(+III)), elemental arsenic (As(0)), and arsenate (As(+V))-occur in both organic and inorganic forms in the environment.9 The environment can include arsenic in a variety of oxidation states. However, inorganic trivalent arsenite (As(III)) and pentavalent arsenate (As(V)) oxyanions are more commonly found in natural waters.10
In India, arsenic contamination levels are concerning in several regions, leading to decreasing crop yields. While plants do not need As, its uptake disrupts their metabolism, causing disorders within the plant system. This disruption results in stunted growth, the generation of oxidative stress, and various morphological changes at the cellular level.11,12 The toxicity of arsenic is evident in its interference with aerobic phosphorylation, where it competes with phosphate in adenosine triphosphate (ATP) formation, leading to the formation of unstable adenosine diphosphate (ADP) and disrupting energy flow within cells.13 Previous research has indicated that arsenic exposure results in reduced plant growth and yield, as well as symptoms such as wilting, yellowing, and necrosis at the leaf margins, along with diminished photosynthetic activity.14-16 Additionally, soil arsenic has been shown to affect the uptake and accumulation of minerals in the aerial parts of plants.17 Exposure to arsenate (As(V)) leads to the induction of oxidative stress, characterized by increased lipid peroxidation and damage to cellular membranes.18 The heavy metal stress (lead, chromium, and manganese) has been reported to induce ultra-structural damage in the roots and shoots of V. faba and gram seedlings.19,20 V. faba is easy to cultivate, cost-effective, and has been used in genotoxicity and phytotoxicity studies,21 it also has demonstrated high sensitivity to arsenic toxicity, exhibiting morphological alterations and significant changes in antioxidant enzyme activities. However, there has been a lack of comprehensive studies on the morphological, biochemical, and cytological effects of toxic metals like As in V. faba L.
Mitigating arsenic (As) toxicity in plants is crucial for enhancing agricultural productivity and ensuring food safety. Several strategies can be employed to reduce arsenic absorption and its harmful effects on plant health. Agronomic practices, such as the amendment of organic matter and phosphate fertilizers, can lower arsenic availability in the soil, decreasing its uptake.21-23 In addition to enhancing nutrient uptake and strengthening antioxidant defenses, plant growth-promoting rhizobacteria (PGPR) can assist plants in overcoming arsenic stress. It has also been demonstrated that nitric oxide helps plants like V. faba improve their antioxidant defense mechanisms by reducing oxidative stress brought on by arsenic.24,25
PGPR, which are closely associated with and colonize plant roots, have been extensively documented for their numerous benefits to crop plants. One of PGPR’s main advantages is its potential to increase yield and biomass.26 Moreover, PGPR is essential for engaging defense systems in plants, which increases their resistance to abiotic stressors, especially those associated with climate and chemically modified soils.27 The current study examines the adverse effects of As on the morphological, biochemical, and cytological parameters of V. faba L., but more importantly to study the mitigation of As-toxicity by PGPR.
Faba bean seeds
The Swarna Gaurav faba bean seeds were purchased from ICAR-IARI, New Delhi, India. The seeds were surface sterilized by soaking in 0.1% (v/v) mercuric chloride for 10 min with occasional shaking and then rinsed thoroughly, 4-5 times with sterilized distilled water.
Isolation and characterization of arsenic-tolerant PGPR bacteria (CKVF1)
The CKVF1 was isolated from the rhizosphere soil of faba bean (V. faba L.) plants cultivated at the botanical gardens of M.J.P. Rohilkhand University, Bareilly (Uttar Pradesh, India). The rhizosphere soil was mixed with 0.85% saline solution in a 1:10 ratio. The resulting suspension was serially diluted and inoculated onto nutrient agar media enriched with sodium arsenate heptahydrate salt (Himedia Pvt. Ltd., India) at 100 ppm concentration until arsenic-tolerant single colonies were obtained.28 A total 5 bacterial isolates were obtained out of which only one isolate CKVF1 survived till the highest As-concentration (750 ppm). The CKVF1 was tested for the plant growth-promoting (PGP) characteristics,29 which include the formation of siderophores, hydrogen cyanide (HCN), phosphate solubilization, and indole-3-acetic acid (IAA).
The arsenic-tolerant bacterial isolate (CKVF1) was then characterized using physiological and biochemical tests, including the IMViC, nitrate reduction, and various enzymatic activities using standard methods.30-32 For molecular identification, DNA extraction was performed followed by the amplification of the 16S rRNA gene. Following PCR product purification, nucleotide sequences were identified and added to the GenBank database (accession number OR921377).33 The resultant sequence was analyzed using BLAST analysis to closely comparable sequences in GenBank. The 16S rRNA gene sequences from related bacterial strains were aligned with CKVF1 sequence from the NCBI database using ClustalW within MEGA11. Following alignment, the optimal nucleotide substitution model was selected based on the lowest Akaike Information Criterion score. This model was then applied to construct a maximum likelihood phylogenetic tree of the bacterial isolates. Tree robustness was evaluated using a bootstrap analysis with 1,000 replicates.34-36
Experimental design
Faba bean seeds were soaked for 24 hours at room temperature and then placed for germination in sterilized soil containing sodium arsenate heptahydrate salt (Himedia Pvt. Ltd., India) at a concentration of 50 mg kg-1 soil. Untreated seeds were also germinated in soil without As. Seedlings of V. faba at the 3-4 leaf stage, both control (untreated) and those exposed As were transferred to the greenhouse and allowed to acclimatize before the experiment. The experiment was carried out in triplicate, and the seedlings were subsequently exposed to the following treatments, with five plants per pot: (1) control (untreated seeds without PGPR), (2) untreated seeds with PGPR, (3) As-treated seeds without PGPR, and (4) As-treated seeds with PGPR. Three pots of each set were irrigated with 200 milliliters of demineralized water at field capacity on the day of sowing. Then, every day for the duration of the experiment, all of the pots were routinely irrigated at field capacity using either 1/4 N Hoagland solution or distilled water. For PGPR inoculation, a 20 ml cell suspension of CKVF1 at a concentration of 9.0 log10 CFU ml-1 was used to moisten the CKVF1 treatment group’s soil.37,38 The physical characteristics of the soil used in the experiment are given in Table 1. The young leaves were collected from three replicates (pooled from five plants per replicate), immediately frozen in liquid nitrogen, and used for further biochemical analyses.
Table (1):
Physico-chemical analysis of rhizospheric soil
Parameters |
Values |
|---|---|
pH |
6.9 |
Electrical Conductivity (EC) |
0.39 mS/cm3 |
Organic Carbon (OC) |
0.22% |
N |
49.5 kg/ha |
P |
9 kg/ha |
K |
156 kg/ha |
S |
11.45 ppm |
Zn |
0.78 ppm |
B |
0.61 ppm |
Fe |
3.44 ppm |
Mn |
1.56 ppm |
Cu |
0.48 ppm |
Assessment of morphological, physiological, and biochemical parameters of V. faba leaves
Chlorophyll Content, Moisture Content, and Nodulation
3.0 ml of cooled 80% acetone (v/v) were used to extract chlorophyll from 0.3 grams of fresh V. faba leaf samples. Chlorophyll concentration (mg g-1 FW) was determined using a Systronics double beam spectrophotometer to measure absorbance at 663 nm and 645 nm for chl a and b, respectively.39 Weight measurements were taken at regular intervals until a constant weight was reached after 1 g of fresh V. faba leaves were heated in a hot air oven set at 80 °C to assess the moisture content.40,41 Nodulation was assessed by uprooting three replicates (pooled from five plants per replicate) on the 35th day post-inoculation, and the number of nodules was counted.41,42
Proline content
Proline was extracted from 0.1 g of leaf tissue using 3% sulfosalicylic acid and quantified using the acid-ninhydrin method.43,44 The absorbance of the chromophore was measured at 520 nm and proline content was calculated using a standard curve.45-47
Malondialdehyde (MDA) content
MDA was determined by homogenizing 0.3 g of fresh leaves with 5% TCA. The homogenate was centrifuged and the supernatant combined with TBA. After boiling and centrifugation, the supernatant’s absorbance was measured at 532 and 600 nm. The non-specific absorbance at 600 nm was removed from the 532 nm value, and MDA concentration was estimated using an attenuation coefficient of 115 mM-1 cm-1.48
Antioxidant enzyme extraction
Frozen leaf tissues (500 mg) were crushed in an extraction buffer containing 1 mM EDTA, 0.05% Triton X-100, 2% polyvinyl pyrrolidone (PVP), and 1 mM ascorbate in 50 mM potassium phosphate buffer (pH 7.8).47 The homogenate was centrifuged at 10,000 g for 20 minutes, and the supernatant was kept at -20 °C for subsequent enzyme assays. The activities of different antioxidants, including SOD, CAT, GPoX, and APX, were assessed as described in the previous study.48,49 All experiments included three replicates of each treatment.
Total phenolic compounds
Phenolics were extracted from 100 mg of dried leaf and stem powder (pooled from five plants) with 50% methanol, followed by centrifugation at 10,000 rpm for 10 minutes. The supernatant was mixed with 0.5 mL of Folin-Ciocalteu reagent50-52 and 2 mL of 20% sodium carbonate solution. After incubation in the dark at room temperature for 2 hours, absorbance was measured at 720 nm. Results were calculated using gallic acid as the standard and expressed as mg gallic acid equivalents per gram of dry weight.53-55
Specific phenolic compounds
Specific phenolic compounds, including gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, rutin, ferulic acid, quercetin, kaempferol, and salicylic acid, were analyzed in leaf and stem tissues using HPLC (Shimadzu Prominence system).56,57 Quantification was performed against respective standards, and results were expressed as µg g-1 of dry weight (DW).58
Microscopic study of V. faba
Light microscopy
V. faba L. stem cross-sections from each of the three treatments were hand-cut, and their anatomical tissue characteristics were analyzed for histological alterations. The samples were fixed and rehydrated in FAA solution for one week before being preserved in an alcohol-glycerol solution (70% ethyl alcohol: glycerol in a 1:1 ratio). Safranin and fast green stains were applied to the sections prior to the examination.58-60
Transmission Electron Microscopy
Changes at the ultra-cellular level of cell components in all three treatments were studied using a TEM. The second leaf samples from control, treated, and PGPR-amended plants were fixed in a solution of 3% glutaraldehyde and 2% formaldehyde prepared in 0.1 M sodium cacodylate buffer (pH 7.2) for 2 hours at 4 °C.59,61,62 After fixation, samples were washed three times with 0.1 M sodium cacodylate buffer and then fixed in 1% osmium tetroxide for an additional 2 hours. The washing was repeated once with sodium cacodylate, dehydrated through a series of acetone solutions (15%-100%), and embedded in Spurr’s epoxy resin (Electron Microscopy Sciences, Hatfield, PA). Semi-thin sections (400 nm thick) of the leaf samples were cut using a Leica EM UC6 microtome, transferred to glass slides, and adhered by heating before being stained with Epoxy Tissue Stain (Electron Microscopy Sciences, Hatfield, +PA). Ultrathin sections (60-80 nm thick) were also cut with the Leica EM UC6 microtome and post-stained with uranyl acetate and lead citrate. The sections were examined using a Phillips Morgagni 268 transmission electron microscope (FEI Company, Hillsboro, OR) at an accelerating voltage of 80 kV.63-65
Data analysis
The experiments were arranged in a completely randomized design. Each treatment consisted of 5 replicates, and all experiments were repeated thrice. The data were statistically analyzed using one-way ANOVA, and means were compared using Tukey’s honestly significant difference (HSD) test at P = 0.05.66-68
Bacterial Isolation and characterization
The isolate CKVF1 exhibited negative IMViC tests and positive nitrate reduction. It demonstrated various enzymatic activities, such as catalase, oxidase, amylase, protease, and lipase production, but was negative for urease and gelatin hydrolysis. The results (Table 2), highlight the biochemical and plant growth-promoting (PGP) traits of the As-tolerant CKVF1 isolate, which could withstand up to 100 ppm of sodium arsenate (As III). The isolate demonstrated a high capacity for producing indole-3-acetic acid (IAA), at concentration 135 ± 4 µg/mL, the isolate exhibited the ability to solubilize phosphate, at a rate of 2.6 ± 0.529 mg/L, it also showed a strong siderophore production capacity of 77.67 ± 2.516%. The isolate tested positive for hydrogen cyanide (HCN) production and positive for ammonium production.
Table (2):
Characteristic biochemical and PGPR traits of CKVF1
| Trait | Test | Result |
|---|---|---|
| IMViC and Nitrate reduction | ||
| Indole | Negative | |
| Methyl Red (MR) | Negative | |
| Voges-Proskauer (VP) | Negative | |
| Citrate | Negative | |
| Nitrate reduction | Positive | |
| Enzymatic activities | ||
| Catalase | Positive | |
| Oxidase | Positive | |
| Urease | Negative | |
| Gelatin hydrolysis | Negative | |
| Amylase | Positive | |
| Protease | Positive | |
| Lipase | Positive | |
| Plant growth-promoting traits | ||
| IAA concentration (µg/mL) | 135 ± 4 | |
| IAA concentration (µg/mL) in presence of As (V) | 290 ± 5 | |
| Concen. of soluble phosphate (mg/L) | 26 ± 0.529 | |
| Siderophore production (%) | 77.67 ± 2.516 | |
| HCN production | Positive | |
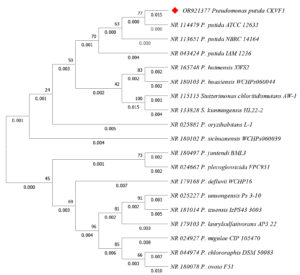
The molecular identification of CKVF1 (GenBank Acc. No. OR921377.1) by BLASTn analysis and 16S rRNA sequencing revealed that the isolate showed the highest nucleotide similarity with P. chlororaphis in the clade of P. putida as shown in Figure 1.
Figure 1. Phylogenetic tree constructed using the neighbor-joining method based on 16S rRNA gene sequences of Pseudomonas and Stutzerimonas strains, including reference sequences retrieved from GenBank. The evolutionary history was inferred using the Neighbor-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. This analysis involved 19 nucleotide sequences. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 1498 positions in the final dataset. Evolutionary analyses were conducted in MEGA11
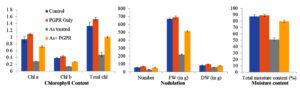
Chlorophyll content in V. faba leaves
The chlorophyll content in the control plants was increased by the amendment of CKVF1 (PGPR) as shown in Table 3 and Figure 2. Total percent increase was calculated as follows-
Percentage Change
PGPR Only vs Control:
Chl a: (1.092-0.938)/0.938*100 ≈+16.4%
Chl b: (0.437-0.388)/0.388*100 ≈+12.6%
Total Chl: (1.530-1.326)/1.326*100 ≈+15.4%
As + PGPR vs As Treated:
Chl a: (: 0.723-0.312)/0.312*100 ≈+131%
The results showed that chlorophyll content was increased in the PGPR-treated plants compared to the control plants. The arsenic treatments in the plants resulted in a drastic decrease in the chlorophyll content (Table 3 and Figure 2); however, the PGPR amendment overcame the adverse effects of arsenic by a huge difference (>100% increment compared to As alone).
Figure 2. Effects of different treatments on chlorophyll content, nodulation, and moisture content in plants. Chl a, chl b, total chl, nodule number, nodule fresh weight (FW), nodule dry weight (DW), and total moisture content were measured under four treatments: Control (blue), PGPR Only (orange), As treated (gray), and As + PGPR (yellow). As-treatment (gray) markedly reduced all measured parameters compared to control (blue) and PGPR Only (orange), while co-application of PGPR (As + PGPR, yellow) alleviated these negative effects. Data are presented as mean ± Stdev
Table (3):
Biological parameters in V. faba under As stress and PGPR treatment comparison to control plants. The results are shown as Mean ± Stdev. N = Number of nodules; FW = fresh weight (in g); DW = dry weight (in g)
| Chlorophyll Content (mg/g) | Nodulation | Moisture content (%) | |||||
|---|---|---|---|---|---|---|---|
| Chl a | Chl b | Total Chl | N | FW | DW | ||
| Control | 0.938 ± 0.09 | 0.388 ± 0.024 | 1.326 ± 0.114 | 55.67 ± 3.055 | 668.57 ± 11.68 | 81.53 ± 6.71 | 87.3 ± 2.62 |
| PGPR Only | 1.092 ± 0.026 | 0.437 ± 0.027 | 1.530 ± 0.053 | 68.33 ± 3.055 | 689.2 ± 15.98 | 96.53 ± 3.69 | 89.13 ± 2 |
| As treated | 0.312 ± 0.077 | 0.136 ± 0.0138 | 0.292 ± 0.088 | 28.67 ± 3.51 | 217.27 ± 7.77 | 58.7 ± 1.70 | 51.13 ± 2.7 |
| As + PGPR | 0.723 ± 0.255 | 0.277 ± 0.009 | 1.0001 ± 0.023 | 54 ± 4.36 | 513.43 ± 8.88 | 76.03 ± 5.05 | 79.73 ± 1.79 |
Nodulation
The nodulation parameters, including the average number of nodules (N), fresh weight (FW), and dry weight (DW), were compared between the control group and the PGPR treatment. The percent change was calculated as done in chlorophyll content, the PGPR treatment increased 22.7%, 3.9%, and 18.4% for N, FW, and DW, respectively, compared to the control plants. Additionally, the adverse effects of arsenic on N, FW, and DW were mitigated by PGPR treatment, showing improvements of 88%, 136.5%, and 29.5%, respectively (Table 3 and Figure 2).
Moisture content
The moisture content in the PGPR-only plants increased by 2% compared to the control plants. However, the negative effects of arsenic on the moisture content of V. faba plants were alleviated by 55.9% with PGPR treatment (Table 3 and Figure 2).
Antioxidative enzymes and proline content
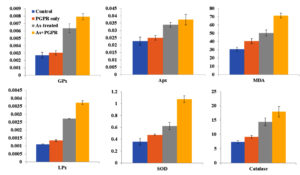
The effects of PGPR treatment on antioxidative enzyme activities were analyzed using one-way ANOVA. All parameters showed statistically significant differences (p < 0.001). The strongest effect was observed in MDA (F = 904.75, p < 2 × 10-10), followed by SOD (F = 118.01, p< 5.82 × 10-7). PGPR treatment induced substantial increases in antioxidative enzyme activities compared to control, with the highest percentage increases observed in SOD (32.31%) and Proline (31.78%). Even the smallest increase, seen in Apx (9.95%), was statistically significant (F = 23.67, p < 0.001). The mean values with standard deviations (Table 4) and their respective percent changes are indicated in Figure 3.
Figure 3. Impact of different treatments on antioxidative enzyme activities and oxidative stress markers in V. faba plants. Bar graphs represent the mean values for GPx, Apx, MDA, LPx, SOD, and catalase across four treatment groups: Control (blue), PGPR only (orange), As-treated (gray), and As + PGPR (yellow). Data are shown as mean ± Stdev
Table (4):
The antioxidant enzyme activities (catalase, SOD, Gpx, MDA, Apx) and proline content in plants (Mean ± stdev): control, PGPR-only, As-treated, and As + PGPR
Treatment Group |
Catalase (µmol H2O2/min/g) |
SOD (U/g) |
GPx (U/g) |
MDA (U/g) |
Apx (U/g) |
Proline (µmol/g) |
|---|---|---|---|---|---|---|
Control |
7.686 ± 0.442 |
0.358 ± 0.057 |
0.0027 ± 0.0004 |
0.0011 ± 0.0000 |
0.022 ± 0.002 |
30.35 ± 2.66 |
PGPR-only |
9.784 ± 0.455 |
0.474 ± 0.010 |
0.0031 ± 0.0004 |
0.0014 ± 0.0001 |
0.025 ± 0.001 |
40.78 ± 2.65 |
As-treated |
14.412 ± 1.234 |
0.622 ± 0.055 |
0.0063 ± 0.0006 |
0.0027 ± 0.0001 |
0.033 ± 0.001 |
50.27 ± 2.69 |
As+PGPR |
17.274 ± 1.846 |
0.484 ± 0.002 |
0.0078 ± 0.0005 |
0.0038 ± 0.0002 |
0.037 ± 0.003 |
71.39 ± 3.56 |
Based on the post-hoc analysis (Tukey’s HSD, a = 0.05), all parameters showed significant differences between treatments. PGPR treatment significantly mitigated As-induced stress across all enzymes and the most pronounced effects were in SOD and Proline activities. All pairwise comparisons between As+PGPR and other treatments were statistically significant (p < 0.05). Control and PGPR-only treatments showed fewer significant differences between each other.
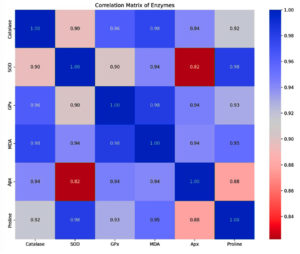
The correlation heatmap analysis was also done to check the relationships between different antioxidative enzyme activities and how changes in one enzyme’s activities might be related to changes in another (Figure 4). With correlation coefficients of r = 0.963 and r = 0.977, respectively, catalase shows a substantial positive connection with GPx and MDA, indicating possible co-regulation or comparable reactions to oxidative stress. Proline and SOD have a strong connection (r = 0.981), suggesting that proline accumulation and SOD activity may be related. Proline is known to improve stress tolerance. The substantial correlation (r = 0.982) between GPx and MDA further supports the coordinated antioxidative response. Additionally, MDA and Apx have a strong correlation (r = 0.945), indicating they may be tightly related. A strong association (r = 0.929) between Apx and Proline, suggests that Apx may play a part in regulating proline levels under stress.
Figure 4. Correlation matrix illustrating the interrelationships of antioxidative enzymes and non-enzymatic antioxidants based on Pearson’s correlation coefficients (r). Color intensity indicates the strength and direction (positive/blue, negative/red) of the correlations
Total phenolic and specific phenolic compounds
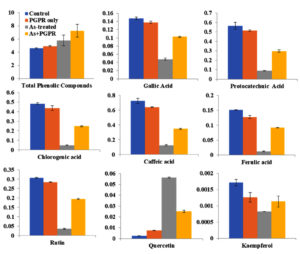
In present study, the effects of different treatments on the concentrations of specific phenolic compounds, including total phenolics, gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, rutin, ferulic acid, quercetin, and kaempferol was also examined. The results indicated that the combination of As and PGPR yielded the highest levels of total phenolics (8.282 U) and individual compounds such as gallic acid (0.297 U), protocatechuic acid (0.609 U), chlorogenic acid (0.496 U), caffeic acid (0.728 U) (Figure 5). In contrast, the control group exhibited lower concentrations across all measured compounds, with total phenolics averaging 4.669 and gallic Acid at 0.146. Percent change calculations showed that the As + PGPR treatment resulted in a 67% increase in total phenolics compared to the control group. Gallic Acid concentrations increased by 108%, while protocatechuic acid increased 528% under the same treatment. Chlorogenic acid levels increased by 935%, and caffeine concentrations rose by 1079%. Rutin levels increased by 861%, ferulic acid by 1107%, quercetin by 1866%, and kaempferol by 125% (Figure 5).
Figure 5. Effects of different treatments on total phenolic content and specific phenolic compounds in V. faba plants. Bar graphs display mean values (±Stdev) for total phenolic compounds, gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, ferulic acid, rutin, quercetin, and kaempferol under four treatment conditions: Control (blue), PGPR only (orange), As-treated (gray), and As + PGPR (yellow). Data are presented as mean ± Stdev
Microscopy of V. faba
Light microscopy
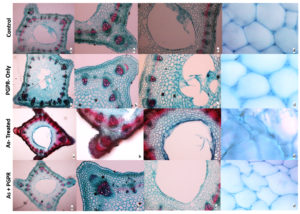
Under light microscopy, the micrograph of the stem of V. faba at 10X showed a well-arranged ring of vascular bundles. The pith cavity or central canal was also visible at 40X. Additionally, well-organized hexagonal cells, such as pith cells, appeared at 60X in healthy or control plants (Figure 6). Similar structural features were observed in the control plant treated with PGPR (Figure 6). In contrast, As-treated plants showed increased lignification in the cells, and the micrograph showed that cells retained more stains. The pith cavity broadened and morphologically cells were undefined (Figure 6). However, the plants treated with the combination of both showed organized vascular bundles, well-defined pith cavities, and morphology of the pitch cells (Figure 6).
Figure 6. Light micrograph of V. faba stem. a- stem morphology at 10X; b & c- vascular bundles and pith cavities at 40X; d- cell shape and structure at 60X magnifications
TEM
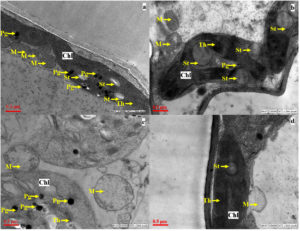
TEM analysis of the leaf from a healthy control plant showed typical mature cells with well-defined, elongated chloroplasts, organized in an ellipsoid shape. These chloroplasts exhibited closely stacked thylakoids and contained numerous large starch vacuoles. Additionally, well-developed mitochondria were observed in the selected leaf region (Figure 7a). In the control plant treated with PGPR, the chloroplasts showed well-organized, stacked thylakoids and larger starch vacuoles (Figure 7b). In contrast, the As-treated plant showed poorly defined chloroplasts, lacking starch vacuoles, its mitochondria appeared swollen and faint (Figure 7c). However, plants treated with both arsenic and PGPR exhibited well-organized chloroplasts containing starch vacuoles, with mitochondria showing signs of recovery (Figure 7d).
The majority of culturable arsenic (As)-resistant plant growth-promoting (PGP) bacteria have been isolated from soil, the rhizosphere of mangrove ecosystems, domestic wastewaster and industrial wastewater.69-71 In Contrast, this study successfully isolated and characterized an As-resistant plant growth-promoting rhizobacterium (PGPR), P. putida strain CKVF1, from the rhizosphere soil of V. faba L. plants cultivated at the botanical gardens of M.J.P. Rohilkhand University, Bareilly, India. Among five bacterial isolates, CKVF1 demonstrated remarkable tolerance, thriving even at As concentrations as high as 750 ppm. This finding is significant given the increasing prevalence of As contamination in agricultural soils, particularly in regions relying on arsenic-laden groundwater for irrigation.3 The characterization of CKVF1 included a series of physiological and biochemical tests, which confirmed its identity as P. putida. Molecular identification through 16S rRNA gene sequencing further validated this classification, with the sequence deposited in the GenBank database (accession number OR921377). This aligns with previous studies that have identified Pseudomonas species as effective PGPR capable of enhancing plant growth and mitigating stress.72,73 The study demonstrated that arsenate exposure leads to significant physiological and biochemical stress in V. faba, as evidenced by stunted growth, yellowing of leaves, and reduced chlorophyll content.74 These symptoms are indicative of oxidative stress, which is a common response to heavy metal toxicity. The increase in MDA level further confirms lipid peroxidation resulting from oxidative damage due to As treatment.75 Elevated proline levels in arsenate-treated plants suggest an adaptive response to osmotic stress, while the activities of antioxidant enzymes such as catalase, guaiacol peroxidase, ascorbate peroxidase, and superoxide dismutase were significantly altered, indicating an attempt by the plants to counteract oxidative stress.76,77 Studies have shown that P. putida can enhance plant growth and alleviate stress caused by heavy metals, including arsenate, through various mechanisms. For instance, the presence of the arsH gene in P. putida has been linked to increased tolerance against oxidative stress induced by arsenic compounds, suggesting a role in protecting cellular integrity under such conditions.78
Additionally, the application of PGPR like P. putida has been demonstrated to improve physiological responses in plants facing abiotic stressors, reinforcing the importance of microbial interactions in plant health.79 The enhancement of antioxidant enzyme activities in PGPR-treated plants further supports the notion that these bacteria can effectively scavenge reactive oxygen species and mitigate oxidative damage.71
PGPR can employ several mechanisms to detoxify heavy metals like As, aiding in plant resilience and health. One key mechanism involves enhancing the antioxidant activity of plants by upregulating enzymes such as CAT, GPx, APx, and SOD, which scavenge reactive oxygen species (ROS) generated during heavy metal stress, thereby reducing oxidative damage to cellular components.80 PGPR also induce stress-responsive pathways, modulating signaling molecules like nitric oxide to further bolster antioxidant defenses.81 Additionally, PGPR produce secondary metabolites that chelate heavy metals, reducing their bioavailability and toxicity in plants while promoting nutrient uptake and overall plant health.82,83 Arsenic’s toxicity disrupts cellular energy production by interfering with aerobic phosphorylation, a critical process for generating ATP. Due to their structural similarities, arsenic competes with phosphate in ATP formation. However, the resulting adenosine diphosphate-arsenate is unstable and readily hydrolyzes, preventing efficient energy storage. This leads to reduced net ATP production, disrupting energy-dependent cellular processes and inhibiting sulfhydryl-containing enzymes involved in energy metabolism. This interference with energy metabolism affects cell viability and overall organismal health
The findings from this study align with these mechanisms, as P. putida CKVF1 significantly enhanced the antioxidant defenses of V. faba under arsenate stress. The observed increases in antioxidant enzyme activities in CKVF1-treated plants indicate effective mitigation of oxidative stress caused by arsenic exposure. Moreover, CKVF1 improved plant growth parameters, restored nodulation, and preserved cellular integrity, demonstrating its role in enhancing nutrient uptake and promoting overall plant health under heavy metal stress. These results are particularly relevant given the increasing soil contamination with heavy metals due to anthropogenic activities, which threaten agricultural productivity and food safety.3,4 By leveraging such beneficial microbes, sustainable agricultural practices can be developed to mitigate heavy metal toxicity and improve crop resilience. Arsenic’s toxicity disrupts cellular energy production by interfering with aerobic phosphorylation, a critical process for generating ATP.13 Due to their structural similarities, arsenic competes with phosphate in ATP formation. However, the resulting ADP-As is unstable and readily hydrolyzes, preventing efficient energy storage leading to reduced net ATP production, disrupting energy-dependent cellular processes and inhibiting sulfhydryl-containing enzymes involved in energy metabolism. This interference with energy metabolism affects cell viability and overall organismal health.
Mitigating arsenic (As) toxicity in plants is essential for improving agricultural productivity and ensuring food safety, particularly in contaminated regions. Various strategies, including agronomic practices, can help reduce arsenic uptake and its harmful effects on plant health. The amendment of organic matter improves soil properties and can immobilize arsenic, while phosphate fertilizers compete with arsenate for uptake sites, thereby lowering arsenic availability to plants.21-23 These approaches offer practical means to decrease arsenic absorption, contributing to safer crop production and enhanced soil health. Building on the importance of agronomic practices in mitigating As-toxicity, nitric oxide (NO) has also been shown to play a crucial role in enhancing plant defense mechanisms against As-induced oxidative stress. In plants such as V. faba, NO acts as a signaling molecule that stimulates the antioxidant system, leading to increased activities of enzymes like SOD, CAT, and APX. These enzymes help scavenge ROS generated by As-exposure, thereby reducing cellular damage and maintaining redox balance.24,25
The study also highlighted the significant impact of arsenate on nodulation in V. faba. Nodulation is critical for nitrogen fixation and overall plant health; thus, the observed improvement in nodulation with the application of P. putida CKVF1 suggests that this PGPR not only enhanced plant resilience but also promoted beneficial symbiotic relationships with nitrogen-fixing bacteria. This aspect is particularly important for improving soil fertility and crop yield in arsenic-contaminated areas, as effective nodulation can significantly enhance nitrogen availability to plants, thereby supporting their growth and productivity in challenging environments.84
The ability of CKVF1 to improve nodulation under arsenate stress underscores its potential role as a bio-stimulant in agricultural practices, especially in regions where soil contamination poses a threat to crop production. By fostering beneficial interactions between plants and nitrogen-fixing bacteria, CKVF1 can contribute to sustainable agricultural practices aimed at enhancing soil fertility and crop resilience in arsenic-affected areas. This finding aligns with previous research that emphasizes the importance of PGPR in enhancing nodulation and nitrogen fixation, which are vital for maintaining soil health and agricultural productivity.85,86
The microscopic analysis of V. faba L. provided compelling evidence of the structural and ultrastructural impacts of arsenate stress and the protective role of P. putida CKVF1. Light microscopy revealed significant disruptions in the vascular tissues of arsenate-treated plants, including reduced cell size, disorganized vascular bundles, and damaged parenchyma cells. These anatomical changes are indicative of impaired water and nutrient transport, which likely contributed to the stunted growth observed in arsenate-exposed plants.87,88 In contrast, CKVF1-treated plants exhibited well-organized vascular tissues and larger cell sizes, suggesting that PGPR application mitigates arsenic-induced damage by promoting tissue integrity and functionality.
Transmission electron microscopy (TEM) further highlighted the ultrastructural damage caused by As stress. As-treated plants displayed swollen chloroplasts with disrupted thylakoid membranes, unevenly shaped mitochondria, and increased vacuolation, all of which are hallmarks of oxidative stress and cellular dysfunction.19,88,89 These changes compromise photosynthetic efficiency and energy metabolism, exacerbating the negative effects of arsenic toxicity. However, CKVF1 treatment preserved organelle integrity, as evidenced by well-structured chloroplasts with intact thylakoid arrangements and functional mitochondria. This preservation is critical for maintaining photosynthesis and energy production under heavy metal stress.90,91 The microscopic findings also align with biochemical analyses that showed reduced MDA levels and normalized antioxidant enzyme activities in CKVF1-treated plants, indicating alleviation of oxidative damage. The restoration of nodulation in PGPR-treated plants further underscores CKVF1’s role in fostering symbiotic relationships with nitrogen-fixing bacteria, which is vital for nitrogen acquisition and soil fertility in contaminated environments.85,86 These structural and ultrastructural improvements highlight the multifaceted benefits of P. putida CKVF1 in mitigating arsenic toxicity through enhanced cellular resilience and biochemical homeostasis.
Overall, the integration of light and electron microscopy findings provides a comprehensive understanding of how CKVF1 mitigates heavy metal stress at both anatomical and subcellular levels. These results emphasize the potential application of PGPR as a sustainable strategy to enhance plant health and productivity in arsenic-contaminated soils.17,92
One potential pathway for arsenate bioremediation in V. faba could involve bio-volatilization, as CKVF1 demonstrated significant mitigation of the harmful effects associated with arsenic exposure. Bio-volatilization, a process where As is converted into volatile forms by microbial activity, has been proposed as an effective detoxification mechanism in contaminated soils.93,94
Additionally, CKVF1’s ability to enhance antioxidant enzyme activities, such as CAT, GPoX, and superoxide dismutase SOD, suggests its role in reducing oxidative stress caused by arsenic-induced ROS.81,95,96
Exploring how CKVF1 interacts with other plant systems and soil microbiomes could provide insights into its broader applicability for improving crop resilience and productivity in arsenic-contaminated environments. This aligns with previous studies highlighting the importance of PGPR in enhancing plant growth, nutrient uptake, and stress tolerance under heavy metal contamination.81,97 Further research into CKVF1’s molecular mechanisms, including its potential for bio-volatilization and other bioremediation pathways, could pave the way for innovative strategies to manage soil contaminants effectively while ensuring agricultural sustainability.98,99
The present study on P. putida strain CKVF1 reveals its significant potential as a plant growth-promoting rhizobacterium for combating As-toxicity and enhancing crop resilience. To maximize its applications, several research directions can be pursued viz., investigating the genetic basis of CKVF1’s As-tolerance, examining CKVF1’s synergistic relationships with other beneficial soil microorganisms to understand its broader impact on soil ecosystem health, conducting field trials across various As-contaminated agricultural regions in India to validate its effectiveness in diverse real-world conditions, assessing CKVF1’s bio-volatilization capabilities and nutrient uptake enhancement across different crop species, and developing scalable bioformulations to facilitate widespread agricultural application. CKVF1-based approaches may offer a sustainable and environmentally considerate means to support soil fertility and crop productivity in As-affected areas, although additional studies would be necessary to better understand their long-term impact on sustainable agriculture. This microbial strategy could significantly contribute to India’s food security goals while promoting healthier agroecosystems and reducing dependence on chemical interventions.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Raessler M. The Arsenic Contamination of Drinking and Groundwaters in Bangladesh: Featuring Biogeochemical Aspects and Implications on Public Health. Arch Environ Contam Toxicol. 2018;75(1):1-7.
Crossref - Srivastava S. Arsenic in Drinking Water and Food. 2019.
Crossref - Chakraborti D, Singh SK, Rahman MM, et al. Groundwater Arsenic Contamination in the Ganga River Basin: A Future Health Danger. Int J Environ Res Public Health. 2018;15(2):180.
Crossref - Marghade D, Mehta G, Shelare S, Jadhav G, Nikam KC. Arsenic Contamination in Indian Groundwater: From Origin to Mitigation Approaches for a Sustainable Future. Water (Switzerland). 2023;15(23):4125.
Crossref - Bhattacharya AK. Arsenic Contamination in the Groundwater of West Bengal, Jharkhand and Bihar with a Special Focus on the Stabilization of Arsenic-Laden Sludge from Arsenic Filters. Electron J Geotech Eng. 2019;24(2019):1-23.
- Shrivastava A, Barla A, Yadav H, Bose S. Arsenic Contamination in Shallow Groundwater and Agricultural Soil of Chakdaha Block, West Bengal, India. Front Environ Sci. 2014;2:50.
Crossref - Dickin SK, Schuster-Wallace CJ, Qadir M, Pizzacalla K. A review Health Risks and Pathways of Wastewater Exposure to wastewater use in agriculture. Environ Health Perspect. 2016;124(7):900-909.
Crossref - Nicomel NR, Leus K, Folens K, Van Der Voort P, Laing GD. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int J Environ Res Public Health. 2015;13(1):62.
Crossref - Chaudhary MM, Hussain S, Du C, Conway BR, Ghori MU. Arsenic in Water: Understanding the Chemistry, Health Implications, Quantification and Removal Strategies. Chem Engineering. 2024;8(4):78.
Crossref - De Francisco P, Martin-Gonzalez A, Rodriguez-Martin D, Diaz S. Interactions with Arsenic: Mechanisms of Toxicity and Cellular Resistance in Eukaryotic Microorganisms. Int J Environ Res Public Health. 2020;18(22):12226.
Crossref - Hasanuzzaman M, Bhuyan MHMB, Parvin K, et al. Regulation of ROS Metabolism in Plants Under Environmental Stress: A Review of Recent Experimental Evidence. Int J Mol Sci. 2020;21(22):8695.
Crossref - Raza A, Habib M, Kakavand SN, et al. Phytoremediation of Cadmium: Physiological, Biochemical, and Molecular Mechanisms. Biology. 2020;9(7):177.
Crossref - Shaji E, Santosh M, Sarath KV, Prakash P, Deepchand V, Divya BV. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci Front. 2021;12(3):101079.
Crossref - Sinha D, Datta S, Mishra R, et al. Negative Impacts of Arsenic on Plants and Mitigation Strategies. Plants. 2023;12(9):1815.
Crossref - Szaloki T, Szekely A, Valkovszki NJ, Tarnawa A, Jancso M. The Reaction of Rice Growth to the Arsenic Contamination Under Various Irrigation Methods. Plants. 2024;13(9):1253.
Crossref - Zemanova V, Popov M, Pavlikova D, et al. Effect of Arsenic Stress on 5-Methylcytosine, Photosynthetic Parameters and Nutrient Content in Arsenic Hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol. 2020;20(1):2325.
Crossref - Sandil S, Ovari M, Dobosy P, et al. Effect of Arsenic-Contaminated Irrigation Water on Growth and Elemental Composition of Tomato and Cabbage Cultivated in Three Different Soils, and Related Health Risk Assessment. Environ Res. 2021;197:111098.
Crossref - Hu Y, Li J, Lou B, et al. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules. 2020;10(2):240.
Crossref - Dey U, Mondal NK. Ultrastructural Deformation of Plant Cell Under Heavy Metal Stress in Gram Seedlings. Cogent Environ Sci. 2016;2(1):1196472.
Crossref - Sadee BA, Foulkes ME, Hill SJ. A study of arsenic speciation in soil, irrigation water and plant tissue: A case study of the broad bean plant, Vicia faba. Food Chem. 2016;210: 362-370.
Crossref - Alavi E, Tajadod G, Jafari Marandi S, Arbabian S. Vicia faba seed: a bioindicator of phytotoxicity, genotoxicity, and cytotoxicity of light crude oil. Environ Sci Pollut Res Int. 2023;30(8):21043-21051.
Crossref - Gandhi N, Sridhar J, Pallavi A, et al. Germination, Growth, Physiological and Biochemical Response of Pigeon Pea (Cajanus cajan) Under Varying Concentrations of Copper (Cu), Lead (Pb), Manganese (Mn) and Barium (Ba). Int J Res Rev. 2020;7(3):3.
- Punshon T, Jackson BP, Meharg AA, Warczack T, Scheckel K, Guerinot ML. Understanding Arsenic Dynamics in Agronomic Systems to Predict and Prevent Uptake by Crop Plants. Sci Total Environ. 2017;581-582:209-220.
Crossref - Chen Y, Han YH, Cao Y, Y-Zhu G, Rathinasabapathi B, Ma LQ. Arsenic Transport in Rice and Biological Solutions to Reduce Arsenic Risk from Rice. Front Plant Sci. 2017;8:268.
Crossref - Tang Z, Zhao FJ. The Roles of Membrane Transporters in Arsenic Uptake, Translocation and Detoxification in Plants. Crit Rev Environ Sci Technol. 2020;51(21):2449-2484.
Crossref - Molina-Favero C, Creus MC, Lanteri ML, et al. Nitric Oxide and Plant Growth Promoting Rhizobacteria: Common Features Influencing Root Growth and Development. Adv Bot Res. 2007;46:1-33.
Crossref - Waheed Z, Iqbal S, Irfan M, Jabeen K, Ilyas N, Al-Qahtani WH. Isolation and Characterization of PGPR Obtained from Different Arsenic-Contaminated Soil Samples and Their Effect on Photosynthetic Characters of Maize Grown Under Arsenic Stress. Environ Sci Pollut Res. 2024;31(12):18656-18671.
Crossref - Chieb M, Gachomo EW. The Role of Plant Growth Promoting Rhizobacteria in Plant Drought Stress Responses. BMC Plant Biol. 2023;23(1):4403.
Crossref - Bhat MA, Mishra AK, Jan S, et al. Plant Growth Promoting Rhizobacteria in Plant Health: A Perspective Study of the Underground Interaction. Plants. 2023;12(3):629.
Crossref - Dave A, Sharma RK. The Isolation and Characterization of Plant Growth Promoting Rhizobacteria. In: Sharma I, Sharma A, Bhardwaj R, Sirhindi G, eds. PGPR (Plant Growth Promoting Rhizobacteria) for Plant Stress Management, New York: Nova Science Publisher. 2023:1-17.
Crossref - Gupta V, Buch A. Pseudomonas aeruginosa Predominates as Multifaceted Rhizospheric Bacteria with Combined Abilities of P-Solubilization and Biocontrol. J Pure Appl Microbiol. 2019;13(1):319-328.
Crossref - Cervantes-Vazquez TJA, Valenzuela-Garcia AA, Cervantes-Vazquez MG, et al. Morphophysiological, Enzymatic, and Elemental Activity in Greenhouse Tomato Saladette Seedlings from the Effect of Plant Growth-Promoting Rhizobacteria. Agronomy. 2021;11(5):1008.
Crossref - Khan N, Bano A, Rahman MA, Guo J, Kang Z, Babar MA. Comparative Physiological and Metabolic Analysis Reveals a Complex Mechanism Involved in Drought Tolerance in Chickpea (Cicer arietinum L.) Induced by PGPR and PGRs. Sci Rep. 2019;9(1):38702.
Crossref - Shabaan M, Asghar HN, Zahir ZA, Zhang X, Sardar MF, Li H. Salt-Tolerant PGPR Confer Salt Tolerance to Maize Through Enhanced Soil Biological Health, Enzymatic Activities, Nutrient Uptake and Antioxidant Defense. Front Microbiol. 2022;13:901865.
Crossref - Santosa S, Sutarno S, Purwanto E, Suranto S, Sajidan S. Molecular Characterization of Plant Growth Promoting Rhizobacteria Using 16S rRNA Sequences in the Organic Rice Field of Sukorejo Village, Central Java, Indonesia. Biodiversitas. 2018;19(6):2157-2162.
Crossref - Hassan WS, Abdulrazzaq KM, Al-Obaidi QT, Al-Azow KA. Molecular Detection of Anaplasma platys in Dogs in Nineveh Province, Iraq. Iraqi J Vet Sci. 2024;38(3):677-682.
Crossref - Hutter G, Schlagenhauf U, Valenza G, et al. Molecular Analysis of Bacteria in Periodontitis: Evaluation of Clone Libraries, Novel Phylotypes and Putative Pathogens. Microbiology. 2003;149(1):67-75.
Crossref - Wu D, Jospin G, Eisen JA. Systematic Identification of Gene Families for Use as “Markers” for Phylogenetic and Phylogeny-Driven Ecological Studies of Bacteria and Archaea and Their Major Subgroups. PLoS ONE. 2013;8(10):77033.
Crossref - Kohler J, Caravaca F, Roldan A. Effect of Drought on the Stability of Rhizosphere Soil Aggregates of Lactuca sativa Grown in a Degraded Soil Inoculated with PGPR and AM Fungi. Appl Soil Ecol. 2009;42(2):160-165.
Crossref - Nautiyal CS, Govindarajan R, Lavania M, Pushpangadan P. Novel Mechanism of Modulating Natural Antioxidants in Functional Foods: Involvement of Plant Growth Promoting Rhizobacteria NRRL B-30488. J Agric Food Chem. 2008;56(12):4474-4481.
Crossref - Kumari R, Ashraf S, Bagri GK, Khatik SK, Bagri DK, Bagdi DL. Extraction and Estimation of Chlorophyll Content of Seed Treated Lentil Crop Using DMSO and Acetone. J Pharmacogn Phytochem. 2018;7(3):249-250.
- Chan EWC, Lim YY, Wong SK, et al. Effects of Different Drying Methods on the Antioxidant Properties of Leaves and Tea of Ginger Species. Food Chem. 2009;113(1):166-172.
Crossref - Wanyo P, Siriamornpun S, Meeso N. Improvement of Quality and Antioxidant Properties of Dried Mulberry Leaves with Combined Far-Infrared Radiation and Air Convection in Thai Tea Process. Food Bioprod Process. 2011;89(1):22-30.
Crossref - Bhattacharjya S, Chandra R. Effect of Inoculation Methods of Mesorhizobium ciceri and PGPR in Chickpea (Cicer arietinum L.) on Symbiotic Traits, Yields, Nutrient Uptake and Soil Properties. Legume Res. 2013;36(4):331-337.
- Forlani G, Funck D. A Specific and Sensitive Enzymatic Assay for the Quantitation of L-Proline. Front Plant Sci. 2020;11:582026.
Crossref - Zahra S, Amin B, Ali Y, Mehdi Y. The Salicylic Acid Effect on the Tomato (Lycopersicum esculentum Mill.) Germination, Growth and Photosynthetic Pigment Under Salinity Stress (NaCl). J Stress Physiol Biochem. 2010;2(3):35-41.
- Cha-Um S, Kirdmanee C. Effect of Salt Stress on Proline Accumulation, Photosynthetic Ability and Growth Characters in Two Maize Cultivars. Pak J Bot. 2009;41(1):87-98.
- Khan MH, Panda SK. Alterations in Root Lipid Peroxidation and Antioxidative Responses in Two Rice Cultivars Under NaCl-Salinity Stress. Acta Physiol Plant. 2008;30(1):81-89.
Crossref - Janknegt PJ, Rijstenbil JW, van de Poll WH, Gechev TS, Buma AGJ. A Comparison of Quantitative and Qualitative Superoxide Dismutase Assays for Application to Low Temperature Microalgae. J Photochem Photobiol B. 2007;87(3):218-226.
Crossref - Anjum NA, Sharma P, Gill SS, et al. Catalase and Ascorbate Peroxidase-Representative H2O2-Detoxifying Heme Enzymes in Plants. Environ Sci Pollut Res. 2016;23(19):19002-19029.
Crossref - Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viegas RA, Silveira JAG. Superoxide Dismutase, Catalase and Peroxidase Activities Do Not Confer Protection Against Oxidative Damage in Salt-Stressed Cowpea Leaves. New Phytol. 2004;163(3):563-571.
Crossref - Ainsworth EA, Gillespie KM. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat Protoc. 2007;2(4):875-877.
Crossref - Dominguez-Lopez I, Perez M, Lamuela-Raventos RM. Total (Poly)phenol Analysis by the Folin-Ciocalteu Assay as an Anti-Inflammatory Biomarker in Biological Samples. Crit Rev Food Sci Nutr. 2023;64(27):10048-10054.
Crossref - Siti Mahirah Y, Rabeta MS, Antora RA. Effects of Different Drying Methods on the Proximate Composition and Antioxidant Activities of Ocimum basilicum Leaves. Food Res. 2018;2(5):421-428.
Crossref - Ojha S, Raj A, Roy A, Roy S. Extraction of total phenolics, flavonoids and tannins from Paederia foetida l. Leaves and their relation with antioxidant activity. Pharmacognosy Journal. 2018;10(3):541-547.
Crossref - Soltanabad MH, Bagherieh-Najjar MB, Baghkheirati EK, Mianabadi M. Ag-conjugated nanoparticle biosynthesis mediated by Rosemary leaf extracts corre-lates with plant antioxidant activity and pro-tein content. Int J Nanosci Nanotechnol. 2018;14(4):319-325.
- Vinueza D, Allauca AAA, Bonilla GAP, Leon KLA, Lopez SPA. Assessment of the diuretic and urinary electrolyte effects of hydroalcoholic extract of Oreocallis grandiflora (Lam.) R. Br. in Wistar albino rats. Pharmacologyonline. 2018;1:117-127.
- Rodriguez-Gomez R, Vanheuverzwjin J, Souard F, et al. Determination of three main chlorogenic acids in water extracts of coffee leaves by liquid chromatography coupled to an electrochemical detector. Antioxidants. 2018;7(10):143.
Crossref - Sarker U, Oba S. Polyphenol and flavonoid profiles and radical scavenging activity in leafy vegetable Amaranthus gangeticus. BMC Plant Biology. 2020;20(1):1-12.
Crossref - Yang XJ, Dang B, Fan MT. Free and bound phenolic compound content and antioxidant activity of different cultivated blue highland barley varieties from the qinghai-tibet plateau. Molecules. 2018;23(4):879.
Crossref - Espinosa F, Castro MP. On the use of herbarium specimens for morphological and anatomical research. Botany Letters. 2018;165(3-4):361-367.
Crossref - Graham L, Orenstein J. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat Protoc. 2007;2:2439-2450.
Crossref - Dassanayake RP, Falkenberg SM, Stasko JA, Shircliff AL, Lippolis JD, Briggs RE. Identification of a reliable fixative solution to preserve the complex architecture of bacterial biofilms for scanning electron microscopy evaluation. PLoS ONE. 2020;15(5):1-15.
Crossref - Shami GJ, Chen Z, Cheng D, Wisse E, Braet F. On the long-term storage of tissue for fluorescence and electron microscopy: lessons learned from rat liver samples. Histochemistry and Cell Biology. 2024;163(1):12.
Crossref - Burattini S, Falcieri E. TEM: A precious evergreen approach to cell biology and pathology. AIP Conf Proc. 2020;1:2257.
Crossref - Dumancic E, Vojta L, Fulgosi H. Beginners guide to sample preparation techniques for transmission electron microscopy. Periodicum Biologorum. 2023;125(1-2):123-131.
Crossref - Zhang H, Wang C, Zhou G. Ultra-Microtome for the Preparation of TEM Specimens from Battery Cathodes. Microscopy and Microanalysis. 2020;26(5):867-877.
Crossref - Nanda A, Mohapatra BB, Mahapatra APK, Mahapatra APK, Mahapatra APK. Multiple comparison test by Tukey’s honestly significant difference (HSD): Do the confident level control type I error. International Journal of Statistics and Applied Mathematics. 2021;6(1):59-65.
Crossref - Simmons-Elliott J, Tolosa T, Zebelo S. Plant growth-promoting rhizobacteria (PGPR) modulates sweet corn-corn earworm interactions. Crop Protection. 2023;169(April):106246.
Crossref - Sonmez N, Gultekin P, Turp V, Akgungor G, Sen D, Mijiritsky E. Evaluation of five CAD/CAM materials by microstructural characterization and mechanical tests: A comparative in vitro study. BMC Oral Health. 2018;18(1):1-13.
Crossref - Mallick I, Bhattacharyya C, Mukherji S, et al. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth-promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. The Science of the Total Environment. 2018;610-611:1239-1250.
Crossref - Maslennikova D, Koryakov I, Yuldashev R, Avtushenko I, Yakupova A, Lastochkina O. Endophytic Plant Growth-Promoting Bacterium Bacillus subtilis Reduces the Toxic Effect of Cadmium on Wheat Plants. Microorganisms. 2023;11(7):1653.
Crossref - Prasad M, Madhavan A, Babu P, et al. Alleviating arsenic stress affecting the growth of Vigna radiata through the application of Klebsiella strain ASBT-KP1 isolated from wastewater. Front Microbiol. 2024;15:1-15.
Crossref - Backer R, Rokem JS, Ilangumaran G, et al. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci. 2018;871:1-17.
Crossref - Singh A, Yadav VK, Gautam H, et al. The role of plant growth promoting rhizobacteria in strengthening plant resistance to fluoride toxicity: a review. Front Microbiol. 2023;14:1-12.
Crossref - El-Bouzidi L, Khadra A, Zefzoufi M, et al. In vivo protective effects of vitamin C against cyto-genotoxicity induced by Dysphania ambrosioides aqueous extract. Open Chemistry. 2024;22(1).
Crossref - Lin A, Zhang X, Zhu YG, Zhao FJ. Arsenate-induced toxicity: Effects on antioxidative enzymes and dna damage in Vicia faba. Environ Toxicol Chem. 2008;27(2):413-419.
Crossref - Duquesnoy I, Champeau GM, Evray G, Ledoigt G, Piquet-Pissaloux A. Enzymatic adaptations to arsenic-induced oxidative stress in Zea mays and genotoxic effect of arsenic in root tips of Vicia faba and Zea mays. Comptes Rendus – Biologies. 2010;333(11-12):814-824.
Crossref - Kabbadj A, Makoudi B, Mouradi M, Pauly N, Frendo P, Ghoulam C. Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS ONE. 2017;12(12):1-19.
Crossref - Paez-Espino AD, Nikel PI, Chavarria M, de Lorenzo V. ArsH protects Pseudomonas putida from oxidative damage caused by exposure to arsenic. Environ Microbiol. 2020;22(6):2230-2242.
Crossref - Reva ON, Weinel C, Weinel M, et al. Functional genomics of stress response in Pseudomonas putida KT2440. J Bacteriol. 2006;188(11):4079-4092.
Crossref - Shukla P, Singh AK. Nitric oxide mitigates arsenic-induced oxidative stress and genotoxicity in Vicia faba L. Environ Sci Pollut Res. 2015;22(18):13881-13891.
Crossref - Nahar K, Rhaman MS, Parvin K, et al. Arsenic-Induced Oxidative Stress and Antioxidant Defense in Plants. Stresses. 2022;2(2):179-209.
Crossref - Narayanan Z, Glick BR. Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms. 2022;10(10):1-18.
Crossref - Qin H, Wang Z, Sha W, Song S, Qin F, Zhang W. Role of Plant-Growth-Promoting Rhizobacteria in Plant Machinery for Soil Heavy Metal Detoxification. Microorganisms. 2024;12(4):700.
Crossref - Jach ME, Sajnaga E, Ziaja M. Utilization of Legume-Nodule Bacterial Symbiosis in Phytoremediation of Heavy Metal-Contaminated Soils. Biology. 2022;11(5):676.
Crossref - Abbas G, Murtaza B, Bibi I, et al. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int J Environ Res Public Health. 2018;15(1):59.
Crossref - Ahmed A, Sara Taha A, Sundas RQ and, Man-Qun W. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics. 2021;9(3):42.
Crossref - El-Sappah AH, Zhu Y, Huang Q, et al. Plants’ molecular behavior to heavy metals: from criticality to toxicity. Front Plant Sci. 2024;15:1-20.
Crossref - Hu Z, Zhao C, Li Q, et al. Heavy Metals Can Affect Plant Morphology and Limit Plant Growth and Photosynthesis Processes. Agronomy. 2023;13(10):2601.
Crossref - Keyster M, Niekerk LA, Basson G, et al. Decoding heavy metal stress signalling in plants: Towards improved food security and safety. Plants. 2020;9(12):1-26.
Crossref - Maleva MG, Nekrasova GF, Borisova GG, Chukina NV, Ushakova OS. Effect of heavy metals on photosynthetic apparatus and antioxidant status of Elodea. Russ J Plant Physiol. 2012;59(2):190-197.
Crossref - Rai R, Agrawal M, Agrawal SB. Impact of heavy metals on physiological processes of plants: With special reference to the photosynthetic system. In: Singh A, Prasad S, Singh R, eds. Plant responses to xenobiotics. Springer, Singapore. 2016.
Crossref - Mahimairaja S, Bolan NS, Adriano DC, Robinson B. Arsenic Contamination and its Risk Management in Complex Environmental Settings. Advances in Agronomy. 2005;86:1-82.
Crossref - Vocciante M, Grifoni M, Fusini D, Petruzzelli G, Franchi E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl Sci. 2022;12(3):1231.
Crossref - Ghosh PK, Maiti TK, Pramanik K, Ghosh SK, Mitra S, De TK. The role of arsenic-resistant Bacillus aryabhattai MCC3374 in the promotion of rice seedlings growth and alleviation of arsenic phytotoxicity. Chemosphere. 2018;211:407-419.
Crossref - Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9(8):1-52.
Crossref - Kumar A, Yadav PK, Singh A. An overview on emerging and innovative technologies for regulating arsenic toxicity in plants. In: Srivastava PK, Singh R, Parihar P, Prasad SM, eds. Arsenic in plants: Uptake, Consequences an Remediation Techniques. John Wiley & Sons, Inc. 2022.
Crossref - Andrade LAD, Santos CHB, Frezarin ET, Sales LR, Rigobelo EC. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms. 2023;11(4):1088.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.