ISSN: 0973-7510
E-ISSN: 2581-690X
The study mediates silver nanoparticles (AgNPs) synthesis from Euphorbia geniculata and is evaluated for its multifunctional applications in healthcare, agriculture, and environmental sustainability. The AgNPs showed bactericidal activity against an array of human pathogens, including Escherichia coli, Salmonella typhi, and Bacillus cereus, with a 1.3 cm zone of inhibition and a 1.1 cm zone of inhibition against Staphylococcus aureus. The antioxidant potential of AgNPs were authenticated with the DPPH assay, which demonstrated scavenging activity of 43.22% at 75 mg. Plant growth promotion of AgNPs was evaluated, which showed an increase in the root and shoot length. The AgNPs also displayed dye degradation efficacy with a time interval of 24 hours. The AgNPs were biophysically characterized via spectroscopic analysis, which depicted a maximum peak at 418 nm. FTIR analysis revealed the functional groups at different frequencies, with major groups identified as hydroxyl and carboxyl groups. The size distribution pattern of AgNPs was studied with DLS analysis, showing the size of the particles as 201 nm. The morphological characterisation using TEM showed polydispersity from 10 nm to 100 nm. Additionally, XRD results proved the crystalline structure of synthesised AgNPs, showing the 2θ peaks at 38°, 44°, 64°, and 77°. The Phyto metabolomic studies of Euphorbia geniculata showed 40 different active phytocomponents in the methanolic extract. Some of the major metabolites include derivatives of 1-butanol, oleic acid, and n-hexadecanoic acid through GC-MS analysis. Overall, the study demonstrates the multifunctional properties of nanoparticles with profound activities.
Euphorbia geniculata, Silver Nanoparticles, Dye Degradation, Plant Growth Promotion
Recent advances in nanoscience have revolutionized multiple scientific disciplines by enabling the production of multifunctional nanoparticles with enhanced physicochemical and biological potential.1 Among various nanomaterials, AgNPs have gained importance due to their unique optical, catalytic, and biological characteristics.2 AgNPs exhibit a broad spectrum of bioactivities, including potent antibacterial, antifungal, antiviral, antioxidant, and anticancer properties. Thus, leading to applications in healthcare, agriculture, and environmental remediation.3
A critical aspect in realising the potential of AgNPs lies in the synthesis protocol, which not only dictates the particle’s morphology and bioactivity but also determines its environmental footprint. Green synthesis approaches using biological systems have become evident as sustainable substitutes to conventional chemical and physical procedures in this context.4 Plant-mediated synthesis, in particular, offers numerous advantages such as simplicity, cost-effectiveness, scalability, and environmental benignity.5 Moreover, plants serve as a dual source of both reducing and stabilising agents through their diverse phytochemical constituents, eliminating the need for toxic reagents.6
Green-synthesised AgNPs are emerging as pivotal components in next-generation biomedical technologies due to their superior biocompatibility, surface functionalization potential, and controlled physicochemical attributes.7 A study demonstrated the synthesis of AgNPs by mixing aqueous extracts of Paullinia cupana Kunth and AgNO3 (1 mM) in a 1:9 ratio and heating at 70 °C for 180 mins. These nanoparticles were effective against several bacterial and fungal strains.8 Pirtarighat et al.9 synthesized AgNPs by treating Salvia spinosa extract with AgNO3 (1 mM) in the ratio 1:9 and stirring it continuously for 6 hours. The inhibitory function of these nanoparticles was effective against Gram- and Gram+ bacteria. In another study, antifungal AgNPs were produced by dropwise adding Avena fatua extract to AgNO3 stock solution and stirring on a magnetic plate for 3 hours.10 In similar terms, Asif et al.11 synthesized AgNPs by dropwise adding Moringa oleifera leaves extract in 100 ml AgNO3 solution in the temperature range 60 °C-80 °C for an hour.
3 mM AgNO3 treated with Azadirachta indica extract in the ratio 1:5, followed by incubation in the dark for 24 hours, synthesized AgNPs which were strong inhibitors of P. aeruginosa.12 Phanse et al.13 reported confirmation of AgNPs by mixing AgNO3 and Tinospora cordifolia leaf extract in the ratio 8:2 followed by heating at 75 °C for 25 mins.
These nanoparticles have been successfully integrated into multifunctional wound dressings that exhibit enhanced antimicrobial efficacy, accelerated epithelialization, and prevent wound infection, thereby facilitating rapid tissue regeneration.14-16 Furthermore, their surface-modifiable nature enables conjugation with therapeutic agents, making them efficient carriers in drug delivery systems, particularly for site-specific cancer therapy and antimicrobial interventions.17-20 In tissue engineering, AgNPs are increasingly incorporated into bioactive scaffolds to promote cell adhesion, proliferation, and extracellular matrix remodelling, while concurrently protecting implant-associated infections.21 Moreover, their unique surface plasmon resonance properties have facilitated their application in high-sensitivity biosensors for the early detection of biomarkers, pathogens, and environmental toxins.22,23
Additionally, the intrinsic antioxidant potential of AgNPs offers therapeutic benefits in reducing oxidative stress, a key contributor to chronic and degenerative diseases. Beyond medical applications, AgNPs synthesized via green routes have shown promise in agricultural systems, where they function as intelligent nano-formulations for sustainable crop management.24-26 These include nano-enabled pesticides with controlled release profiles and enhanced target specificity. Recent studies highlight nano-fertilizers that optimize nutrient bioavailability and uptake efficiency, and nanoscale diagnostic platforms for real-time detection of plant pathogens through colorimetric, electrochemical, or fluorescence-based sensing modalities. Such precision agriculture interventions not only enhance crop yield and quality but also reduce agrochemical runoff and environmental toxicity, aligning with the principles of circular bioeconomy and sustainable farming practices.27,28 Moreover, environmental applications of AgNPs are increasingly being recognised, particularly in wastewater treatment, where their catalytic properties facilitate the decomposition of hazardous synthetic dyes like methylene blue (MB) and crystal violet (CV) into less toxic byproducts.29,30 These technologies represent a fast-advancing field of applied research with significant societal impact.
Among various plant species explored for nanoparticle biosynthesis, the genus Euphorbia stands out due to its rich reservoir of bioactive metabolites, including flavonoids, terpenoids, phenolics, and alkaloids.31 These metabolites reduce metal ions and act as stabilizing agents for the nanoparticles, thereby enhancing their physicochemical stability and biological efficacy.32 While extensive studies have focused on several Euphorbia species, the role of Euphorbia geniculata in nanoparticle biosynthesis remains underexplored. However, preliminary phytochemical profiling of Euphorbia geniculata reveals a repertoire of secondary metabolites comparable to other Euphorbia species, suggesting its potential as an effective biogenic source.33 The integration of Euphorbia geniculata-mediated AgNPs could open new avenues in combating multidrug-resistant (MDR) pathogens, an escalating global health concern.34-36
Given these multidimensional applications, the current research seeks to achieve the synthesis of multifunctional AgNPs using Euphorbia geniculata as a green biogenic source. This approach not only harnesses the phytochemical richness of the plant but also lines up with the principles of sustainable nanotechnology, offering a novel strategy for addressing contemporary challenges in health, agriculture, and environmental management.
The healthy plant materials of Euphorbia geniculata were obtained from an abundantly growing area of Mysuru and transported to the laboratory for further processing. The chemicals used in this study were of analytical grade, including AgNO3 (Sigma-Aldrich), DPPH (2,2-Diphenyl-1-Picrylhydrazyl) (SRL), Crystal violet (Sigma Aldrich), Methylene Blue (Sigma Aldrich), Methanol (CH3OH) (Merck), and Ascorbic acid (C6H8O6) (Sigma-Aldrich). Additionally, seeds of Vigna radiata and Cicer arietinum were obtained from the local market.
Methods
Plant processing
The healthy plant materials were repeatedly washed to remove any residual impurities. Following this, the plant materials were processed to obtain leaf extract by boiling 20 g in 100 ml of sterile water at 80 °C until the colour changed, and the filtrate was separated.37
AgNPs and their characterisation
The AgNPs were synthesised by treating AgNO3 with plant extract in a 9:1 ratio with slight modifications as reported in Lima et al.8 The mixture was processed at an elevated temperature above 60 °C. A transition from yellow to reddish-brown was observed, which was then subjected to Ultracentrifugation. The AgNPs obtained in the pellet were analysed through UV-visible spectroscopy for confirmation. Fourier Transform Infrared Spectroscopy (FTIR) analysis identified functional groups between 400 to 4000 cm-1. X-ray Diffraction (XRD) was conducted to check the crystal characteristics. This experiment used an X-ray diffractometer to check the 2q angles from 20 degrees to 100 degrees. The morphological analysis and size distribution of AgNPs were studied using TEM (Transmission Electron Microscopy)38 and DLS (Dynamic Light Scattering), respectively.
Antibacterial Activity of Euphorbia geniculata-Mediated AgNPs
The bactericidal activity was assessed by evaluating the AgNPs in the agar well diffusion method. Uniform swabbing of test pathogens followed by punching of agar to create wells, which were seeded with AgNPs at a 50 mg/mL concentration. Incubation was carried out at 37 °C for 24 hours. A clear zone of inhibition across the well showed the bactericidal activity, which was compared with standard antibiotics.39
Antioxidant Assay of Euphorbia geniculata-Mediated AgNPs
The antioxidant potential of Euphorbia geniculata-mediated AgNPs were assessed using the DPPH assay. Here, ascorbic acid was set as a standard antioxidant. AgNPs solutions ranging from 25, 50, and 75 µg/mL were prepared by dispersing the Euphorbia geniculata-mediated AgNPs in 1 mL of methanol. The activity was measured by dissolving DPPH solution at a 1 mg /ml concentration, and the activity was measured at 517 nm.40 The activity was measured with the following formula
% inhibition = A-B / A × 100
A: Control absorbance
B: Sample
Plant Growth Promotion Assay and Chlorophyll Content Measurement of Euphorbia geniculata-Mediated AgNPs
The potential of Euphorbia geniculata-mediated AgNPs to promote plant growth was assessed using Vigna radiata and Cicer arietinum seeds. The seeds were surface sterilized and soaked at a concentration of 10 mg/ml of nanoparticles overnight.38-41 Sterilised soil (approximately 50 g) was distributed into sterile containers, and both AgNPs-treated and control seeds were sown separately. The containers were kept in a well-lit environment to ensure favourable growth conditions for 7 days. Upon completion of the growth period, the plants were gently removed to prevent damage to their root and shoot structures. Measurements of root and shoot lengths were then recorded to evaluate the influence of AgNPs treatment on plant development.42
Chlorophyll content was determined by mechanically grinding the leaf material of the plants using chloroform. The content was measured at 645 and 663 nm.43 This analysis provided insights into the physiological effects of the Euphorbia geniculata-mediated AgNPs on chlorophyll production and overall plant health.
- Chlorophyll a (Chl A)
Chl A = 12.25 × A663 – 2.79 × A645
- Chlorophyll b (Chl B)
Chl B = 21.50 × A645 – 5.10 × A663
- Total Chlorophyll (Total Chl)
Total Chl = 20.2 × A645 + 8.02 × A663
Dye Degradation Assay of Euphorbia geniculata-Mediated AgNPs
The photodegradation potential of Euphorbia geniculata-mediated AgNPs was evaluated using selected dyes, crystal violet (CV), and methylene blue (MB). The stock solutions were prepared with 1 mg/ml, and 10 µL of the Euphorbia geniculata-mediated AgNPs were mixed with the dyes, and incubated under sunlight for 24 hrs. The degradation potential was measured at 663 nm for MB and 590 nm for CV. After 24 hours, the final absorbance values were recorded to evaluate the extent of degradation, with a decrease indicating successful nanoparticle-mediated degradation.38,44
Soxhlet Extraction and GC-MS Analysis of Euphorbia geniculata Crude Extract
The Soxhlet extraction of Euphorbia geniculata was carried out as per the procedure outlined by Hatami.45 A 20 g sample with 250 ml of methanol was subjected to extraction using a standard Soxhlet apparatus. This process was conducted at 65 °C. The obtained crude extract was studied via GC-MS, which used a fused silica column, and the carrier gas was helium. The reaction was performed at 250 °C.46 The resulting spectral data were compared with the NIST 2017 GC-MS library for compound identification.47
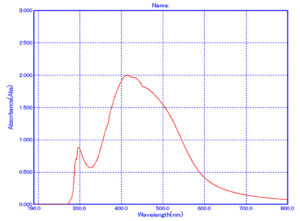
The results of Euphorbia geniculata-mediated AgNPs were visually verified by the change in colour to reddish-brown, which might be due to the excitation of electrons. This was confirmed with UV-visible spectroscopy, with a distinct peak at 418 nm, a standard absorption wavelength for AgNPs, as shown in Figure 1.
Characterization of Euphorbia geniculata-mediated AgNPs
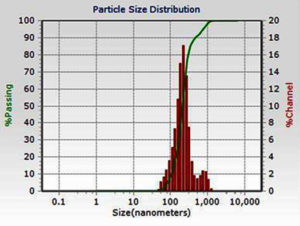
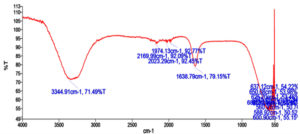
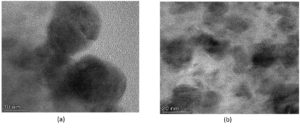
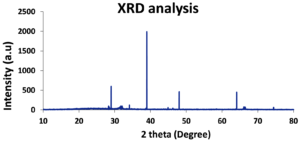
The DLS analysis of Euphorbia geniculata-mediated AgNPs revealed a polydisperse size distribution, which displayed an average size of 201 nm with a zeta potential of 0.8 mV, as shown in Figure 2. These functional groups were confirmed by specific absorption peaks within the 600 cm-1 to 4000 cm-1 as shown in Figure 3. The TEM analysis further checked the morphological characteristics of polydisperse AgNPs with various sizes from 10 to 100 nm, as shown in Figure 4. The FTIR analysis identified several functional groups, including hydroxyl (-OH), alkenes (C=C), carbonyl (C=O), alkynes (C≡C), and halogenated groups (-C-Br, -C-Cl), which are likely involved as capping agents in the nanoparticle synthesis. The XRD analysis confirmed the crystalline nature of the synthesized AgNPs, with distinct diffraction peaks corresponding to the structure of metallic silver. The observed peaks at 38° (111), 44° (200), 64° (220), and 77° (311) are in strong agreement with the standard reference pattern for silver (JCPDS file No. 04-0783), validating the presence of elemental silver in the sample as shown in Figure 5.
Figure 4. (a) TEM analysis of the nanoparticles measurement with 10 nm scale range; (b) TEM analysis of the nanoparticles measurement with 20 nm scale range
Antimicrobial activity of Euphorbia geniculata-mediated AgNPs
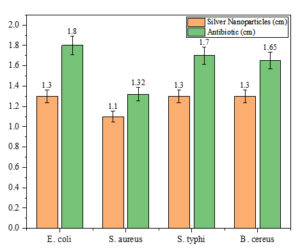
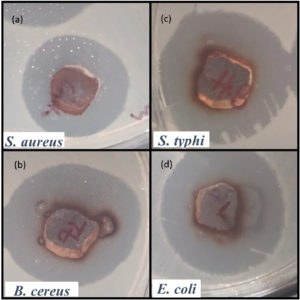
The agar well-diffusion assay revealed significant antimicrobial activity of Euphorbia geniculata-mediated AgNPs against the selected human pathogens. Amoxicillin served as the control in the study. The zone of inhibition, measured in centimetres, indicated comparable activity of AgNPs across all pathogens, with the highest inhibition observed against Escherichia coli, Salmonella typhi, and Bacillus cereus at 1.3 cm each. Staphylococcus aureus exhibited a slightly lower inhibition zone of 1.1 cm. The comparative results, presented in Figure 6, highlight the antimicrobial potential of Euphorbia geniculata-mediated AgNPs, with zones of inhibition observed in Figure 7.
Figure 6. Antimicrobial activity of Euphorbia geniculata-Mediated AgNP’s and amoxicillin against pathogens via well diffusion
Figure 7. The inhibition zones in well diffusion assay by Euphorbia geniculata-Mediated AgNP’s. (a) Activity against S. aureus, (b) Activity against B. cereus, (c) Activity against S. typhi, (d) Activity against E. coli
Antioxidant Activity of Euphorbia geniculata-Mediated AgNPs
The DPPH assay demonstrated the potent antioxidant activity of Euphorbia geniculata-mediated AgNPs, which showed inhibition percentages of 33.28%, 35.01%, and 43.22%, at 25, 50, and 75 µg/mL, respectively. The reduction in free radicals, as evidenced by the decreasing optical density (OD) values at 517 nm, confirms the significant antioxidant potential of the AgNPs. These findings, detailed in Table 1, highlight the efficacy of the nanoparticles in neutralizing free radicals under the tested conditions.
Table (1):
DPPH Activity of Euphorbia geniculata-Mediated AgNP’s
| Time | Sample (mg/mL) | OD (517 nm) | Percentage |
|---|---|---|---|
| 1 hour | 25 | 1.812 | 33.28% |
| 50 | 1.765 | 35.01% | |
| 75 | 1.542 | 43.22% |
Plant growth promotion
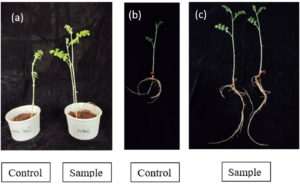
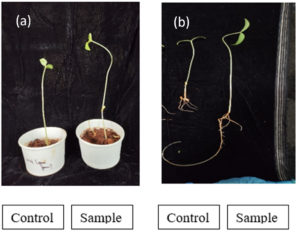
The application of Euphorbia geniculata-mediated AgNPs significantly enhanced the growth of Cicer arietinum and Vigna radiata seeds compared to untreated seeds shown in Figure 8 and Figure 9. In Cicer arietinum, nanoparticle-treated seeds showed an increase in root and shoot length of 24 and 22 cm, respectively. In Vigna radiata, nanoparticle-treated seeds displayed root lengths of 22 cm and shoot heights of 23 cm. Both seed growths were compared with the control group of seeds.
Figure 8. Growth promotion of plant Cicer arietinum by Euphorbia geniculata-Mediated AgNP’s wherein, 8a shows the shoot length measurement. 8b & 8c shows the root length
Figure 9. Growth promotion of plant Vigna radiata by Euphorbia geniculata-Mediated AgNP’s wherein, 9a shows the shoot length measurement. 9b shows the root length
Chlorophyll measurement
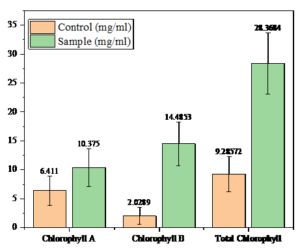
The nanoparticle-treated Cicer arietinum plants exhibited a significant increase in chlorophyll A, with 10.375 mg/mL compared to the control. Similarly, chlorophyll B levels were higher in the treated sample by 14.4853 mg/mL than in the control, which showed 2.0289 mg/mL. The total chlorophyll content was also enhanced in the treated plants, with a value of 28.3684 mg/mL compared to the control’s 9.28572 mg/mL. This indicates that nanoparticles significantly promote chlorophyll production in Cicer arietinum as detailed in Table 2 and Figure 10.
Table (2):
Chlorophyll measurement of Cicer arietinum
Chlorophyll |
Control (mg/mL) |
Sample (mg/mL) |
|---|---|---|
Chlorophyll A |
6.411 |
10.375 |
Chlorophyll B |
2.0289 |
14.4853 |
Total Chlorophyll |
9.28572 |
28.3684 |
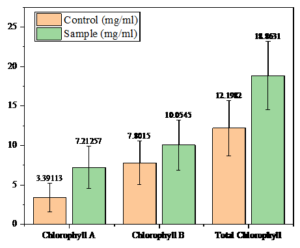
In Vigna radiata, the nanoparticle-treated plants showed enhanced chlorophyll A content with 7.21257 mg/mL compared to the control. In similar terms, Chlorophyll B levels were also increased in the treated sample with 10.0545 mg/mL compared to the control, which showed 7.8015 mg/mL. The total chlorophyll content was higher in the treated plants, which showed 18.8631 mg/mL, than in the control, displaying 12.1982 mg/mL, highlighting the positive effect of nanoparticles on chlorophyll accumulation in Vigna radiata as detailed in Table 3 and Figure 11.
Table (3):
Chlorophyll measurement of Vigna radiata
Chlorophyll |
Control (mg/mL) |
Sample (mg/mL) |
|---|---|---|
Chlorophyll A |
3.39113 |
7.21257 |
Chlorophyll B |
7.8015 |
10.0545 |
Total Chlorophyll |
12.1982 |
18.8631 |
Dye degradation assay of Euphorbia geniculata-mediated AgNPs
AgNPs exhibited significant dye degradation for both crystal violet and methylene blue, though with varying efficiencies. For crystal violet, the degradation was more pronounced, showing a 14.4% reduction in absorbance after 2 hours and 28.2% after 24 hours. In contrast, the control sample exhibited absorbance values of 1.339 (2 hours) and 1.292 (24 hours), with lesser degradation observed.
Methylene blue degradation was comparatively lower, with 8.96% after 1 hour, 14.76% after 2 hours, and 23.4% after 24 hours. The control sample for methylene blue showed absorbance values of 1.718 (1 hour), 1.675 (2 hours), and 1.447 (24 hours), demonstrating a slower degradation rate compared to crystal violet. While both CV and MB underwent degradation, among the tested dyes, CV demonstrated a relatively higher percentage of degradation, as shown in Table 4.
Table (4):
Dye degradation analysis of Euphorbia geniculata-Mediated AgNP’s
| Dye | Duration | Control Absorbance | Sample Absorbance | Degradation Percentage (%) |
|---|---|---|---|---|
| Crystal
violet |
2 hours | 1.339 | 1.147 | 14.4 |
| 24 hours | 1.292 | 0.924 | 28.2 | |
| Methylene
blue |
1 hour | 1.718 | 1.564 | 8.96 |
| 2 hours | 1.675 | 1.428 | 14.76 | |
| 24 hours | 1.447 | 1.109 | 23.4 |
GC-MS analysis
The methanolic extract from Euphorbia geniculata revealed 40 bioactive compounds, indicating its potential to bear different compounds, as shown in Table 5 and Figure 12. Some of the major compounds are derivatives of 1-butanol, oleic acid, and n-hexadecanoic acid. These findings highlight the broad pharmacological potential of Euphorbia geniculata, suggesting its role in possible drug development.
Table (5):
Identified phytochemicals in crude methanol leaf extract of Euphorbia geniculata
Name |
Formula |
Activity |
Molecular Weight |
|
|---|---|---|---|---|
1. |
1-Butanol, 3-methyl-, formate |
C6H12O2 |
Antimicrobial Aromatic compound |
116.0837297 |
2. |
2-Propenoic acid, 2 pentyl ester |
C8H14O2 |
NA |
142.09938 |
3. |
1-Butanol, 3-methyl-, acetate |
C7H14O2 |
Flavour |
130.09938 |
4. |
CH3C(O)CH2CH2OH |
C4H8O2 |
Flavour and Fragrance |
88.0524297 |
5. |
1-Butanethiol, 3-methyl |
C5H12S |
Odor |
104.0659714 |
6. |
2-Butanone, 4-(acetyloxy) |
C6H10O3 |
Fragrance and Flavour |
130.062994 |
7. |
Acrylic acid isoamyl ester |
C8H14O2 |
NA |
142.09938 |
8. |
Methyl tridecyl ether |
C14H30O |
NA |
214.229666 |
9. |
1-Dodecanol, 2-octyl-, acetate |
C22H44O2 |
Fragrance |
340.334131 |
10. |
2-Isopropyl-4-methylhex-2-enal |
C10H18O |
Fragrance and Flavour |
154.135765 |
11. |
Cyclopentane, decyl |
C15H30 |
NA |
210.234751 |
12. |
Cyclododecanone |
C12H22O |
Fragrance and Flavour |
182.167066 |
13. |
2-Octyldecyl acetate |
C20H40O2 |
NA |
312.30283 |
14. |
Cyclohexanone, 4-ethyl-3,4-dimethyl |
C10H18O |
NA |
154.135765 |
15. |
Oleic Acid |
C18H34O2 |
Antibacterial Antioxidant |
282.25588 |
16. |
Pentadecanoic acid |
C15H30O2 |
Anticancer |
242.22458 |
17. |
12-Hydroxydodecanoic acid |
C12H24O3 |
Antimicrobial |
216.1725445 |
18. |
Z-8-Methyl-9-tetradecenoic acid |
C15H28O2 |
Antifungal |
240.20893 |
19. |
n-Hexadecanoic acid |
C16H32O2 |
Antibacterial Antifungal |
256.24023 |
20. |
Undecanoic acid |
C11H22O2 |
Antifungal |
186.16198 |
21. |
Cyclopentaneundecanoic acid |
C16H30O2 |
Antioxidant |
254.22458 |
22. |
Z-10-Tetradecen-1-ol acetate |
C16H30O2 |
Fragrance and Flavour |
254.22458 |
23. |
Methyl Z-11-tetradecenoate |
C15H28O2 |
Antibiotic resistance |
240.20893 |
24. |
Methyl9-heptadecenoate or 9-17:1 |
C18H34O2 |
NA |
282.25588 |
25. |
Methacrylic acid, nonadecyl ester |
C23H44O2 |
NA |
352.334131 |
26. |
9-Octadecenamide, (Z) |
C18H35NO |
Anticancer |
281.271864 |
27. |
10-Octadecenoic acid, methyl ester |
C19H36O2 |
NA |
296.27153 |
28. |
1-Aminononadecane, N-trifluoroacetyl |
C21H40F3NO |
Corrosion inhibition |
379.3062 |
29. |
Cyclopropaneoctanoic acid, 2-hexyl-, methyl ester |
C18H34O2 |
NA |
282.25588 |
30. |
Methyl myristoleate |
C15H28O2 |
NA |
240.20893 |
In the present study, AgNPs were synthesised by Euphorbia geniculata. The plant was chosen based on the reports, which suggested that they are one of the prominent sources of diverse phytocomponents. This chemical diversity acts as efficient reducing and stabilising agents to mediate the production of AgNPs.48 The noticeable colour transition from pale yellow to reddish brown serves as a preliminary visual confirmation of AgNPs formation. This is well documented with previous reports, which justifies the synthesis process.49-52 Further, this was substantiated by UV-visible spectroscopy results, which revealed a sharp absorption peak at 418 nm, confirming and characterising the surface plasmon resonance band for AgNPs. This coincides with the AgNPs UV absorbance from the green synthesis protocol.50-54 Studies report absorbance from 400 to 450 nm, which corresponds to polydispersity and spherical AgNPs.55,56 Further characterisation using the DLS analysis of AgNPs revealed a polydisperse size distribution with a zeta potential of +0.8 mV. Such a broad size distribution is commonly observed in plant-mediated nanoparticle synthesis, where the complex mixture of phytochemicals adheres to the nanoparticles to make them more stable. Al-Shabib et al.57 reported the production of AgNPs from Tamarix nilotica shoot extract, where the DLS analysis showed the polydispersity of the nanoparticles. This was further confirmed with TEM analysis of AgNPs, which also displayed the polydisperse morphology, with particle sizes ranging from 10 to 100 nm. This observation is consistent with findings from plant-mediated nanoparticle synthesis in a recent scientific report.57 The FTIR results of AgNPs showed several key functional groups, including hydroxyl (-OH), alkenes (C=C), carbonyl (C=O), alkynes (C≡C), and halogenated groups (-C-Br, -C-C-Cl). These functional groups are indicative of the phytocomponents like terpenoids, saponins, alkaloids, and phenolics present in Euphorbia geniculata, which aid in attenuating the desired activity.58 These functional groups are mainly reported from nanoparticles produced from plant extracts, which confirms the consistency and role of these phytocomponents.59 The presence of hydroxyl groups has been substantiated in AgNPs synthesized using Ocimum sanctum,60 and the hydroxyl and C=O groups in AgNPs synthesized from Azadirachta indica61 confirmed their role in bio-reduction and surface functionalization. The XRD analysis characteristic peaks were consistently confirmed with previous studies, on biologically synthesised AgNPs, further supporting the crystallinity of AgNPs.62,63 The peak at 38° is notably intense, indicating a preferential growth orientation, which is a common feature in silver nanoparticle synthesis via plant-mediated reduction methods.5,64,65 These results corroborate previous findings in green production of nanoparticles, where, unlike chemical and physical production, there might be extracted intensities that might correspond to the presence of phytocomponents as documented in various studies.64,66,67
The well-diffusion assay displayed the highest efficacy against Escherichia coli, Salmonella typhi, and Bacillus cereus, each showed similar inhibition zones of 1.3 cm, while Staphylococcus aureus showed only 1.1 cm of inhibition. Thus, AgNPs have broad-spectrum bactericidal properties. The findings are consistent with previous reports of plant-mediated AgNPs,51,68,69 which exhibited a strong bactericidal effect against both Gram+ and Gram- bacteria. In similar terms, Dutt et al.70 reported bactericidal properties from AgNPs produced from Azadirachta indica, which suppressed the proliferation of E. coli and S. aureus. The mode of action of the nanoparticles is reported, wherein the activity is dependent on size, surface charge, shape, surface coating, agglomeration, solubility, and stability. The smaller size and high surface area of the AgNPs likely enhanced their interaction with bacterial cells, resulting in significant bactericidal effects.71,72
The antioxidant capacity of the AgNPs, evaluated using the DPPH radical scavenging assay, revealed substantial activity across varying concentrations (25, 50, and 75 µg/mL). This dose-dependent response aligns with the results of Syed et al.72 The results obtained are in line with the report where Euphorbia granulata mediated AgNPs were produced.73 The results obtained from the studies recommend natural antioxidants against oxidative stress associated with degenerative diseases.74
The seed germination and plant growth studies on Cicer arietinum and Vigna radiata increased the shoot and root measurements, indicating the influence of positive impact of nanoparticles. Additionally, the nanoparticles enhanced chlorophyll content, corroborating the findings of Timi et al.75 that substantiate their role in promoting photosynthetic efficiency and overall plant vitality.
Further, the dye degradation efficiency of AgNPs showed the moderate degradation potential of nanoparticles. The efficiency can be improved by optimizing various parameters, which will be the subject of interest in future studies. The process of degradation aligns with reports of Seerangaraj et al.,76 and Saha et al.77 To barcode the phytoconstituents present in Euphorbia geniculata, Soxhlet extraction and GC-MS was carried out which revealed the presence of myriad compounds among which some of the compounds where 1-Butanol, 3-methyl, formate, with notable antimicrobial and aromatic properties78 oleic acid, known for its antibacterial and antioxidant activities,79 n-hexadecanoic acid with established anti-inflammatory property80 antibacterial and antifungal effects,81 and 9-octadecenamide, (Z), recognized for its anticancer potential.82,83 The identification of these compounds emphasizes the therapeutic potential of Euphorbia geniculata, positioning it as a sustainable source for bioactive agents.
The study demonstrates the green synthesis of AgNPs and their potential applications. This approach is efficient, eco-friendly, and cost-effective. It also contributes valuable scientific information on the multifunctional uses of green-synthesized AgNPs. In this study, the AgNPs were well characterized and subjected to a thorough preliminary investigation. Based on the results obtained, future research can focus on optimizing the exact concentration needed for field applications and agricultural greenhouse studies. Further studies can also explore the mechanism behind their antimicrobial properties. In addition, upcoming research may aim to identify the specific phytocomponents responsible for the synthesis and biological activities of the AgNPs.
ACKNOWLEDGMENTS
The authors would like to thank Sri Jayachamarajendra College of Engineering, JSS Science and Technology University, Mysore and Karnataka State Open University, Mysore, for providing the infrastructure for the present study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SL and SB conceptualized the study. LRM, PA, KGR, SL and HS resource acquisition. SL and VJG performed data curation. MH, LRM and VJG applied methodology. MH performed data investigation. SB and R performed formal analysis. MNNP and KM performed supervision and project administration. SB wrote the original draft. SR, SNR, HS and MNNP wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Malik S, Muhammad K, Waheed Y. Nanotechnology: A revolution in modern industry. Molecules. 2023;28(2):661.
Crossref - Beyene HD, Werkneh AA, Bezabh HK, Ambaye TG. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain Mater Technol. 2017;13(1):18-23.
Crossref - Jain K, Takuli A, Gupta TK, Gupta D. Rethinking nanoparticle synthesis: A sustainable approach vs. traditional methods. Chem Asian J. 2024;19(1):e202400701.
Crossref - Soni V, Choudhary M, Singh R, et al. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ Res. 2021;202(1):111622.
Crossref - Amtaghri S, Akdad M, Slaoui M, Eddouks M. Traditional uses, pharmacological, and phytochemical studies of Euphorbia: A review. Curr Top Med Chem. 2022;22(17):1553-1570.
Crossref - Mavundza EJ, Street R, Baijnath H. A review of the ethnomedicinal, pharmacology, cytotoxicity, and phytochemistry of the genus Euphorbia in Southern Africa. S Afr J Bot. 2022;144(1):403-418.
Crossref - Khan RA, Khan HU, Shahnaz, et al. Eco-benign synthesis of multifunctional silver nanoparticles using Leptochloa fusca for enhanced biomedical and catalytic applications. Mater Res Express. 2025;12(1):015006.
Crossref - Lima AKO, Souza LMdS, Reis GF, et al. Synthesis of Silver Nanoparticles Using Extracts from Different Parts of the Paullinia cupana Kunth Plant: Characterization and In Vitro Antimicrobial Activity. Pharmaceuticals. 2024;17(7):869.
Crossref - Pirtarighat S, Ghannadnia M, Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J Nanostruct Chem. 2019;9:1-9.
Crossref - Qureshi AK, Farooq U, Shakeel Q, et al. The Green Synthesis of Silver Nanoparticles from Avena fatua Extract: Antifungal Activity against Fusarium oxysporum f.sp. lycopersici. Pathogens. 2023;12(10):1247.
Crossref - Asif M, Yasmin R, Asif R, Ambreen A, Mustafa M, Umbreen S. Green Synthesis of Silver Nanoparticles (AgNPs), Structural Characterization, and their Antibacterial Potential. Dose Response. 2022;20(1):15593258221088709.
Crossref - Senthilkumar P, Rashmitha S, Veera P, Ignatious CV, Priya CS, Samrot AV. Antibacterial Activity of Neem Extract and its Green Synthesized Silver Nanoparticles against Pseudomonas aeruginosa. J Pure Appl Microbiol. 2018;12(2):969-974.
Crossref - Phanse NV, Venkataraman K, Kekre PA, Shah S, Parikh S. Phyto-assisted synthesis of silver nanoparticles using Tinospora cordifolia leaf extract and their antibacterial activity: An eco-friendly approach. Braz J Sci. 2024;3(2):57-65.
Crossref - Jangid H, Singh S, Kashyap P, Singh A, Kumar G. Advancing biomedical applications: An in-depth analysis of silver nanoparticles in antimicrobial, anticancer, and wound healing roles. Front Pharmacol. 2024;15:1438227.
Crossref - Heidari F, Raoufi Z, Abdollahi S, Asl HZ. Antibiotic delivery in the presence of green AgNPs using multifunctional bilayer carrageenan nanofiber/sodium alginate nanohydrogel for rapid control of wound infections. Int J Biol Macromol. 2024;277(Pt 1):134109.
Crossref - El-Rafie HM, Maghraby HR, Sleem AA, Abdelfattah MS. Wound-healing cotton dressings containing greenly fabricated silver nanoparticles from Ficus binnendijkii hydroethanolic leaves extract. Res J Pharm Technol. 2024;17(9):4427-4436.
Crossref - Prasher P, Sharma M, Mudila H, et al. Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloid and Interface Science Communications. 2020;35:100244.
Crossref - Prashob Peter KJ. Multi-functional silver nanoparticles for drug delivery: A review. Int J Curr Pharm Rev Res. 2017;9(8):1-5.
- Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6(3):399-408.
Crossref - Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6: 399-408.
Crossref - Marsich E, Bellomo F, Turco G, Travan A, Donati I, Paoletti S. Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: preparation, characterization, and biological properties. J Mater Sci Mater Med. 2013;24(7):1799-1807.
Crossref - Zhu M, Qian G, Ding G, Wang Z, Wang M. Plasma resonance of silver nanoparticles deposited on the surface of submicron silica spheres. Mater Chem Phys. 2006;96(2-3):489-493.
Crossref - Kavitha KS, Baker S, Rakshith D, et al. Plants as green source towards synthesis of nanoparticles. Int Res J Biol Sci. 2013;2(6):66-76.
- Kumar B, Smita K, Cumbal L, Debut A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J Biol Sci. 2017;24(1):45-50.
Crossref - Kale SK, Parishwad GV, Husainy ASN, Patil AS. Emerging agriculture applications of silver nanoparticles. ES Food & Agroforestry. 2021;3:17-22.
Crossref - Sadak MS. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull Natl Res Cent. 2019;43(1):1.
Crossref - Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346-356.
Crossref - Korbekandi H, Iravani S, Abbasi S. Production of nanoparticles using organisms. Crit Rev Biotechnol. 2009;29(4):279-306.
Crossref - Bennet RD, Raji P, Divya KM, et al. Green Synthesis and Antibacterial Activity Studies of Silver Nanoparticles from the Aqueous Extracts of Euphorbia hirta, J. Pure Appl. Microbiol., 2020;14(1):301-306.
Crossref - Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10(3):507-517.
Crossref - Latansio de Oliveira T, Reder Custodio de Souza A, Dias Fontana P, et al. Bioactive secondary plant metabolites from Euphorbia umbellata (PAX) Bruyns (Euphorbiaceae). Chemistry & Biodiversity. 2022;19(12):e202200568.
Crossref - Loo YY, Rukayadi Y, Nor-Khaizura MA-R, et al. In vitro antimicrobial activity of green-synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front Microbiol. 2018;9:1555.
Crossref - Heinemann MG, Rosa CH, Rosa GR, Dias D. Biogenic synthesis of gold and silver nanoparticles used in environmental applications: A review. Trends Environ Anal Chem. 2021;30:e00129.
Crossref - Samberg ME, Loboa EG, Oldenburg SJ, Monteiro-Riviere NA. Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine. 2012;7(8):1197-1209.
Crossref - Mahapatra DK, Bharti SK. Research progress and new insights in the biosynthesis of silver nanoparticles with particular applications. Chemical Nanoscience and Nanotechnology. 2019:195-240.
Crossref - Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and catalytic applications. Mater Today Chem. 2017;32:55-61.
Crossref - Arif M, Ullah R, Ahmad M et al., Green Synthesis of Silver Nanoparticles Using Euphorbia wallichii Leaf Extract: Its Antibacterial Action against Citrus Canker Causal Agent and Antioxidant Potential. Molecules. 2022;27(11):3525.
Crossref - Ranjini HK, Manju K, Shayista H, Raj SN, Baker S, Prasad A. Phytogenic Silver Nanoparticles from Callicarpa macrophylla and their Biological Activities. J Pure Appl Microbiol. 2024;18(4):2636-2644.
Crossref - Ashraf JM, Ansari MA, Khan HM, Alzohairy MA, Choi I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on Acanthamoeba castellanii. J Microbiol Immunol Infect. 2020;53(4):569-576.
Crossref - Sadeghi B, Garmaroudi FS, Hashemi M, et al. Comparison of the antibacterial activity on the nanosilver shapes: nanoparticles, nanorods, and nanoplates. Adv Powder Technol. 2012;23(1):22-26.
Crossref - Jeetkar TJ, Khataokar SP, Indurkar AR, Pandit A, Nimbalkar MS. A review on plant-mediated synthesis of metallic nanoparticles and their applications. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2022;13(3):033004.
Crossref - Roy P, Das B, Mohanty A, Mohapatra S. Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. Appl Nanosci. 2017;7(8):843-850.
Crossref - Birla SS, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Lett Appl Microbiol. 2009;48(2):173-179.
Crossref - Matei PM, Martin-Gil J, Iacomi BM, Perez-Lebena E, Barrio-Arredondo MT, Martin-Ramos P. Silver nanoparticles and polyphenol inclusion compounds composites for Phytophthora cinnamomi mycelial growth inhibition. Antibiotics. 2018;7(3):76.
Crossref - Hatami A. Phytochemical profiling and antibacterial activities of Ziziphora tenuior root extracts: a molecular docking against VanA of vancomycin-resistant enterococci. 3 Biotech. 2024;14:1-11.
Crossref - Santhoshkumar J, Rajeshkumar S, Kumar SV. Phyto-assisted synthesis, characterization and applications of gold nanoparticles – A review. Biochem Biophys Rep. 2017;11:46-57.
Crossref - Ramesh M, Anbuvannan M, Viruthagiri G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136(Pt B):864-870.
Crossref - James O, Friday ET. Phytochemical composition, bioactivity, and wound healing potential of Euphorbia heterophylla (Euphorbiaceae) leaf extract. Int J Pharm Biomed Res. 2010;1(1):54-63.
- Song JY, Kim BS. Biological synthesis of bimetallic Au/Ag Nanoparticle using persimmon (Diopyros kaki) leaf extract, Korean J Chem Eng. 2008;25:808-811.
Crossref - Geoprincy G, Srri BNV, Poonguzhali U, Gandhi NN, Renganathan SA. A review on green synthesis of silver nanoparticles. Asian J Pharm Clin Res. 2013; 6(1):8-12.
- Al-Malki WF, Alharbi NS. Ficus Plant-Mediated Silver Nanoparticles: Synthesis, Optimization, Characterization, and Biomedical Applications. J Pure Appl Microbiol. 2025;19(1):74-99.
Crossref - Al-zoubi OM. Bio-Synthesis of Silver Nanoparticles Utilizing Yanbu’s Indigenous Medicinal Herbs and Plants: Antimicrobial Activities Evaluation. J Pure Appl Microbiol. 2025;19(1):485-497.
Crossref - Chhatre A, Solasa P, Sakle S, Thaokar R, Mehra A. Color and surface plasmon effects in nanoparticle systems: Case of silver nanoparticles prepared by microemulsion route. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2012;404:83-92.
Crossref - GnanaJobitha G, Annadurai G, Kannan C. Green synthesis of silver nanoparticles using Elettaria cardamom and assessment of its antimicrobial activity. Int J Pharma Sci Res. 2012;3(3):323-330.
- Rathod D, Golinska P, Wypij M, Dahm H, Rai M. A new report of Nocardiopsis valliformis strain OT1 from the alkaline Lonar crater of India and its use in the synthesis of silver nanoparticles with special reference to the evaluation of antibacterial activity and cytotoxicity. Med Microbiol Immunol. 2016;205(5):435-447.
Crossref - Ahmed S, Saifullah, Ahmad M, Swami BL, Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci. 2016;9(1):1-7.
Crossref - Al-Shabib NA, Husain FM, Nadeem M, et al. Bio-inspired facile fabrication of silver nanoparticles from in vitro grown shoots of Tamarix nilotica: Explication of its potential in impeding growth and biofilms of Listeria monocytogenes and assessment of wound healing ability. RSC Adv. 2020;10(50):30139-30149.
Crossref - Doughari JH. Phytochemicals: extraction methods, basic structures and mode of action as potential chemotherapeutic agents. Rijeka, Croatia: INTECH Open Access Publisher. 2012:1-33.
Crossref - Dauthal P, Mukhopadhyay M. Noble metal nanoparticles: plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind Eng Chem Res. 2016;55(36):9557-9577.
Crossref - Singhal G, Bhavesh R, Kasariya K, Sharma AR, Singh RP. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res. 2011;13:2981-2988.
Crossref - Kumari SA, Patlolla AK, Madhusudhanachary P. Biosynthesis of silver nanoparticles using Azadirachta indica and their antioxidant and anticancer effects in cell lines. Micromachines. 2022;13(9):1416.
Crossref - Singh J, Dutta T, Kim KH, Rawat M, Samddar P, Kumar P. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol. 2018;16(1):84.
Crossref - Kharissova OV, Dias HR, Kharisov BI, Perez BO, Perez VMJ. The greener synthesis of nanoparticles. Trends Biotechnol. 2013;31(4):240-248.
Crossref - Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plant extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17-28.
Crossref - Gurunathan S, Park JH, Han JW, Kim JH. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: targeting p53 for anticancer therapy. Int J Nanomed. 2015;10:4203-4227.
Crossref - Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76-83.
Crossref - Lakshmi S, Bhat A, Shriya, et al. Synthesis of enzyme-enriched zinc oxide nanoparticles using Lantana camara L. fruit extract for detoxification of phenol and derivatives. Hybrid Advances. 2024;7:100326.
Crossref - Arya A, Tyagi PK, Bhatnagar S, et al. Biosynthesis and assessment of antibacterial and antioxidant activities of silver nanoparticles utilizing Cassia occidentalis L. seed. Sci Rep. 2024;14:7243.
Crossref - Akhtar Y, Rashid A, Atif M, et al. Green Synthesis of Silver Nanoparticles Using Doxycycline: Evaluation of Antimicrobial Potential and Organ-specific Toxicity. J Pure Appl Microbiol. 2024;18(4):2496-2506.
Crossref - Dutt Y, Pandey RP, Dutt M, et al. Synthesis and biological characterization of phyto-fabricated silver nanoparticles from Azadirachta indica. J Biomed Nanotechnol. 2022;18:2022-2057.
Crossref - Qais FA, Ahmad I, Altaf M, et al. Biofabricated silver nanoparticles exhibit broad-spectrum antibiofilm and antiquorum sensing activity against gram-negative bacteria. RSC Adv. 2021;11(23):13700-13710.
Crossref - Syed B, Yashavantha Rao HC, Nagendra-Prasad MN, et al. Biomimetic synthesis of silver nanoparticles using endosymbiotic bacterium inhabiting Euphorbia hirta L. and their bactericidal potential. Scientifica. 2016;2016:1-7.
Crossref - Periyasami G, Palaniappan S, Karuppiah P, et al. Biogenic silver nanoparticles fabricated by Euphorbia granulata forssk’s extract: investigating the antimicrobial, radical scavenging, and catalytic activities. J Nanomater. 2022;2022(1):3864758.
Crossref - Mohanta YK, Panda SK, Jayabalan R, Sharma N, Bastia AK, Mohanta TK. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front Mol Biosci. 2017;4:14.
Crossref - Timi D, Gopalakrishnan S, Maino M. Characterization and antimicrobial assessment of photosynthesized silver nanoparticles using aqueous leaf extract of Euphorbia geniculata. Indian J Sci Technol. 2017;11(47):1-7.
Crossref - Seerangaraj V, Sathiyavimal S, Shankar SN, et al. Cytotoxic effects of silver nanoparticles on Ruellia tuberosa: Photocatalytic degradation properties against crystal violet and Coomassie Brilliant Blue. J Environ Chem Eng. 2021;9(2):105088.
Crossref - Saha J, Begum A, Mukherjee A, Kumar S. A novel green synthesis of silver nanoparticles and their catalytic action in the reduction of methylene blue dye. Sustain Environ Res. 2017;27(5):245-250.
Crossref - Antoce OA, Antoce V, Mori N, Yasui S, Kobayashi A, Takahashi K. Calorimetric evaluation of the antimicrobial properties of 1, 3-butanediol and 1, 2-pentanediol on various microorganisms. Netsu Sokutei. 1998;25(1):2-8.
Crossref - Saravanakumar K, Chelliah R, Ramakrishnan SR, Kathiresan K, Oh DH, Wang MH. Antibacterial and antioxidant potentials of non-cytotoxic extract of Trichoderma atroviride. Microb Pathog. 2018;115:338-342.
Crossref - Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M. Anti inflammatory property of n hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Des. 2012;80(3):434-439.
Crossref - Hawar SN, Taha ZK, Hamied AS, AL-Shmgani HS, Sulaiman GM, Elsilk SE. Antifungal activity of bioactive compounds produced by the endophytic fungus Paecilomyces sp. (JN227071.1) against Rhizoctonia solani. Int J Biomater. 2023;2023(1):1-8.
Crossref - Mongalo N, Soyingbe O, Makhafola T. Antimicrobial, cytotoxicity, anticancer and antioxidant activities of Jatropha zeyheri Sond. roots (Euphorbiaceae). Asian Pac J Trop Biomed. 2019;9(7):307-314.
Crossref - Maungchanburi S, Chaithada P, Rattanaburi S, et al. Antiproliferative Activity and GC- MS Analysis from the Leaves Extract of Different Cultivars Carica Papaya. ASEAN Journal of Scientific and Technological Reports. 2012;27(6):e254526-e254526.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.