Aspergillus fumigatus is a pathogenic fungus that causes fatal infectious human disease known as aspergillosis. Fungus A. fumigatus is capable of causing infections in various body parts of humans, but it primarily infects the lungs and causes pulmonary infections known as allergic bronchopulmonary aspergillosis (ABPA). Subsequent dissemination of A. fumigatus into the deeper body parts may also contribute to the development of invasive infections in other vital organs. Therefore, the complexity and spectrum of aspergillosis majorly include allergic and invasive infections. To dominate the human body and escape the human immune system, A. fumigatus produces a number of virulence factors as well as pathogenicity determinants to establish disease. These pathogenic moieties of A. fumigatus includes allergens, toxins, hydrophobins, integrins, mannans/galactomannans, lectins, adherins and many other proteins with unknown functions. Combinatorially, these components make A. fumigatus a successful pathogen for humans, although, additionally the A. fumigatus pathogenicity has also been influenced by the metabolism of nitrogen/amino acids, folate synthesis, metal ions, cell wall biosynthesis as well as protein degradation mechanisms. A number of tools and techniques are being used to provide an accurate and timely laboratory diagnosis of aspergillosis. Such comprehensive tools include microscopic examination, culture, and antigen detection methods, serological assays and molecular techniques (DNA probes and molecular typing), G-test and D-mannitol detection and others. However, identifying fungi and assessing antifungal susceptibility by detecting galactomannans and Aspergillus-DNA are also useful. However, certain limitations exist in determining the circulating biomarkers that can be addressed via upcoming approaches such as lateral flow devices and next-generation sequencing. According to current understandings, the biology and clinical impact of A. fumigatus have been complex; therefore, updated information about virulence mechanisms, clinical manifestations, and diagnosis intricacies is essentially required for effective and better management and treatment of Aspergillus-induced infections.
Aspergillosis, Allergen, Diagnosis, Pathogenicity, Virulence
Aspergillus fumigatus is a type of saprotrophic fungus that lives in soil & the air and grows on decaying organic matter. It has been taxonomically reported that around 23 Aspergillus species have reproduced sexually, whereas 10 species divide by asexual mode.1 The sexually reproduced Aspergillus species has been categorized under the genus Neosartorya and exhibits self-incompatible (heterothallic) breeding systems.2 The A. fumigatus isolates manifest as colonies with dark blue green hues on culture media, characterized by densely packed conidiophores intertwined with aerial hyphae. Microscopically, these colonies boast columnar and compact conidial heads, with relatively short, smooth, and often green-hued conidiophores. The diameter of vesicles can range from 20-30 µm, and they are typically subclavate with fertile tissue at the top. The phialides, which are carried on the vesicle itself, usually have pigmentation and are approximately 6-8 µm×2-3 µm in size. When seen in large quantities, conidia have a greenish hue, a smooth to sub-echinulate texture, and a globose to sub-globose shape, with a diameter between 2.0 and 3.5 µm.3 However, strains isolated from animals or humans often have restricted sporulation and tend to undergo micromorphological changes. These variations can include branched conidiophores, elongate or septate phialides, and conidia that are quite varied in size or shape. Importantly, this kind of variation has already caused the incorrect categorization of several apparently “New” species derived from clinical samples.4 Subsequent phylogenetic and molecular analyses revealed that all of these strains were A. fumigatus. Taxa belonging to this group include the uniquely shaped A. fumigatus, A. fumigatiaffinis, A. novofumigatus, and A. viridinutans.5 A. fumigatus sensu stricto stands out with wider conidiophore stipes, typically ranging from 3.5 to 10 µm in width, and subclavate vesicles, in contrast, the other taxa feature narrower stipes and more sub-globose vesicles. Additionally, A. fumigatus sensu stricto thrives at 50 °C but has restricted growth at 10 °C; the other taxa exhibit growth or germination at 10 °C but fail to grow at 50 °C. The nuanced distinctions continue with growth ratios at different temperatures on specific culture media.5 Further molecular analyses revealed the existence of a cryptic species, A. fumigatus “occulturn”, which has morphological similarities to A. fumigatus. However, consistent differences remain elusive because of limitations in figuring out the significant distinctions between the clinical and soil that exhibit altered susceptibility to antifungal drugs compared to that of A. fumigatus. This could be overcome by employing molecular typing analysis tools.6
Virulence determinants of A. fumigatus
Assessing the associated mechanisms predicts a pathogen’s virulence. However, such mechanisms in A. fumigatus are complex and still not completely understood.7 The adherins of A. fumigatus includes rodA-mediated adhesion, adhesin proteins, AfCalAp, laminin-binding, and hyphal adherence molecules. A. fumigatus hyphae is also a potential adherin that enhances the pathogenicity by adhering to host cells. However, galactosaminogalactan (GAG) has been reported as a critical mediator of hyphal adherence, whereas certain exclusive properties of GAG intermediate the A. fumigatus hyphal adherence to various host factors and leads to the virulence.8 A. fumigatus thermotolerance capacity and limitless growth dynamics can boost adaptability to row in a wide temperature range. However, a research outcome has provided the clues to decipher gene expression profiles and temperature sensing information to get a deep insight about the regulatory networks responsible for the thermotolerance.9 However, 2 proteins (a-1,2-mannosyltransferase and CgrA) are critical in the A. fumigatus thermotolerance and virulence. Whereas a-1,2-mannosyltransferase disrupts the fungal cell wall architecture and retards its growth at increasing temperatures, occasionally, at temperatures higher than 25 °C A. fumigatus growth decreases. Also, in a study conducted on a rat model of invasive aspergillosis, the disruption of A. fumigatus cgrA gene reduced the severity of the disease.10
The nutritional needs of A. fumigatus include amino acids, nitrogen metabolism, extracellular enzymes, and the suppression of carbon catabolism. The pathogenicity of A. fumigatus is strongly influenced by its nitrogen metabolism; in fact, 23% of the nitrogen absorption and metabolism genes are necessary for virulence.11 Animal models of invasive pulmonary aspergillosis have shown that A. fumigatus is less virulent when gene deletions affecting the production of specific amino acids, such as histidine, lysine, tyrosine, tryptophan, isoleucine/valine, methionine, cysteine, and alanine, are present. The virulence of A. fumigatus depends on two processes: the synthesis of folate and the degradation of proteins. The para-aminobenzoic acid (PABA) synthase gene is necessary for folate synthesis.12 Metabolites such as propionyl-CoA are produced during protein breakdown, contributing to nutrient absorption. 2-Methylacitrate synthase deficiency reduces fungal development and invasive infections in mice with impaired immune systems. The development and pathogenicity of A. fumigatus depend on assimilation of inorganic elements such as zinc and iron. In the fungus, processes (reductive iron assimilation and siderophore-mediated absorption) are two high-affinity mechanisms required for the acquisition and utilization of metal ions. In mouse models of aspergillosis conducted study has applied the genetic alterations to target the gene (HapX) involved in siderophore production that leads to alterations in the iron homeostasis regulation and ultimately reduced the disease burden and highlights the role of a pathogenicity related gene in A. fumigatus.13 One must note that the maintenance of cell wall integrity is also an important virulence-relevant factor in the pathogen A. fumigatus, which was investigated in a gene deletion study. This study highlights the correlation of specific cell wall synthesis genes with fungal pathogenicity.14 Therefore, knowing more about the cell wall maintenance processes and associated genes will provide insight for designing new therapeutic interventions to combat different forms of aspergillosis.
Molecular interactions of A. fumigatus with the host immune system
Unique molecular characteristics of A. fumigatus make possible to tackle and escape from the host immune system. Certainly, the pigmented cell wall of A. fumigatus conidia, it possible the fungus from reactive oxygen species (ROS) produced by host immune cells (neutrophils and macrophages) that results in the dominance of the pathogen over the host immune cells.15 In animal models with normal or impaired immune systems, removing the pksP gene is essential for melanin production and results in a less dangerous strain that does not produce melanin. This antiapoptotic protein blocks fungal programmed cell death pathways, increasing survival under oxidative stress and amplifying virulence in mice. It has been proposed that A. fumigatus expresses AfBIR1 to impede the immune response. Mortality rates, fungal loads, and inflammatory monocyte, neutrophil, and leukocyte counts in the lungs all increase in tandem with this increase in virulence.16 Enzymes such as catalases and superoxide dismutase are also involved in detoxifying reactive oxygen species (ROS) generated by the host immune system. Although immunocompromised rats show less pathogenicity after double deletion of the cat1 and cat2 genes, murine aspergillosis models show no less virulence after triple deletion of the sod1, sod2, and sod3 genes; however, this deletion improves the efficiency of fungal clearance by alveolar macrophages.17 Among the chemicals secreted by A. fumigatus, gliotoxin has received the most attention for its potential to modulate the immune system. Macrophage phagocytosis, T-lymphocyte proliferation, mast cell activation, and the cytotoxic T-cell response are all immunological responses that gliotoxin suppresses. In chemotherapy mouse models of invasive lung aspergillosis, organisms deficient in the rglT gene exhibited decreased virulence, suggesting a strong self-protection mechanism against gliotoxin. The hyphae of A. fumigatus secrete GAG when the infection is active. This substance masks the cell wall β-glucan, triggers neutrophil apoptosis, and controls host defense cells.18
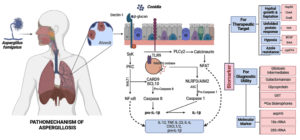
A. fumigatus reacts to its surroundings by activating several signaling pathways associated with its pathogenicity and cellular response. Protein kinase-A, dependent on cyclic AMP (cAMP), can influence pathogenesis by affecting carbon sources and environmental stress. More importantly, for virulence, the ability to detect host-specific stresses that attack the cell wall of the fungus is essential. When cells are stressed by osmotic or cell wall issues, mitogen-activated protein kinases (MAPKs) coordinate how cells react through the high osmolarity glycerol (HOG) and cell wall integrity (CWI) pathways.19 Auxiliary proteins such as PtcB phosphatase and SchA kinase indirectly control virulence, whereas Mkk2 is directly involved. The calcium-calcineurin route is pivotal to the biology of A. fumigatus, impacting proliferation, antifungal resistance, and virulence; maintaining calcium homeostasis is critical for controlling fungal signaling pathways.20 We can disrupt the genes encoding mucin virulence factor A and the downstream transcription factor crzA to decrease A. fumigatus pathogenicity. Two important parts of the unfolded protein response (UPR) are ireA and hacA. They control how proteins enter the endoplasmic reticulum (ER) and affect virulence-related features. For A. fumigatus to survive in its host, it must adapt to low oxygen levels. Changes in morphology and oxygen levels drive immune responses and biofilm development. Oxygen availability influences changes in A. fumigatus cell wall composition and immune cell cytokine release. Oxygen sensing affects both biofilm structure and resistance to antifungals. Proteins that respond to alkaline pH, like PacC, stop epithelial damage and invasion. This is another way that A. fumigatus adjusts to the alkaline pH of mammalian lungs. Mutants deficient in pacC exhibit less virulence and less damage to lung epithelial cells.21 Figure 1 pictorially presents the interaction of A. fumigatus with host immune cells to establish infection, the subsequent roles of virulence factors and pathogenic determinants, and their utility for diagnostic and therapeutic purposes. Table provides a list of potential pathogenic determinants of aspergillosis.
Table:
List of reported A. fumigatus proteins with potential role in pathogenesis
Accession No. |
Gene ID |
Protein Name |
Protein Description |
Ref. |
|---|---|---|---|---|
Afu2g04010 |
tspB |
Alpha, alpha-trehalose-phosphate synthase subunit |
Trehalose biosynthesis protein. Trehalose is important for conidial survival. |
[60] |
Afu5g04170 |
hsp90 |
Molecular chaperone and allergen |
Transcriptional activation of heat shock protein 90. |
[61] |
Afu2g17580 |
arp1 |
Conidial pigment biosynthesis scytalone dehydratase |
Conidial DHN melanin biosynthesis gene. |
[62] |
Afu2g17560 |
arp2 |
Conidial pigment biosynthesis 1,3,6,8-tetrahydroxy naphthalene reductase |
Conidial DHN melanin biosynthesis gene. |
[63] |
Afu2g17550 |
ayg1 |
Conidial pigment biosynthesis protein |
Conidial melanin biosynthesis gene. |
[64] |
Afu4g00860 |
dprA |
Cell surface protein, putative |
Dehydrin-like protein for oxidative stress response and conidia stress tolerance. |
[65] |
Afu6g12180 |
dprB |
Hypothetical protein |
Dehydrin-like protein for osmotic and pH stress responses and conidia stress tolerance. |
[66] |
Afu7g04520 |
dprC |
Hypothetical protein |
Dehydrin-like protein for tolerance against freezing. |
[67] |
Afu4g10860 |
vosA |
Hypothetical protein |
Regulatory protein involved in conidiation. |
[68] |
Afu1g01970 |
velB |
VeA-like protein |
Regulatory protein involved in conidiation. |
[68] |
Afu1g12490 |
veA |
Sexual development activator protein |
Regulatory protein involved in conidiation. |
[69] |
Afu2g05340 |
gel4 |
1,3-beta-glucanosyltransferase, putative |
An important protein for the elongation of 1,3-β-glucan chains |
[62] |
Afu4g06820 |
ecm33 |
GPI-anchored cell wall organization protein |
Mutant conidia have an increased diameter together with an increased concentration of chitin in the cell wall. |
[70] |
Afu3g00270 |
eglC |
GPI-anchored cell wall beta-1,3-endoglucanase |
β-(1,3)-Glucanosyltransferase with β-(1,6)-branching activity. It cleaves and transfers the β-(1,3)-glucan chain towards another glucan chain by a β-(1,6)-glucan linkage. |
[71] |
Afu2g01170 |
gel1 |
1,3-beta-glucanosyltransferase |
1,3-β-Glucanosyltransferases with an essential role in the elongation of 1,3-β-glucan chains. |
[72] |
Afu4g08960 |
gel1 |
GPI anchored cell wall protein, putative |
GPI-anchored protein. |
[70] |
Afu2g01870 |
chsA |
Class I chitin synthase |
Chitin biosynthesis protein. |
[73] |
Afu8g05630 |
chsF |
Class III chitin synthase |
Chitin biosynthesis protein. |
[73] |
Afu3g14420 |
chsG |
Class IV chitin synthase |
Involved in chitin biosynthesis. |
[73] |
Afu2g13440 |
chsE |
Class V chitin synthase |
Chitin biosynthesis; required for average hyphal growth. |
[73] |
Afu2g13430 |
csmB |
Class VI chitin synthase |
Chitin biosynthesis and hyphal tip growth. |
[73] |
Afu5g08540 |
aspA |
Septin, putative |
A gene for septin is involved in development. |
[74] |
Figure 1. Diagram showing Aspergillus fumigatus exposure to human alveolar cells via inhalation followed by interactions of conidia with host immune cells to establish infection. Subsequent role of virulence factors or pathogenic determinant molecules for diagnostic and therapeutic utility
Clinical spectrum of A. fumigatus induced diseases
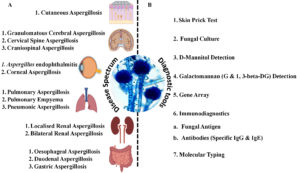
The pathological responses caused by A. fumigatus vary in severity; the clinical course may include primary as well as secondary infections. The spores of A. fumigatus may bypass the upper respiratory tract defense and reach the distal bronchial airway and pulmonary alveoli because of their smaller size (<5 µm) and aerodynamic properties.22 In this region, the host relies primarily upon phagocytic cells to efficiently remove spores. If phagocyte cells are unable to clear spores quickly, germination and fungal colonization may occur. The principal phagocytic cells responsible for maintaining sterility in the lower respiratory tract are pulmonary alveolar macrophages. However, other cells, including polymorphonuclear leukocytes and lymphocytes, are known to play important roles in the removal of Aspergilli. Aspergillosis refers to tissue invasion or allergic disease caused by A. fumigatus. The infection can be primary or secondary, varying in severity and clinical course. Clinical responses include superficial infections of the skin, nails, and other sites; colonization of preformed cavities and debilitated tissues; invasive, inflammatory, granulomatous, and necrotizing lung infections; disseminated disease involving various organs; allergies due to the presence of conidia; and toxicity due to the ingestion of foods contaminated with mycotoxins or other metabolites produced by Aspergilli. The clinical manifestations of aspergillosis and allied diseases are largely determined by the host’s immunological and physiological state. There are several clinical types of aspergilloses, including pulmonary diseases, CNS aspergillosis, PNS aspergillosis, Aspergillus endocarditis, and cutaneous aspergillosis as summarized in Figure 2 diagrammatically.23-25
Figure 2. (A) Diagrammatic representation of the vast spectrum of diseases caused by Aspergillus fumigatus affecting major human organs; (B) Available diagnostic methods widely used in combination for the detection of Aspergillus-induced infections in suspected patients
Pulmonary aspergillosis
A. fumigatus causes pulmonary diseases that can be classified into three categories depending on the host’s atopic or immunocompromised status. Two types of allergic aspergillosis can develop due to prolonged and intense exposure to Aspergillus spores: allergic bronchopulmonary aspergillosis (ABPA) and bronchial asthma or pulmonary eosinophilia.26 A chest X-ray will show transient shadows and periods of lung consolidation. Compared to the mucous plugs of ABPA, which include few hyphae, the progressive cough of obstructive aspergillosis exclusively consists of Aspergillus hyphae and can occasionally produce bronchial casts. Bronchoscopy revealed fungal casts entirely blocking some airways, while chest X-rays revealed areas of collapse. As soon as these obstructions disappear, the bronchial mucosa looks normal. It is likely a harbinger of Aspergillus tracheobronchitis, a locally invasive pseudomembranous fungal infection.27 The fungus aspergilloma forms a tight clump of mycelia encased in fibrous walls and invades preexisting cavities, most commonly tubercular cavities. Most aspergillomas are small, solitary nodules that range in diameter from 8 to 10 cm. On radiographs, they look like a well-defined movable opacity inside a cavity, accompanied by what is commonly called an air crescent or Monod’s sign at the top boundary.28 An important source of morbidity and mortality in immunocompromised hosts, invasive aspergillosis is becoming more common. This is particularly true in individuals with acute leukemia, bone marrow, solid organ transplantation, or even AIDS. Treatment with antifungal agents, such as amphotericin B and the azole derivatives itraconazole and voriconazole, did not reduce the exceedingly high attributable mortality rate. The most common occurrence of invasive aspergillosis is death in approximately 85% of patients. A rare and mild lung infection known as critical necrotizing pulmonary aspergillosis (CNPA) can develop in people with compromised local defences as a result of a history of respiratory illness. Although pulmonary or systemic symptoms are almost always present in CNPA patients, the most prevalent symptom in aspergilloma patients, hemoptysis, is described in approximately 10% of CNPA patients and is seldom an isolated symptom. While aspergilloma grows noninvasively in healthy hosts by colonizing an existing cavity, Candida nonsporulans pulmonary aphthous (CNPA) grows noninvasively in immunocompromised hosts by creating its own cavity. Radiographs initially show consolidation zones in the upper lobe; subsequently, cavitation develops over the course of weeks or months.1,29
CNS aspergillosis
A. fumigatus is the most common causative agent of central nervous system aspergillosis, a dangerous fungal illness that affects 5% of cases. Possible symptoms include meningitis, granulomas, the rhino-cerebral form, and abscesses. Intracranial mass lesions, meningitis, granulomas, and ventriculitis are most commonly found. Other clinical symptoms include encephalitis, meningoencephalitis, stroke-like syndrome, intracranial space-occupying lesions, skull base syndrome, and intraorbital space-occupying lesions, which can be observed in CNS aspergillosis. Most cases of Aspergillus meningoencephalitis occur in people who have been on immunosuppressants for a long time, who have disseminated invasive aspergillosis, or who have undergone a bone marrow or solid organ transplant and rejected the new tissue. If an HIV-positive patient presents with vague neurological symptoms, a diagnosis of central nervous system aspergillosis should be considered.30
Paranasal sinuses aspergillosis
A. fumigatus may colonize and invade the paranasal sinus, leading to four types of nasal and paranasal sinus aspergillosis: allergic, noninvasive, invasive, and fulminant. The allergic form is a combination of Type I and Type III hypersensitivity to A. fumigatus antigens, while the noninvasive form results from aspergilloma formation and chronic sinusitis. The invasive form is malignant neoplasia that is slow-progressing and locally destructive. Fulminant disease is angioinvasive, rapidly destructive, and often fatal, spreading through bone to adjacent areas such as the orbit of the eye and the brain. In some countries, A. flavus is the common causative agent of paranasal sinus mycosis. Symptoms are usually nonspecific, with patients presenting with proptosis and polypoid lesions in the nose.31
Aspergillus endocarditis
Aspergillus endocarditis is a fungal infection that can occur in immunocompromised patients, particularly those who with prior cardiac surgery. It is the most common fungal organism after Candida infection in endocarditis patients after cardiothoracic surgery. Patients present with fever, multiple embolic strokes, and large fungal vegetation on heart valves. Risk factors include hyperalimentation, antibiotic therapy, intravenous drug abuse, concomitant bacterial endocarditis, and immunosuppression. Echocardiography reveals vegetation in most cases. Patients generally have a poor prognosis, and successful treatment relies on antifungal therapy and surgical removal of infected tissue.32
Cutaneous aspergillosis
This kind of aspergillosis is visible on the skin. It can manifest as primary cutaneous aspergillosis caused by direct external inoculation from objects such as arm boards, external catheter sites, or surgical or other traumas. Primary cutaneous aspergillosis can manifest as nodules, papules resembling molluscum, plaques, and ulcers. Early detection of primary cutaneous aspergillosis combined with medical and surgical treatment can eliminate the infection. This helps treat locally destructive diseases and prevent the spread of aspergillosis. Patients with pulmonary aspergillosis can develop secondary cutaneous aspergillosis, which usually occurs on the chest wall and is caused by the spread of an invasive skin disease. Most cases of cutaneous aspergillosis are found in cancer patients with low white blood cell (WBC) counts (neutropenic). It has also become a major infection in people with weak immune systems, such as newborns, people who have had organ transplants or have a disease called chronic granulomatous disease, people with autoimmune diseases who take corticosteroids, and people who have burned wounds. Aspergillosis of the skin is very common in people with HIV.33
Current laboratory diagnosis protocol
The laboratory diagnosis of A. fumigatus infection necessitates a multifaceted approach, given the complexities involved in fungal detection. Microscopic examination of clinical specimens provides direct visualization of septate hyphae, offering rapid but potentially specific identification. Sabouraud’s dextrose agar culture allows for the growth and characterization of A. fumigatus; however, the time required for this process and the potential for false negatives, especially in patients receiving antifungal therapy, underscore the need for complementary methods.34 Antigen detection methods, such as the galactomannan assay, leverage the presence of fungal cell wall components, but the risk of false positives due to cross-reactivity poses challenges. Molecular techniques, particularly polymerase chain reaction (PCR), amplify specific A. fumigatus DNA, enhancing sensitivity; however, they are very tedious and costly methods and require specialized equipment. Despite these diagnostic modalities, limitations persist. No single test offers both high sensitivity and specificity, emphasizing the importance of a comprehensive diagnostic strategy.35 An accurate clinical correlation with patient history, symptoms, and imaging findings can directly influence and subsequently enhance the diagnostic accuracy. However, knowledge of prior antifungal therapy and test sensitivity potentiates the role of patient’s medical history. Furthermore, tissue biopsy in cases of invasive infections can provide valuable information about a patient’s condition and other associated clinical circumstances. Therefore, A. fumigatus infection diagnosis requires careful integration of the various laboratory methods as well as focusing more on the respective applicability by the clinicians to draw a correlation with clinical findings in the patients.36
Direct examination
Various samples are collected to determine the presence of infection; these may include sputum, fluid from bronchoalveolar lavage (BAL), or a biopsy. The hyaline septate hyphae are 3-6 µm in diameter, and branches in two directions can be observed in clinical samples directly by preparing 10% KOH. Dichotomous acute-angle branching is used to spread out hyphae. Many times, the sections that branch out resemble fingers. Many hyphae spread throughout the tissue, frequently in parallel or radial lines, in invasive aspergillosis. Tangled masses of hyphae are the growth media for Aspergilli that inhabit cavitary lesions in the lungs. Hyphae may exhibit peculiar characteristics, such as enlargement as large as 12 µm in diameter or the absence of apparent septa, similar to Aspergillus fungal balls, if the infection persists for an extended period of time. Signs such as septate hyphae or vesicles in clinical specimens are necessary for clinical isolates to prove their significance in culture. However, direct results do not indicate that the infecting organism is Aspergillus or another kind of hyalohyphomycete, such as Fusarium or Scedosporium. In this case, the fungus needs to be grown in a laboratory so that a specific diagnosis can be made.36
Fungal culture
Culturing A. fumigatus on Sabouraud dextrose agar supplemented with antibiotics for up to 4 weeks and without cycloheximide for up to 4 weeks are two methods for growing this species. Positive urine cultures always indicate invasive aspergillosis and, more specifically, kidney abscesses that have spread throughout the body. Although endocarditis patients sometimes have positive blood cultures, A. fumigatus is rarely detected in urine, blood, or CSE. Exoantigen testing is invaluable for genus-level identification and, in the case of A. fumigatus, species-level identification. Histopathological analysis of patient tissue specimens has been strongly advised as a secondary method of cultural isolation and to connect its clinical and pathological importance. The isolation of the fungus from lung secretions and the clinical course of chronic necrotizing pulmonary aspergillosis (CNPA) typically support a diagnosis of the condition. It is generally challenging to obtain the histologic proof necessary for diagnostic confirmation of local lung tissue invasion by septate hyphae, which is typical of Aspergillus species. Compared to autopsy results, diagnostic yields for locally invasive aspergillosis from transbronchial and percutaneous biopsies are poor. When sputum is present, the sensitivity of such culture is likely on the order of 50% to 60%, and in many situations, it is not even available for culture. Cultures of the mucosal secretions from the respiratory tracts collected by bronchoalveolar lavage, bronchial washing, or brushing almost always yield similar results. Sputum samples exhibiting growth of A. fumigatus without negative serological finding can be considered as an oral colonization. However, positive sputum growth from the BAL fluid cultures can be considered a potentially accurate diagnostic tool in most cases; however, in a few interesting cases, it did not correlate with immunodiagnosis.37-39
Immunodiagnosis
Immunological tests are very important for diagnosing different types of aspergilloses, especially aspergilloma, where many patients have precipitated IgG antibodies. A positive skin test for Aspergillus antigens and high serum levels of IgE and specific IgE and IgG antibodies are needed to diagnose allergic bronchopulmonary aspergillosis. Detecting circulating antibodies or Aspergillus antigens helps determine what caused an infection in people whose cultures were negative, whose infections were covered by or misdiagnosed as something else, or who had other diseases simultaneously. Serology, which uses techniques such as indirect immunofluorescence, immunoelectrophoresis, immunoelectrodiffusion, enzyme-linked immunosorbent assay, enzyme-linked immunofiltration assay, and immunoblotting, may be an alternative to isolating cells in the culture.40
Detection of antibody
Immunological tests are crucial for diagnosing various forms of aspergillosis, especially in cases such as aspergilloma, where precipitating IgG antibodies against Aspergillus are common. For allergic bronchopulmonary aspergillosis (ABPA), specific diagnostic criteria include positive skin test reactions to Aspergillus antigens, elevated IgE levels, and specific IgE and IgG precipitating antibodies in the patient’s serum. These markers indicate an immune response to Aspergillus allergens, providing strong evidence for ABPA diagnosis. The detection of antibodies (IgE and IgE against Aspergillus antigens) in the sera of patients could be considered the gold standard when World Health Organization-International Union of Immunological Society (WHO-IUIS)-certified allergens (Asp f1-4 and 6) have been used for component-resolved diagnostic platforms (ImmunoCAP), but cross-reactivity with other fungal allergens has made their universal diagnostic utility questionable. Immunological tests are also helpful when conventional culture-based methods face limitations, such as negative cultures, or when other microorganisms or diseases mask Aspergillus growth. Serological testing, which uses various immunological techniques, can help diagnose patients with coexisting fungal infections or complex clinical presentations. By examining the patient’s immune response through serological tests, proper diagnosis can be established, treatment can be guided, and the progression of aspergillosis can be monitored, ultimately improving patient care and outcomes.41
Detection of antigen
Detecting Aspergillus species antibodies can be challenging, especially in patients with compromised immune systems. Factors such as immunosuppression, antifungal therapy, or infection progression can lead to the absence of detectable antibodies. In such cases, fungal antigen identification can provide an early and accurate diagnosis. Tests such as latex agglutination, radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and BALISA have been widely used to detect soluble antigens in clinical samples. Latex agglutination involves using latex beads coated with antibodies specific to Aspergillus antigens. When mixed with patient samples, Aspergillus antigens can cause agglutination or clumping. Radioimmunoassay (RIA) a susceptible technique that uses radiolabelled antibodies to detect specific antigens. ELISA is another quantitative method used due to its sensitivity and specificity. BALISA is a specialized immunological test used to detect antigens in bronchoalveolar lavage fluid and is particularly useful in cases of pulmonary aspergillosis. After immunoblotting, Aspergillus antigens were separated based on molecular weight and probed with specific antibodies. Aspergillus galactomannan is a common target for detection, as its presence in patient samples has been associated with invasive aspergillosis. An ELISA designed to detect Aspergillus galactomannan is a valuable tool for early diagnosis. However, a negative result from antigen detection tests does not definitively rule out the diagnosis of aspergillosis. Clinical judgment, a combination of diagnostic methods, and careful consideration of the patient’s clinical presentation remain crucial in the overall diagnostic process for aspergillosis.42,43
Molecular techniques
Molecular techniques have become highly sensitive and specific methods for diagnosing fungal infections, even when traditional methods such as fungal culture or serological assays fail. These methods provide rapid results, often on the same day, which is particularly valuable for identifying and treating potentially life-threatening mycotic diseases, especially among immunocompromised patients. DNA probes designed to target specific genetic sequences unique to the pathogen have proven valuable in diagnosing diseases caused by A. fumigatus, which can be fatal, especially in individuals with compromised immune systems. In cases of disseminated infection, serodiagnostic approaches may fail due to anergy, where the immune response is weakened or absent. Molecular techniques based on DNA analysis, such as the nested polymerase chain reaction (PCR) assay, which can detect A. fumigatus DNA, have addressed this limitation. However, it may not differentiate between active infection and colonization. PCR can also be useful for monitoring the response to antifungal therapy, particularly quantitative PCR, which allows for measuring the fungal burden of treatment response. Changes in fungal DNA levels over time were tracked. Overall, molecular techniques have significantly improved the diagnosis of fungal infections caused by Aspergillus species.44,45
Molecular typing
Several molecular typing techniques have been used to meet reproducibility criteria and distinguish A. fumigatus from other types of aspergilli. Some phenotypic traits, such as antigen, enzyme, morphology, and biochemical parameters, can guide these techniques. Genotypic methods include the use of genomic DNA, mitochondrial DNA (mtDNA), and ribosomal DNA (rDNA). Restriction fragment length polymorphism (RFLP) analysis involves the use of enzymes (restriction endonucleases) that cut specific DNA sequences to identify differences in the genome.46
Detection of fungal metabolites
Diagnosing aspergillosis can be challenging, especially in cases where culture-based methods are unavailable. In such situations, detecting distinctive metabolic products in patient fluids or tissues can provide valuable diagnostic information. Several diagnostic approaches have been developed to identify these markers:
G-test for β-(1,3)-D-glucan detection
The G-test, developed by Japanese researchers, is a diagnostic tool that detects the presence of circulating β-(1,3)-D-glucan in patient samples. This assay uses a modified Limulus assay, which was originally designed to detect endotoxins, with a sensitivity of approximately 20 pg/ml. β-(1,3)-D-glucan is a component of fungal cell walls, and its presence in patient samples can indicate invasive fungal infections, including invasive aspergillosis. However, this test does not differentiate between fungal species, such as Candida and Aspergillus. Culture results are unavailable or negative and valuable for confirming invasive mycoses. In some cases of aspergilloma, the G-test may also yield positive results, but clinical evaluation can help distinguish between the conditions.47
D-mannitol detection by gas liquid chromatography
In experimental studies using rats with induced invasive aspergillosis, researchers have identified a high concentration of D-mannitol, a fungal metabolite, in the serum. This finding suggests that D-mannitol has the potential to serve as a diagnostic marker for invasive aspergillosis in humans. Gas liquid chromatography (GLC) detects and quantifies D-mannitol levels in patient serum samples. Elevated D-mannitol concentrations may indicate the presence of invasive aspergillosis.48
Skin tests
Patients who might have allergic bronchopulmonary aspergillosis, atopic dermatitis, or allergic asthma caused by Aspergillus species were given a skin test to determine how they reacted to Aspergillus antigen extracts. Intradermal skin tests were performed on all patients and controls with 0.1 ml of antigen (1000 PNU/ml aspergillin). Within an hour, erythema and wheal are observed in Type I hypersensitivity, Type III-Arthus reactions occur in 4 to 10 hours, and Type IV reactions occur in a duration of more than 5 mm in diameter 24 hours later.49 A list of available diagnostic methods that are usually used in combination for the detection of Aspergillus-induced infections in patients is shown diagrammatically in Figure 2.
Limitations of the current methods used in the diagnosis of A. fumigatus
Advancements in diagnosing A. fumigatus infections have introduced various diagnostic methods, each with inherent limitations that pose challenges to accurate identification and interpretation. Traditional methods such as histology, cytology, and culture, though considered the gold standards, present drawbacks such as time-consuming procedures and the need for skilled labor, which may delay the initiation of therapy, which is crucial for patient survival. Additionally, these methods may lack specificity, leading to potential misidentification and compromising the accuracy of early antifungal therapy, particularly in critically ill patients.50 Nonculture-based approaches such as galactomannan detection and PCR assays offer alternatives but exhibit variability in sensitivity and specificity, contributing to false-positive and false-negative results and complicating the diagnostic process.51 Moreover, DNA analysis from formalin-fixed paraffin-embedded tissue sections faces challenges such as DNA degradation and inhibitory substances, emphasizing the importance of meticulous specimen handling for reliable molecular diagnostics.52 Similarly, studies relying on bronchoalveolar lavage fluid (BALF) for diagnosis are prone to contamination issues, necessitating stringent quality control measures to differentiate between invasive disease and colonization or contamination.53 While promising, serum biomarkers such as galactomannan and β-D-glucan may yield false-positive results, limiting their diagnostic utility. In contrast, novel technologies such as Aspergillus-specific lateral flow devices and pentraxin three monitoring show promise but require robust validation before widespread adoption.54 Siderophore-based diagnostics face challenges in differentiating invasive pulmonary aspergillosis from chronic pulmonary aspergillosis, further complicating diagnostic accuracy.55 In the face of these challenges, the emergence of technologies such as lateral flow device (LFD), MALDI-TOF mass spectrometry, and next-generation sequencing (NGS) herald a new era in Aspergillus diagnostics. The LFD offers quick operational answers with high specificity and sensitivity, complemented by PCR confirmation if needed. MALDI-TOF mass spectrometry promises quicker identification, which is crucial in clinical settings where timely diagnosis is imperative.34,56,57 Moreover, the increase in azole resistance in A. fumigatus, driven by the broad use of azole drugs, underscores the need for innovative approaches. Mutations in the cyp51A gene contribute to resistance, highlighting the importance of detecting antifungal resistance promptly.58 NGS represents a revolutionary step in identifying Aspergillus species and detecting resistance mutations directly, circumventing the limitations of culture-based methods and offering insights into resistance evolution. Despite these advancements, the outlook for invasive aspergillosis remains challenging, with high mortality rates and complex risk factors. However, integrating LFD, MALDI-TOF, and NGS into diagnostic algorithms holds promise for transforming Aspergillus infection management, including the detection of antifungal resistance.59 Continued research and validation efforts are crucial to harnessing the full potential of these technologies and improving patient outcomes in the fight against invasive aspergillosis.
Future prospects of advancements and challenges in diagnosing A. fumigatus Infections
The diagnosis of A. fumigatus infections has significantly evolved with the introduction of various diagnostic methods, yet inherent limitations persist, posing challenges to accuracy and timely treatment. Traditional methods such as histology, cytology, and culture remain the gold standards but are time-consuming, labor-intensive, and require skilled personnel, often delaying critical therapy. These methods also suffer from limited specificity, which can lead to misidentification and compromise early antifungal treatment, especially in critically ill patients. Serological approaches, such as detecting antibodies (IgE and IgG) against Aspergillus antigens, are considered reliable when WHO-IUIS-certified allergens (Asp f1-4 and 6) are used on platforms like ImmunoCAP. However, cross-reactivity with other fungal allergens restricts their universal diagnostic applicability. Nonculture-based methods, including galactomannan detection and PCR assays, provide alternatives but are hampered by variability in sensitivity and specificity, leading to false-positive and false-negative results. Molecular diagnostics involving DNA analysis from formalin-fixed paraffin-embedded tissue face obstacles like DNA degradation and inhibitory substances, underlining the need for meticulous sample handling. Similarly, diagnostic studies using bronchoalveolar lavage fluid (BALF) are vulnerable to contamination, requiring stringent quality control to differentiate between true invasive disease, colonization, and contamination. Serum biomarkers, such as galactomannan and β-D-glucan, show promise but may yield false positives, limiting their diagnostic reliability. Emerging technologies like Aspergillus-specific lateral flow devices (LFD) and pentraxin-3 monitoring demonstrate potential but require robust validation before routine clinical implementation. Siderophore-based diagnostics face challenges in distinguishing invasive pulmonary aspergillosis (IPA) from chronic pulmonary aspergillosis, further complicating diagnostic precision. The emergence of advanced technologies marks a new era in Aspergillus diagnostics. Lateral flow devices (LFD) offer rapid and sensitive detection, providing operational answers quickly, which can be complemented by PCR confirmation for enhanced accuracy. MALDI-TOF mass spectrometry is a transformative tool that enables rapid fungal identification, an essential advantage in clinical settings where timely diagnosis can improve outcomes. Next-generation sequencing (NGS) represents a revolutionary advancement, enabling precise identification of Aspergillus species and direct detection of antifungal resistance mutations, circumventing limitations of culture-based methods. The rising incidence of azole resistance in A. fumigatus, largely due to the widespread use of azole drugs, underscores the importance of detecting resistance promptly. Mutations in the cyp51A gene are a major contributor to resistance, and technologies like NGS provide crucial insights into resistance mechanisms and their evolution. Despite these advancements, the management of invasive aspergillosis remains challenging, with high mortality rates and complex risk factors. The integration of novel diagnostic tools LFD, MALDI-TOF mass spectrometry, and NGS into clinical workflows offers a promising future for improved detection, diagnosis, and management of Aspergillus infections, including antifungal resistance. Continued research, validation, and technological refinement are essential to fully harness these advancements, ultimately improving patient outcomes in combating invasive aspergillosis.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PK, SAM, SA, K and JP wrote the manuscript. SM, AKS, SA and AS reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Latge J-P, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33(1):e00140-18.
Crossref - Lamoth F. Aspergillus fumigatus-Related Species in Clinical Practice. Front Microbiol. 2016;7:683.
Crossref - Mandal V, Adhikary R, Maiti PK, Mandal S, Mandal V. Morpho-biochemical and molecular characterization of two new strains of Aspergillus fumigatus nHF-01 and A. fumigatus PPR-01 producing broad-spectrum antimicrobial compounds. Braz J Microbiol. 2021;52(2):905-917.
Crossref - Krach EK, Wu Y, Skaro M, Mao L, Arnold J. Wild Isolates of Neurospora crassa Reveal Three Conidiophore Architectural Phenotypes. Microorganisms. 2020;8(11):1760.
Crossref - Zeng M, Zhou X, Yang C, et al. Comparative analysis of the biological characteristics and mechanisms of azole resistance of clinical Aspergillus fumigatus strains. Front Microbiol. 2023;14:1253197.
Crossref - dos Santos RAC, Steenwyk JL, Rivero-Menendez O, et al. Genomic and Phenotypic Heterogeneity of Clinical Isolates of the Human Pathogens Aspergillus fumigatus, Aspergillus lentulus, and Aspergillus fumigatiaffinis. Front Genet. 2020;11:459.
Crossref - Earle K, Valero C, Conn DP, et al. Pathogenicity and virulence of Aspergillus fumigatus. Virulence. 2023;14(1):2172264.
Crossref - Croft CA, Culibrk L, Moore MM, Tebbutt SJ. Interactions of Aspergillus fumigatus Conidia with Airway Epithelial Cells: A Critical Review. Front Microbiol. 2016;7:472.
Crossref - Korfanty G, Heifetz E, Xu J. Assessing thermal adaptation of a global sample of Aspergillus fumigatus: Implications for climate change effects. Front Public Health. 2023;11:1059238.
Crossref - Fontaine T, Latge J-P. Galactomannan Produced by Aspergillus fumigatus: An Update on the Structure, Biosynthesis and Biological Functions of an Emblematic Fungal Biomarker. J Fungi. 2020;6(4):283.
Crossref - Perez-Cuesta U, Guruceaga X, Cendon-Sanchez S, et al. Nitrogen, Iron, and Zinc Acquisition: Key Nutrients to Aspergillus fumigatus Virulence. J Fungi. 2021;7(7):518.
Crossref - Desoubeaux G, Cray C. Rodent Models of Invasive Aspergillosis due to Aspergillus fumigatus: Still a Long Path toward Standardization. Front Microbiol. 2017;8:841.
Crossref - Ries LNA, Steenwyk JL, de Castro PA, et al. Nutritional Heterogeneity Among Aspergillus fumigatus Strains Has Consequences for Virulence in a Strain- and Host-Dependent Manner. Front Microbiol. 2019;10:854.
Crossref - Garcia-Rubio R, de Oliveira HC, Rivera J, Trevijano-Contador N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front Microbiol. 2020;10:2993.
Crossref - van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latge J-P. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol 2017;15(11):661-74.
Crossref - Akoumianaki T, Kyrmizi I, Valsecchi I, et al. Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell Host Microbe. 2016;19(1):79-90.
Crossref - Keizer EM, Valdes ID, McCann BL, Bignell EM, Wosten HAB, de Cock H. The Protective Role of 1,8-Dihydroxynaphthalene-Melanin on Conidia of the Opportunistic Human Pathogen Aspergillus fumigatus Revisited: No Role in Protection against Hydrogen Peroxide and Superoxides. MSphere. 2022;7(1):e0087421.
Crossref - Arias M, Santiago L, Vidal-Garcia M, et al. Preparations for Invasion: Modulation of Host Lung Immunity During Pulmonary Aspergillosis by Gliotoxin and Other Fungal Secondary Metabolites. Front Immunol. 2018;9:2549.
Crossref - Silva LP, Horta MAC, Goldman GH. Genetic Interactions Between Aspergillus fumigatus Basic Leucine Zipper (bZIP) Transcription Factors AtfA, AtfB, AtfC, and AtfD. Front Fungal Biol. 2021;2:632048.
Crossref - Shwab EK, Juvvadi PR, Waitt G, et al. The Protein Kinase A-Dependent Phosphoproteome of the Human Pathogen Aspergillus fumigatus Reveals Diverse Virulence-Associated Kinase Targets. MBio. 2020;11(6):e02880-20.
Crossref - Bultman KM, Kowalski CH, Cramer RA. Aspergillus fumigatus virulence through the lens of transcription factors. Med Mycol. 2017;55(1):24-38.
Crossref - Otu A, Kosmidis C, Mathioudakis AG, Ibe C, Denning DW. The clinical spectrum of aspergillosis in chronic obstructive pulmonary disease. Infection. 2023;51(4):813-829.
Crossref - Strickland AB, Shi M. Mechanisms of fungal dissemination. Cell Mol Life Sci. 2021;78(7):3219-38.
Crossref - Zhang M, Sun D, Liu G, Wu H, Zhou H, Shi M. Real-time in vivo imaging reveals the ability of neutrophils to remove Cryptococcus neoformans directly from the brain vasculature. J Leukoc Biol. 2016;99(3):467-473.
Crossref - Davis JM, Huang M, Botts MR, Hull CM, Huttenlocher A. A Zebrafish Model of Cryptococcal Infection Reveals Roles for Macrophages, Endothelial Cells, and Neutrophils in the Establishment and Control of Sustained Fungemia. Infect Immun. 2016;84(10):3047-3062.
Crossref - Sisodia J, Bajaj T. Allergic Bronchopulmonary Aspergillosis. Statpearls Publishing 2023.
- Knutsen AP, Temprano J, Bhatla D, Slavin RG. Hypersensitivity Pneumonitis and Eosinophilic Lung Diseases. Kendig’s Disorders of the Respiratory Tract in Children, Elsevier. 2019:944-967.
Crossref - Rebanta K Chakraborty, Krishna M Baradhi. Aspergilloma. Statpearls. 2022.
- Robin C, Cordonnier C, Sitbon K, et al. Mainly Post-Transplant Factors Are Associated with Invasive Aspergillosis after Allogeneic Stem Cell Transplantation: A Study from the Surveillance des Aspergilloses Invasives en France and Societe Francophone de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transplant. 2019;25(2):354-361.
Crossref - Miceli MH. Central Nervous System Infections Due to Aspergillus and Other Hyaline Molds. J Fungi. 2019;5(3):79.
Crossref - Chakrabarti A, Kaur H. Allergic Aspergillus Rhinosinusitis. J Fungi 2016;2(4):32.
Crossref - Hatlen TJ, Filler SG, Bayer A, Shah S, Shodhan S, Van TT. Aspergillus endocarditis diagnosed by fungemia plus serum antigen testing. Med Mycol Case Rep. 2019;23:1-3.
Crossref - Merad Y, Derrar H, Belmokhtar Z, Belkacemi M. Aspergillus Genus and Its Various Human Superficial and Cutaneous Features. Pathogens. 2021;10(6):643.
Crossref - Stemler J, Tobben C, Lass-Florl C, et al. Diagnosis and Treatment of Invasive Aspergillosis Caused by Non-fumigatus Aspergillus spp. J Fungi. 2023;9(4):500.
Crossref - Bakhtiari MR, Behravan D, BolourchI SM, et al. Laboratory and Diagnosis. 14th ed. Journal of Iranian Association of Clinical laboratory Doctors. 2012;3.
- Patterson TF, Thompson GR, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-60.
Crossref - Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68.
Crossref - Kanaujia R, Singh S, Rudramurthy SM. Aspergillosis: an Update on Clinical Spectrum, Diagnostic Schemes, and Management. Curr Fungal Infect Rep. 2023;17:144-55.
Crossref - Salzer HJF, Prattes J, Hoenigl M. Editorial: Diagnostic Approaches for Aspergillus Infections. Front Microbiol. 2019;10:446.
Crossref - Patel AR, Patel AR, Singh S, Singh S, Khawaja I. Diagnosing Allergic Bronchopulmonary Aspergillosis: A Review. Cureus. 2019;11(4):e4550.
Crossref - Richardson M, Page I. Role of Serological Tests in the Diagnosis of Mold Infections. Curr Fungal Infect Rep. 2018;12(3):127-136.
Crossref - Lass-Florl C, Samardzic E, Knoll M. Serology anno 2021-fungal infections: from invasive to chronic. Clin Microbiol Infect. 2021;27(9):1230-41.
Crossref - Fang W, Wu J, Cheng M, et al. Diagnosis of invasive fungal infections: challenges and recent developments. J Biomed Sci. 2023;30(1):42.
Crossref - Wickes BL, Wiederhold NP. Molecular diagnostics in medical mycology. Nat Commun. 2018;9(1):5135.
Crossref - Freeman Weiss Z, Leon A, Koo S. The Evolving Landscape of Fungal Diagnostics, Current and Emerging Microbiological Approaches. J Fungi. 2021;7(2):127.
Crossref - Arastehfar A, Carvalho A, Houbraken J, et al. Aspergillus fumigatus and aspergillosis: From basics to clinics. Stud Mycol. 2021;100:100115.
Crossref - White SK, Schmidt RL, Walker BS, Hanson KE. (1→3)-b-D-glucan testing for the detection of invasive fungal infections in immunocompromised or critically ill people. Cochrane Database Syst Rev. 2020;2020:CD009833.
Crossref - Skriba A, Pluhacek T, Palyzova A, et al. Early and Non-invasive Diagnosis of Aspergillosis Revealed by Infection Kinetics Monitored in a Rat Model. Front Microbiol. 2018;9:2356.
Crossref - Sunman B, Ademhan Tural D, Ozsezen B, Emiralioglu N, Yalcin E, Ozcelik U. Current Approach in the Diagnosis and Management of Allergic Bronchopulmonary Aspergillosis in Children With Cystic Fibrosis. Front Pediatr 2020;8:582964.
Crossref - Takazono T, Izumikawa K. Recent Advances in Diagnosing Chronic Pulmonary Aspergillosis. Front Microbiol. 2018;9:1810.
Crossref - Egger M, Jenks JD, Hoenigl M, Prattes J. Blood Aspergillus PCR: The Good, the Bad, and the Ugly. J Fungi. 2020;6:18(1):18.
Crossref - Sadamoto S, Mitsui Y, Nihonyanagi Y, et al. Comparison Approach for Identifying Missed Invasive Fungal Infections in Formalin-Fixed, Paraffin-Embedded Autopsy Specimens. J Fungi. 2022;8(4):337.
Crossref - Imbert S, Meyer I, Palous M, et al. Aspergillus PCR in Bronchoalveolar Lavage Fluid for the Diagnosis and Prognosis of Aspergillosis in Patients With Hematological and Non-hematological Conditions. Front Microbiol. 2018;9:1877.
Crossref - Dichtl K, Forster J, Ormanns S, et al. Comparison of b-D-Glucan and Galactomannan in Serum for Detection of Invasive Aspergillosis: Retrospective Analysis with Focus on Early Diagnosis. J Fungi 2020;6(4):253.
Crossref - Yeoh DK, McMullan BJ, Clark JE, Slavin MA, Haeusler GM, Blyth CC. The Challenge of Diagnosing Invasive Pulmonary Aspergillosis in Children: A Review of Existing and Emerging Tools. Mycopathologia. 2023;188(5):731-743.
Crossref - Bao S, Song H, Chen Y, Zhong C, Tang H. Metagenomic next-generation sequencing for the diagnosis of pulmonary aspergillosis in non-neutropenic patients: a retrospective study. Front Cell Infect Microbiol. 2022;12:925982.
Crossref - Hsiao H-H, Liu Y-C, Wang H-C, et al. Comparison of a novel lateral-flow device to galactomannan assay at different time periods for detections of invasive aspergillosis. J Formos Med Assoc. 2022;121(10):2123-2129.
Crossref - Hsu T-H, Huang P-Y, Fan Y-C, Sun P-L. Azole Resistance and cyp51A Mutation of Aspergillus fumigatus in a Tertiary Referral Hospital in Taiwan. J Fungi. 2022;8(9):908.
Crossref - Jiang S, Chen Y, Han S, Lv L, Li L. Next-Generation Sequencing Applications for the Study of Fungal Pathogens. Microorganisms. 2022;10(10):1882.
Crossref - Srivastava S, Maurya NS, Kushwah S, Mani A. Current Promising Therapeutic Targets for Aspergillosis Treatment. J Pure Appl Microbiol. 2021;15(2):484-499.
Crossref - Jia L-J, Kruger T, Blango MG, von Eggeling F, Kniemeyer O, Brakhage AA. Biotinylated Surfome Profiling Identifies Potential Biomarkers for Diagnosis and Therapy of Aspergillus fumigatus Infection. MSphere. 2020;5(4):e00535.
Crossref - Baltussen TJH, Coolen JPM, Zoll J, Verweij PE, Melchers WJG. Gene co-expression analysis identifies gene clusters associated with isotropic and polarized growth in Aspergillus fumigatus conidia. Fungal Genet Biol. 2018;116:62-72.
Crossref - Schmidt H, Vlaic S, Kruger T, et al. Proteomics of Aspergillus fumigatus Conidia-containing Phagolysosomes Identifies Processes Governing Immune Evasion. Mol Cell Proteomics. 2018;17(6):1084-96.
Crossref - Baltussen TJH, Zoll J, Verweij PE, Melchers WJG. Molecular Mechanisms of Conidial Germination in Aspergillus spp. Microbiol Mol Biol Rev. 2020;84(1):e00049-19.
Crossref - Blachowicz A, Chiang AJ, Romsdahl J, Kalkum M, Wang CCC, Venkateswaran K. Proteomic characterization of Aspergillus fumigatus isolated from air and surfaces of the International Space Station. Fungal Genetics and Biology. 2019;124:39-46.
Crossref - Yaakoub H, Mina S, Calenda A, Bouchara J-P, Papon N. Oxidative stress response pathways in fungi. Cell Mol Life Sci. 2022;79(6):333.
Crossref - Ross BS, Lofgren LA, Ashare A, Stajich JE, Cramer RA. Aspergillus fumigatus In-Host HOG Pathway Mutation for Cystic Fibrosis Lung Microenvironment Persistence. MBio. 2021;12(4):e0215321.
Crossref - Park H-S, Lee M-K, Kim SC, Yu J-H. The role of VosA/VelB-activated developmental gene vadA in Aspergillus nidulans. PLoS One. 2017;12(5):e0177099.
Crossref - Tan Y, Wang H, Wang Y, et al. The role of the veA gene in adjusting developmental balance and environmental stress response in Aspergillus cristatus. Fungal Biol. 2018;122(10):952-964.
Crossref - Samalova M, Carr P, Bromley M, et al. GPI Anchored Proteins in Aspergillus fumigatus and Cell Wall Morphogenesis. In: Latgé, JP. (eds) The Fungal Cell Wall. Current Topics in Microbiology and Immunology, vol 425. Springer, Cham. 2020;425:167-86.
Crossref - Aimanianda V, Simenel C, Garnaud C, et al. The Dual Activity Responsible for the Elongation and Branching of b-(1,3)-Glucan in the Fungal Cell Wall. MBio. 2017;8(3):e00619-17.
Crossref - Rizzo J, Chaze T, Miranda K, et al. Characterization of Extracellular Vesicles Produced by Aspergillus fumigatus Protoplasts. MSphere. 2020;5(4):e00476–20.
Crossref - Brauer VS, Pessoni AM, Freitas MS, Cavalcanti-Neto MP, Ries LNA, Almeida F. Chitin Biosynthesis in Aspergillus Species. J Fungi. 2023;9(1):89.
Crossref - Vargas-Muniz JM, Renshaw H, Waitt G, et al. Caspofungin exposure alters the core septin AspB interactome of Aspergillus fumigatus. Biochem Biophys Res Commun. 2017;485(2):221-226.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.