ISSN: 0973-7510
E-ISSN: 2581-690X
The escalating global burden of diabetes underscores the urgent need for sustainable insulin production. This study explores the potential of Arthrospira platensis SPKY1 as an alternative source of insulin, particularly pertinent in regions with high diabetes prevalence like India. Through comprehensive experimentation, factors influencing insulin production in A. platensis SPKY1 are investigated, including growth media composition, pH levels, light conditions, greenhouse cultivation, water types and carbon sources. Results reveal those higher concentrations of specific growth media components, such as NaHCO3, NaNO3, NaCl, K2SO4, and K2HPO4, correlate with increased insulin production. Among these components, K2SO4 at a concentration of 1.4 g L-1 showed the highest insulin production, reaching 27.5 µg g-1. Additionally, the study evaluated the impact of various pH levels, finding that pH 10.0 yielded optimal insulin production, with a peak of 21.3 µg g-1. Blue light exposure stimulated the most significant increase in insulin production, with levels ranging from 5.4 to 25.1 µg g-1. Additionally, enriched seawater proved more effective than regular medium for insulin production. The study also demonstrated that glucose proved to be the optimal carbon source, with insulin production reaching 29.4 µg g-1. The study determines the optimal growth conditions of A. platensis SPKY1 for insulin production on a pilot scale.
Antidiabetic, Anti-glycemic, Arthrospira, Diabetes, Insulin
Diabetes poses a significant and enduring challenge, characterized by elevated blood sugar levels stemming from irregularities in β-cell function affecting insulin activity.1 According to the International Diabetes Federation (IDF), the global prevalence of diabetes reached 537 million individuals in 2021, representing a substantial burden on healthcare systems.2,3 Age-standardized prevalence rates surged by approximately 90.5% from 1990 to 2021, with particularly notable increases of over 90% in regions such as South Asia, Western and Eastern Europe, and over 100% in areas including Central Asia, Southern Latin America, and high-income North America. In contrast, some regions experienced more moderate rises, with increases of less than 30% noted in countries like Mexico and the Philippines, while others saw dramatic spikes of up to 200%, as seen in nations like Egypt, Greenland, and Timor-Leste.4
According to a 2023 study by the Indian Council of Medical Research (ICMR), 11.4% of India’s population, amounting to 101 million individuals, are diagnosed with diabetes. The prevalence of diabetes varied significantly across different states, ranging from 4.8% to 26.4%. Specifically, Jharkhand, Nagaland, Manipur, and Punjab exhibited prevalence rates between 5.0% and 9.9%, while Tamil Nadu, Andhra Pradesh, Karnataka, Maharashtra, Gujarat, and Bihar reported rates from 10.4% to 14.9%. Additionally, Kerala, Madhya Pradesh, Rajasthan, West Bengal, and Himachal Pradesh had prevalence rates exceeding 15%. Diabetes prevalence was significantly elevated in both the southern and northern parts of India, with urban areas consistently demonstrating elevated prevalence rates. Conversely, the central and north eastern regions displayed lower prevalence rates.5
Diabetes has the potential to affect various organ systems in the body, leading to severe complications over time. These complications are typically classified as either microvascular or macrovascular. Microvascular complications involve damage to the nervous system (neuropathy), renal system (nephropathy), and eyes (retinopathy). On the other hand, macrovascular complications include cardiovascular disease, stroke, and peripheral vascular disease. Peripheral vascular disease can result in non-healing bruises or injuries, gangrene, and ultimately, the need for amputation.6 These complications not only significantly impact the morbidity and mortality associated with diabetes but also contribute to the growing costs related to its management.
Currently, the primary therapeutic regimens for type 2 diabetes mellitus (T2DM) involve the injection of insulin-like agents and the oral administration of hypoglycemic agents. However, while these agents are crucial for T2DM treatment, they often come with undesirable side effects.7,8 Insulin has been at the forefront of managing uncontrolled insulin-deficient DM since its discovery.9
Insulin is a vital medication for managing both T1DM and T2DM, recognized by the World Health Organization (WHO) as an Essential Medicine necessary for addressing global health needs and promoting cost-effective healthcare resource utilization.10 Despite being discovered almost a century ago, insulin remains inaccessible to millions due to inadequate availability and excessively high prices.11,12
In India, select insulin products are listed in both national and state Essential Medicines Lists (EMLs), allowing for their free provision in public-sector health facilities.13 However, limited funding for India’s central and state public health systems restricts healthcare coverage for much of the population.14 As a result, diabetes patients often turn to the private sector for healthcare, necessitating out-of-pocket payments.15 Additionally, patients may acquire medications from private-sector online pharmacies or government initiatives like the Jan Aushadhi Scheme (JAS), aimed at providing quality medicines at affordable prices to all.16,17
Exploring alternative sources for insulin production is crucial for overcoming the challenges of cost and accessibility. By investigating microorganisms, plant-based systems, researchers aim to develop more affordable and scalable methods for producing insulin. These innovative approaches have the potential to significantly reduce production costs, making insulin more accessible to individuals worldwide who depend on this essential medication for managing diabetes. Spirulina has garnered attention for its potential anti-diabetic properties. A study suggests that Spirulina may help regulate blood sugar levels by improving insulin sensitivity and reducing insulin resistance.18
Research on Spirulina supplementation for lowering blood sugar levels has not pinpointed the specific molecules responsible for its anti-diabetic effects. However, despite the absence of whole genome sequencing in these studies, a recent investigation identified a potentially beneficial strain, A. platensis SPKY1. This strain was subjected to whole genome sequencing and subsequently submitted to NCBI (GenBank ID: JAWMAM000000000.1). Insulin production was validated using multiple analytical techniques, including protein quantification, SDS-PAGE, 2D gel electrophoresis, and MALDI, all consistently detecting a 6 kDa band, aligning with prior insulin identification studies. The genome size of A. platensis SPKY1 was found to be 5.7 Mb, with a total of 7,731 genes encoding hypothetical proteins. Gene analysis revealed four unique hypothetical proteins, each made up of six amino acid residues, that perfectly match human insulin. Furthermore, eight proteins belonging to the insulinase family were discovered.19 Therefore, the primary aim of this study is to investigate the optimal growth conditions for A. platensis SPKY1 to enhance insulin production on a pilot scale.
The A. platensis SPKY1 used in this study were isolated from Ennore estuary, Chennai, Tamil Nadu. The strain is identified through whole genome sequencing and compared with the NCBI WGS database. A. platensis SPKY1 showed high similarity to A. platensis. However, due to the presence of insulinase, the strain is designated as A. platensis SPKY1.
Unialgal culture
Obtaining unialgal cultures is essential for the study. As a first step, the collected water was filtered using mesh cloth, followed by filtration through a 47-µm pore membrane. The pH of the collected water was then adjusted to 12 and maintained for 48 hours. After incubation, the pH was lowered to 9 using a bicarbonate buffer. To eliminate contamination, the culture was treated with cefoxitin (76.9 µg mL-1) for 48 hours in the dark. Following incubation, the culture was washed by centrifugation at 2000 rpm for 5 minutes. Unialgal A. platensis SPKY1 cultures were obtained through serial dilution (1:1000). Cultures were examined under a microscope to identify contaminants before each experiment. All subsequent experiments utilized cultures derived from this mother culture (control).
Growth conditions
Analytical-grade chemicals were used, and the medium was sterilized at 120 °C for 15 minutes prior to inoculation, with pH checked to ensure suitability. Clean conical flasks were used and maintained at a constant temperature below 30 °C, typically around 21 °C. Stock cultures were maintained on agar slants, and before inoculation, cultures were examined for contaminants under a microscope. For inoculum preparation, a portion of the stock culture was shaken with sterile medium. 50 mL of the suspension were inoculated into freshly prepared Zarrouk’s medium.20 Illumination was provided by cool white fluorescent lamps at a light intensity of 50 µmol photons m-2 s-1, measured using a Lutron Lx-130 light meter, with a 12-hour light-dark cycle. The culture was maintained for 20 days, after which the algae and medium were separated by centrifugation, washed, and the biomass yield was determined following the procedure of Donmez et al.21
Effect of media manipulation
A. platensis SPKY1 was grown in different concentrations of NaHCO3 (14.8-18.8 g), NaNO3 (1.5-3.5 g), NaCl (0.6 to 1.4 g), K2SO4 (0.6 to 1.4 g), K2HPO4 (0.3 to 0.7 g) remaining ingredients were kept constant as regular standard Zarrouk’s medium. Due to production cost concerns, this study examined the impact of varying the composition of Zarrouk’s medium from the standard formula. All medium manipulated cultures were compared with controlled A. platensis SPKY1 culture which was grown in standard Zarrouk’s medium.
Effect of pH
The effect of pH on A. platensis SPKY1 growth and insulin production was evaluated using Zarrouk’s medium adjusted to pH levels ranging from 7.0 to 12.0. At the start of the experiment, the pH was adjusted using 8 M NaOH or 1 N HCl solutions. For the experiment, 2000 mL Erlenmeyer flasks containing 1000 mL of medium were prepared, with the pH adjusted before autoclaving. A two-week-old A. platensis SPKY1 mother culture was uniformly inoculated into all flasks, which were subsequently incubated for 20 days.
Effect of light
To assess the impact of light on insulin production, various lighting conditions were employed using colored LEDs. The LEDs generated light in the following spectral ranges: red (620-680 nm), blue (420-475 nm), green (495-570 nm), and yellow (570-590 nm), with an intensity of 150 µmol photons·m-2s-1 measured using a Lutron LX-130 light meter. Freshly prepared standard culture media (1000 mL) were inoculated with the A. platensis SPKY1 mother culture. Each culture was exposed to illumination from LEDs emitting the designated color. To ensure that only the intended light spectrum reached the cultures, they were housed in specially constructed boxes that excluded external light interference.
Effect of culture system
The effect of the cultivation system on A. platensis SPKY1 growth and insulin production was evaluated using an open cultivation and green house setup. Open system: In this setup, the mother culture was exposed to ambient air and directly illuminated by sunlight in open plastic trays, each with a capacity of 10 L. Greenhouse effect: To study the impact of solar irradiance, greenhouse nets were used to partially cover the trays, reducing light intensity. Both setups (open system and green house trays) were maintained in separate locations within a 10 meter radius to minimize cross-interference and potential contamination.
Effect of water type
5 L of seawater was collected from Ennore estuary. Initially the collected water was filtered with mesh to remove larger and smaller waste particles. The mother culture was inoculated in Zarrouk’s medium prepared using seawater (Enrich medium) and only sea water (without addition of nutrients). Both media were sterilized by using autoclaving before inoculation and growth was compared with mother culture growth in Zarrouk’s medium prepared using distilled water.
Effect of organic carbon sources
In order to identify the optimal growth based on the carbon source availability, various concentrations of organic carbon sources (glucose, fructose, galactose, sucrose and glycerol) were added at different concentrations (0.5-1.5 g L-1) in the standard Zarrouk’s medium.
Insulin estimation
To extract insulin from biomass after applying various strain improvement techniques, a series of steps were followed according to Khanna et al.22 Insulin ELISA kit was purchased from Genei Laboratories Pvt. Ltd., Bangalore. Extracted insulin from all treated culture was estimated by following procedure. Prepare the ELISA plate by setting standard, test sample (diluted at least 1/2 with sample dilution buffer), and control (zero) wells, then aliquot 100 µL of standard solutions into the standard wells, add 100 µL of sample dilution buffer into the control well, and add 100 µL of properly diluted sample into test sample wells. Incubate the plate at 37 °C for 90 minutes, and then wash it 2 times with wash buffer. Carefully add 100 µL of the Biotin-labeled antibody working solution to each well, ensuring not to touch the side walls. Incubate the plate at 37 °C for 60 minutes, then wash it three times using wash buffer. Next, dispense 100 µL of HRP-Streptavidin Conjugate working solution into each well, cover the plate, and incubate at 37 °C for 30 minutes. Wash the plate five times with wash buffer. Following this, add 90 µL of 3,3′,5,5′ -Tetramethylbenzidine (TMB) substrate to each well, cover, and incubate at 37 °C in the dark for 10-20 minutes. To halt the reaction, introduce 50 µL of stop solution into each well, mix thoroughly, and immediately measure the Optical Density (OD) absorbance at 450 nm using a microplate reader.
For data analysis, determine the mean OD 450 nm values from duplicate readings of each standard, control, and sample. Subtract the OD 450 nm blank to obtain the corrected OD 450 nm values. Construct a four-parameter logistic (4PL) standard curve by plotting the mean absorbance for each standard against its concentration. Finally, determine the sample concentration by interpolating the OD 450 nm value from the standard curve, adjusting for any dilution factor applied.23
Statistical analysis
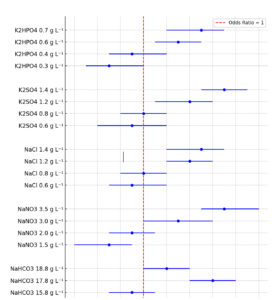
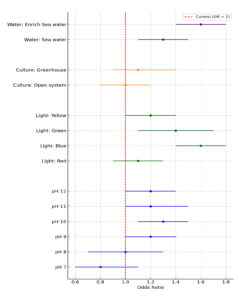
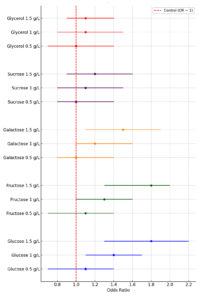
Each experiment in this study conducted in five 1000 mL flask and data were analysed using SPSS software (Version 25). Mean values and standard deviations (SD) of insulin concentration were measured. A two-sided t was used to compare all treated cultures and the absolute difference was analysed and reported with 95% confidence interval. A p-value of less than 0.05 was considered significant. The data were checked for normality, homogeneity of variance, and outliers before conducting the analysis to ensure the accuracy and validity of the analysis. Significance differences in insulin level between different concentrations of each treated cultures were analyzed using one-way ANOVA. The forest plot represents odds ratios (OR) with 95% confidence intervals (CI) for insulin production under different experimental conditions. The red dashed line at OR = 1 indicates the control. OR greater than 1 represents increased insulin production relative to the control, while values below 1 indicate reduced production.
The study investigated the potential of A. platensis SPKY1 as an alternative source of insulin, focusing on optimizing factors influencing its production. Key variables assessed included growth media composition, pH levels, light conditions, greenhouse cultivation, water types, and carbon sources. The data investigates the effect of media manipulation on insulin production, focusing on additives such as NaHCO3, NaNO3, NaCl, K2SO4, and K2HPO4. The results show that cultures treated with 1.4 g L-1 of K2SO4 and 17.8 g L-1 of NaHCO3 produced 72.9% and 60.3% higher insulin production, respectively, compared to the control culture. Conversely, NaCl and K2HPO4 consistently demonstrated enhanced insulin production of 52.8% and 47.7%, respectively, when compared to the control culture. Lower concentrations of these additives often led to significantly decreased insulin production compared to the mother culture (Table 1).
Table (1):
Optimization of insulin production through media manipulation
| Media Manipulation gL-1 | Insulin (µg g) (n = 5) Mean ± SD | p* | p** | Mean Difference from control culture at 20th Day (15.9 µg g-1) Mean (CI) p# | |||
|---|---|---|---|---|---|---|---|
| 5th Day | 10th Day | 15th Day | 20th Day | ||||
| NaHCO3 | |||||||
| 14.8 | 2.4 ± 0.2 | 4.0 ± 0.3 | 8.1 ± 0.1 | 11.4 ± 0.2 | 0.004 | <0.001 | -4.4 (-4.9, -3.9) 0.001 |
| 15.8 | 2.7 ± 0.1 | 5.7 ± 0.1 | 9.5 ± 0.3 | 14.5 ± 0.3 | 0.001 | -1.4 (-2.0, -0.7) 0.012 | |
| 17.8 | 4.3 ± 0.3 | 9.7 ± 0.3 | 14.8 ± 0.4 | 25.5 ± 0.4 | 0.001 | 9.6 (8.8, 10.4) 0.001 | |
| 18.8 | 3.3 ± 0.1 | 8.5 ± 0.4 | 12.3 ± 0.3 | 19.3 ± 0.3 | 0.002 | 3.4 (2.8, 4.0) 0.002 | |
| NaNO3 | |||||||
| 1.5 | 1.8 ± 0.1 | 4.2 ± 0.2 | 6.4 ± 0.2 | 10.5 ± 0.2 | 0.002 | <0.001 | -5.3 (-5.7, -4.9) 0.001 |
| 2.0 | 2.4 ± 0.2 | 5.3 ± 0.2 | 8.5 ± 0.2 | 13.6 ± 0.3 | 0.002 | -2.3 (-2.9, -1.6) 0.004 | |
| 3.0 | 3.8 ± 0.1 | 7.8 ± 0.2 | 13.3 ± 0.4 | 20.4 ± 0.4 | 0.001 | 4.5 (4.2, 4.7) 0.001 | |
| 3.5 | 4.3 ± 0.2 | 9.4 ± 0.3 | 14.9 ± 0.3 | 26.6 ± 0.6 | 0.002 | 10.7 (9.7, 11.6) 0.001 | |
| NaCl | |||||||
| 0.6 | 2.9 ± 0.4 | 6.4 ± 0.2 | 8.8 ± 0.3 | 14.4 ± 0.2 | 0.001 | <0.001 | -1.5 (-1.9, -1.0) 0.006 |
| 0.8 | 3.5 ± 0.2 | 7.4 ± 0.2 | 10.3 ± 0.3 | 15.2 ± 0.3 | <0.001 | -0.6 (-1.1, -0.2) 0.031 | |
| 1.2 | 4.3 ± 0.3 | 8.2 ± 0.3 | 14.4 ± 0.3 | 22.5 ± 0.3 | <0.001 | 6.6 (6.1, 7.1) 0.001 | |
| 1.4 | 5.0 ± 0.3 | 9.1 ± 0.2 | 15.6 ± 0.3 | 24.3 ± 0.3 | <0.001 | 8.4 (7.9, 8.9) 0.001 | |
| K2SO4 | |||||||
| 0.6 | 3.6 ± 0.2 | 5.6 ± 0.3 | 8.4 ± 0.4 | 14.2 ± 0.4 | <0.001 | <0.001 | -1.7 (-2.4, -0.9) 0.010 |
| 0.8 | 3.8 ± 0.3 | 6.7 ± 0.3 | 10.4 ± 0.2 | 15.3 ± 0.3 | <0.001 | -0.6 ( -1.1, -0.3) 0.031 | |
| 1.2 | 4.8 ± 0.2 | 7.6 ± 0.2 | 16.7 ± 0.3 | 21.5 ± 0.3 | <0.001 | 5.6 (5.1, 6.1) 0.001 | |
| 1.4 | 5.3 ± 0.2 | 8.4 ± 0.3 | 18.4 ± 0.2 | 27.5 ± 0.3 | <0.001 | 11.6 (11.1, 12.0) 0.001 | |
| K2HPO4 | |||||||
| 0.3 | 3.3 ± 0.4 | 5.7 ± 0.3 | 8.5 ± 0.2 | 11.3 ± 0.4 | <0.001 | <0.001 | -4.6 (-4.8, -4.3) 0.001 |
| 0.4 | 4.1 ± 0.3 | 6.6 ± 0.3 | 11.7 ± 0.3 | 14.7 ± 0.3 | <0.001 | -1.2 (-1.6, -0.7) 0.007 | |
| 0.6 | 4.6 ± 0.2 | 7.5 ± 0.3 | 15.6 ± 0.3 | 20.6 ± 0.4 | <0.001 | 4.7 (4.2, 5.1) 0.001 | |
| 0.7 | 4.7 ± 0.3 | 7.4 ± 0.3 | 17.5 ± 0.3 | 23.5 ± .4 | <0.001 | 7.6 (7.3, 7.9) 0.001 | |
*- significance from 5th-20th day, **- significance within groups, #- significance between treated and control cultures
Figure 1. Forest plot for odd ratios with 95% CI for insulin production by media optimization cultures compare to control culture
Figure 1 demonstrates the OR and 95% CI for insulin production under various media optimization conditions compared to control cultures (OR = 1). For NaHCO3, insulin production decreases at 14.8 g L-1 (OR = 0.7, CI: 0.5-0.9) and 15.8 g L-1 (OR = 0.9, CI: 0.7-1.1) but significantly increases at 17.8 g L-1 (OR = 1.6, CI: 1.4-1.8), before declining slightly at 18.8 g L-1 (OR = 1.2, CI: 1.0-1.4). For NaNO3, lower concentrations (1.5 g L-1, OR = 0.7, CI: 0.4-0.9; 2 g L-1, OR = 0.9, CI: 0.7-1.1) reduce production, while higher concentrations (3 g L-1, OR = 1.3, CI: 1.0-1.6; 3.5 g L-1, OR = 1.7, CI: 1.5-2.0) enhance it. NaCl shows a steady increase in insulin production from 0.6 g L-1 (OR = 0.9, CI: 0.7-1.2) to 1.4 g L-1 (OR = 1.5, CI: 1.2-1.7). Similarly, K2SO4 improves production, starting at 0.6 g L-1 (OR = 0.9, CI: 0.6-1.2) and peaking at 1.4 g L-1 (OR = 1.7, CI: 1.5-1.9). For K2HPO4, production is lowest at 0.3 g L-1 (OR = 0.7, CI: 0.5-1.0) but increases with concentration, reaching a maximum at 0.7 g L-1 (OR = 1.5, CI: 1.2-1.7)
Table (2):
Growth optimization for insulin production
| Growth conditions | Insulin (µg g-1) (n = 5) Mean ± SD | p* | P** | Mean Difference from control culture at 20th Day (15.9 µg g-1) Mean (CI) p# | |||
|---|---|---|---|---|---|---|---|
| 5th Day | 10th Day | 15th Day | 20th Day | ||||
| pH | |||||||
| 7 | 3.5 ± 0.07 | 5.1 ± 0.1 | 9.0 ± 0.07 | 13.1 ± 0.1 | <0.001 | 0. 025 | -2.8 (-3.0, -2.5) 0.023 |
| 8 | 3.8 ± 0.03 | 6.1 ± 0.08 | 11.2 ± 0.1 | 16.4 ± 0.9 | <0.001 | 0.5 (0.3, 0.7) 0.048 | |
| 9 | 4.4 ± 0.07 | 8.1 ± 0.2 | 14.4 ± 0.1 | 19.5 ± 0.07 | <0.001 | 3.6 (3.3, 3.8) 0.009 | |
| 10 | 4.7 ± 0.3 | 9.8 ± 0.1 | 17.1 ± 0.1 | 21.3 ± 0.4 | <0.001 | 5.4 (4.6, 6.2) 0.001 | |
| 11 | 4.7 ± 0.03 | 7.4 ± 0.1 | 11.1 ± 0.2 | 18.3 ± 0.2 | <0.001 | 2.4 (2.0, 2.8) 0.001 | |
| 12 | 4.1 ± 0.1 | 6.8 ± 0.3 | 8.2 ± 0.06 | 19.1 ± 0.2 | <0.001 | 3.2 (3.0, 3.6) 0.001 | |
| Light colour | |||||||
| Red | 4.5 ± 0.4 | 7.5 ± 0.1 | 11.2 ± 02 | 17.3 ± 0.2 | <0.001 | 0.019 | 1.4 (1.1, 1.8) 0.007 |
| Blue | 5.4 ± 0.4 | 9.1 ± 0.1 | 15.2 ± 0.2 | 25.1 ± 0.6 | <0.001 | 9.2 (8.8, 10.2) 0.005 | |
| Green | 4.6 ± 0.4 | 8.6 ± 0.3 | 14.3 ± 0.3 | 22.4 ± 0.3 | 0.002 | 6.5 (6.0, 7.1) 0.001 | |
| Yellow | 3.4 ± 0.2 | 6.2 ± 0.2 | 10.6 ± 0.3 | 18.4 ± 0.4 | 0.001 | 2.5 (1.8, 3.1) 0.001 | |
| Culture system | |||||||
| Open system | 2.3 ± 0.2 | 5.2 ± 0.2 | 8.3 ± 0.1 | 16.4 ± 0.3 | 0.004 | 0.039 | 0.5 (0.3, 0.6) 0.005 |
| Greenhouse | 4.1 ± 0.2 | 7.5 ± 0.3 | 11.3 ± 0.4 | 17.6 ± 0.1 | 0.001 | 1.7 (1.4, 1.9) 0.003 | |
| Water type | |||||||
| Sea water | 4.9 ± 0.1 | 7.8 ± 0.2 | 14.7 ± 0.1 | 20.5 ± 0.3 | 0.001 | 0.015 | 4.6 (4.0, 5.1) 0.002 |
| Enrich Sea water | 5.6 ± 0.1 | 9.5 ± 0.2 | 16.7 ± 0.1 | 25.4 ± 0.3 | 0.001 | 9.5 (9.1, 9.7) 0.001 | |
*- significance from 5th-20th day, **- significance within groups, #- significance between treated and control cultures
Table 2 illustrates various growth conditions aimed at optimizing insulin production through adjustments in pH levels, light color, culture systems, and water types. The results show that increasing pH generally correlates with higher insulin production. The maximum insulin production (33.9%) was observed at pH 10, compared to the control culture. A similar positive trend was noted with different light colors, with blue light resulting in the most significant increase (57.8%) in insulin production, while yellow light showed the least growth compared to the control culture. Regarding culture systems, the greenhouse system produced 10.7% higher insulin production compared to the control culture. Finally, the highest insulin production (59.7%) was observed in enriched seawater, compared to the control culture.
Figure 2. Forest plot for odd ratios with 95% CI for insulin production by growth optimization cultures compare to control culture
Figure 2 illustrates the odds ratios for insulin production under varying conditions, with the OR = 1 representing the control. For pH levels, insulin production increases with pH, reaching a peak at pH 10 (OR = 1.3, CI: 1.1-1.5) and remaining stable at higher levels. For light color, blue light demonstrates the highest enhancement in insulin production (OR = 1.6, CI: 1.4-1.8), followed by green light (OR = 1.4, CI: 1.1-1.7). Culture systems show a minor increase in production in greenhouses (OR = 1.1, CI: 0.9-1.4) compared to open systems. Water type reveals enriched seawater as most effective (OR = 1.6, CI: 1.4-1.8), while regular seawater also enhances production (OR = 1.3, CI: 1.1-1.5).
Table (3):
Optimization of insulin production by various concentrations of carbon sources
| Carbon source (g L-1) | Insulin (µg g-1) Mean ± SD | p* | p** | Mean Difference from control culture at 20th Day (15.9 µg g-1) Mean (CI) p# | |||
|---|---|---|---|---|---|---|---|
| 5th Day | 10th Day | 15th Day | 20th Day | ||||
| Glucose | |||||||
| 0.5 | 4.4 ± 0.2 | 7.3 ± 0.2 | 11.7 ± 0.2 | 18.1 ± 0.3 | <0.001 | 0.001 | 2.2 (1.4, 2.8) 0.006 |
| 1.0 | 5.4 ± 0.2 | 9.3 ± 0.3 | 16.4 ± 0.1 | 22.4 ± 0.4 | <0.001 | 6.5 (5.5, 7.4) 0.001 | |
| 1.5 | 8.4 ± 0.1 | 12.5 ± 0.1 | 23.3 ± 0.3 | 29.4 ± 0.3 | <0.001 | 13.5 (13.2, 13.9) 0.001 | |
| Fructose | |||||||
| 0.5 | 3.5 ± 0.1 | 6.3 ± 0.2 | 10.3 ± 0.1 | 17.2 ± 0.2 | <0.001 | 0.001 | 1.3 (0.7, 1.9) 0.011 |
| 1.0 | 4.7 ± 0.1 | 8.5 ± 0.2 | 15.0 ± 0.2 | 20.5 ± 0.2 | <0.001 | 4.6 (4.1, 5.0) 0.001 | |
| 1.5 | 7.3 ± 0.1 | 11.6 ± 0.2 | 19.3 ± 0.1 | 28.4 ± 0.3 | <0.001 | 12.5 (12.2, 12.8) 0.001 | |
| Galactose | |||||||
| 0.5 | 3.5 ± 0.2 | 5.5 ± 0.2 | 9.5 ± 0.3 | 16.6 ± 0.3 | <0.001 | 0.001 | 0.7 (0.2, 1.6) 0.049 |
| 1.0 | 5.3 ± 0.2 | 8.0 ± 0.2 | 13.6 ± 0.2 | 18.7 ± 0.2 | <0.001 | 2.8 (2.1, 3.4) 0.003 | |
| 1.5 | 7.2 ± 0.2 | 10.4 ± 0.1 | 17.5 ± 0.3 | 23.4 ± 0.2 | <0.001 | 7.5 (7.2, 7.8) 0.001 | |
| Sucrose | |||||||
| 0.5 | 3.3 ± 0.1 | 4.3 ± 0.2 | 10.4 ± 0.2 | 16.6 ± 0.2 | <0.001 | 0.264 | 0.7 (0.2, 1.3) 0.029 |
| 1.0 | 3.7 ± 0.1 | 5.1 ± 0.2 | 12.5 ± 0.3 | 17.4 ± 0.2 | <0.001 | 1.5 (1.0, 2.0) 0.006 | |
| 1.5 | 3.5 ± 0.4 | 5.4 ± 0.3 | 13.7 ± 0.3 | 18.7 ± 0.3 | <0.001 | 2.7 (1.9, 3.4) 0.004 | |
| Glycerol | |||||||
| 0.5 | 3.5 ± 0.2 | 5.7 ± 0.2 | 11.4 ± 0.2 | 16.1 ± 0.4 | <0.001 | 0.490 | 0.3 (-0.1, 0.6) 0.094 |
| 1.0 | 3.6 ± 0.2 | 5.4 ± 0.4 | 12.4 ± 0.2 | 16.7 ± 0.2 | <0.001 | 0.8 (0.3, 1.3) 0.020 | |
| 1.5 | 3.5 ± 0.3 | 5.6 ± 0.3 | 13.3 ± 0.2 | 17.7 ± 0.2 | <0.001 | 1.8 (1.2, 2.4) 0.005 | |
*– significance from 5th-20th day, **– significance within groups, #- significance between treated and control cultures
Table 3 illustrates various growth conditions aimed at optimizing insulin production through various carbon sources at different concentrations. Result shows that the maximum insulin production compared to the control culture at the 20th day was observed with glucose at 85.9% higher than the control, followed by fructose with a 78.3% increase. Galactose showed a 47.2% enhancement in insulin production, while sucrose resulted in a more modest 17.6% increase. Glycerol produced the least impact, with only an 11.3% increase in insulin production compared to the control culture.
Figure 3. Forest plot for odd ratios with 95% CI for insulin production by various carbon sources treated cultures compare to control culture
Figure 3 demonstrates the odds ratios and 95% confidence intervals for insulin production across various sugar types and concentrations compared to control cultures (OR = 1). Glucose and fructose show a strong dose-dependent increase in insulin production, with glucose peaking at 1.5 g L-1 (OR = 1.8, CI: 1.3-2.2) and fructose at the same concentration (OR = 1.8, CI: 1.3-2.0). Galactose exhibits moderate effects, with the highest OR of 1.5 at 1.5 g L–¹ (CI: 1.1-1.9), while sucrose shows smaller improvements, reaching an OR of 1.2 at 1.5 g L-1 (CI: 0.9-1.6). Glycerol has minimal influence, with OR values remaining relatively stable across concentrations, peaking at 1.1 (CI: 0.9-1.4).
India, with a population exceeding 1.4 billion, has a significant burden of diabetes. According to the IDF, the country has one of the highest numbers of people living with diabetes worldwide, with estimates suggesting over 77 million cases in 2021. This high prevalence of diabetes drives a substantial demand for insulin, which is a vital component in the management of diabetes, especially for T1DM and some cases of T2DM requiring insulin therapy. As awareness and diagnosis rates of diabetes improve, more people are prescribed insulin, leading to increased demand. Despite India’s growing demand for insulin, affordability and accessibility remain significant challenges. Many people with diabetes face economic barriers to accessing insulin, leading to disparities in treatment outcomes.24
The production of insulin using Escherichia coli (E. coli) has been the standard method since the 1980s when recombinant DNA technology revolutionized insulin manufacturing. This approach is used to produce large quantities of human insulin and insulin analogs for diabetes treatment.25,26 However, E. coli-based production has its challenges, including high costs, complex purification processes, and stringent regulatory requirements.27 As a result, there is a growing interest in finding alternative sources for insulin production that are easier to cultivate and more cost-effective.
Spirulina has been harvested for centuries by various indigenous cultures as a valuable food source.28 It is renowned for its rich nutrient profile, making it a popular supplement and a key component in traditional diets.29 Despite its established benefits, it has yet to attract significant attention as a potential source of insulin production. Studies have reported that blood glucose,30,31 and HbA1c,32,33 level was decreased and insulin level,34,35 was increased in human and animal model by Spirulina supplementation. But these studies not isolated and identified any bio molecules responsible for this reduction glucose level.
The first study by Sliva et al.36 identified the presence of insulin in Spirulina maxima; however, the insulin levels in these species were not specified. Subsequently, research by Razique Anwer et al.37 confirmed insulin presence in 16 out of 23 Arthrospira strains screened. But this study evaluated only effect of media alternation for optimizing the insulin production. Recently the A. platensis SPKY1 have been isolated from Ennore estuary shows the presence of insulin and eight insulinase family protein.19 The insulin production by A. platensis SPKY1 was 15.9 µg g-1 at 20th day, which is lesser than the S. platensis (CFTRI, Mysore) reported by Anwer et al.37 In the present study, A. platensis SPKY1 were exposed to various strain improvement strategies to enhance the insulin production.
Comparing the results of insulin production with the findings of Anwer et al.,37 on S. platensis (CFTRI, Mysore). We can observe some parallels and distinctions in how different media components affect insulin production. Both in A. platensis SPKY1 and S. platensis (CFTRI, Mysore) insulin production generally increases with higher concentrations of various media components, demonstrating a dose-response relationship. In A. platensis SPKY1, we see a steady increase in insulin production with higher concentrations of NaHCO3. Specifically, at 17.8 g L-1, insulin production on the 20th day reaches 25.5 µg g-1, significantly exceeding the mother culture. Maximum insulin content (5.6%) with 180 mM NaHCO3 on day 12, was reported in S. platensis (CFTRI, Mysore) indicating that insulin production increases with this component but shows a decline after the peak point. In A. platensis SPKY1 shows that insulin production reaches 26.6 µg g-1 with NaNO3, indicating a steady increase with higher concentrations. Similarly, S. platensis (CFTRI, Mysore) noted the highest increase in insulin content (22.14%) at 55 mM NaNO3 on day 12, suggesting that this compound is effective at promoting insulin production. Our results show that NaCl contributes to insulin production in a more gradual manner compared to other media components. At a 1.4 g L concentration, insulin production reaches 24.3 µg g-1. Anwer et al.,37 do not report specific values for NaCl, highlighting a potential area where our results diverge or offer additional insights. The production of insulin with K2SO4 appears somewhat variable across different concentrations in our data. At 1.4 g L-1, it shows a significant increase in insulin production, reaching 27.5 µg g-1, the highest among all components. S. platensis (CFTRI, Mysore) shows increase in insulin levels of up to 26% with sulfate additions, reinforcing the idea that K2SO4 can significantly boost insulin production. Our data demonstrates that insulin production increases with higher concentrations of K2HPO4, with a maximum of 23.5 µg g-1 at 0.7 g L-1. It was reported that in S. platensis (CFTRI, Mysore) an increase of 3.75% in insulin content when K2HPO4 was added at 5.5 mM, indicating that while K2HPO4 has an impact, it may not be as pronounced as with other components.
In this study, A. platensis SPKY1 insulin production was assessed across various pH levels, with the highest production (21.3 µg g-1) observed at pH 10.0. This can be attributed to the optimal enzymatic activities associated with both photosynthesis and respiration within this pH range.38 Prior research has indicated that a pH of 10.0 fosters an ideal environment for S. platensis growth and nutrient synthesis, resulting in increased biomass, protein, carbohydrate, and pigment production.39 Present findings align closely with those of Abd El-Baky et al.,40 who noted optimal growth and chemical production in S. platensis and S. maxima at pH 10.5. Similarly, Rafiqui et al.,41 reported maximal protein contents in S. platensis and S. fusiformis at pH 9.0 and 10.0, respectively. A study has consistently highlighted pH 10.0 as the point of optimization for S. platensis growth.42
The data suggests that light color plays a significant role in insulin production from A. platensis SPKY1. Across all observations, there is a noticeable increase in insulin production when exposed to different colors of light over time. Blue light appears to stimulate the highest insulin production (5.4 to 25.1 µg g-1). This rapid increase suggests that blue light is particularly effective in enhancing insulin production in A. platensis SPKY1. Notably, red light results in a moderate increase in insulin production, green and yellow light also support insulin production, but to varying extents. Studies have found that blue light enhance the biomass and protein content in Spirulina sp.43-45 In contrast, a study have reported that spirulina growth was observed lesser in blue light compared to orange and white light.46
The results of the present study suggest that a controlled greenhouse system may be more effective than an open system for cultivating Spirulina for insulin production. This suggests that the greenhouse system consistently produces higher insulin levels than the open system. The consistent increase in insulin production in the greenhouse system could be due to better environmental control, allowing for optimized growth conditions and reduced contamination risk. A study reported that Spirulina grown in jars, bags and ponds under greenhouse effect enhance the biomass and protein content.47 Another study shows that S. platensis produced 1.73 g L under greenhouse effect.48
The study investigated the impact of different water types on insulin production in Spirulina. The results demonstrated that enriched sea water led to higher insulin production than regular sea water over the same period. The enriched sea water contains additional essential nutrients and minerals that promote Spirulina growth and metabolic activity, leading to increased insulin synthesis. In contrast, regular sea water has a lower nutrient concentration, which may limit Spirulina growth and thus result in lower insulin production. Similarly, a study reported a large-scale cultivation of Spirulina grown in sea water enriched with NaHCO3 and FeSO4 shows the average biomass (10.3 g) and protein (66.6%) production.49 Another study reported that Spirulina biomass production was enhanced in medium enriched with sea salt (Nacl 40 g L-1). In contrast a study did not find any significant growth in sea water.50
The production of insulin across different carbon sources and varying concentrations over a 20-day period was examined. Across all carbon sources, the insulin production generally increased from the 5th to the 20th day. This suggests that the system is responsive to the carbon source and that the production process becomes more efficient as time progresses.
Glucose had the highest insulin production, especially at the higher concentrations reached 29.4 µg g-1, a significant increase from the mother culture. This indicates that glucose is a potent carbon source for insulin production. Fructose and galactose both had moderate results. Sucrose and glycerol had relatively lower results compared to glucose, fructose, and galactose indicating that these are less efficient carbon sources for insulin production. Research indicated that cyanobacterial growth rates were boosted by all carbon sources except lactose. The highest growth rate of S. platensis occurred on the fourth day of cultivation when glucose, fructose, and succinate were present.51 These findings are consistent with previous studies. The concentration of glucose notably impacted biomass yield, with C. protothecoides and C. saccharophila exhibiting heterotrophic growth and yielding higher biomass when organic compounds were used as carbon sources.52,53 In contrast, other studies found that elevated carbon source concentrations could inhibit microalgal growth.54,55
The top-performing Spirulina strains identified in this study, showing the highest levels of insulin production, will be selected for gene expression analysis and pilot-scale production. By examining these strains, gene expression profiles, we can identify the specific genes responsible for increased insulin synthesis, allowing for targeted genetic modifications or culture optimizations to further boost production. Pilot-scale production will provide insights into the commercial viability of these strains, helping to bridge the gap between laboratory-scale research and full-scale commercial insulin production from Spirulina. This approach could pave the way for innovative, cost-effective solutions to meet the growing demand for insulin, especially in regions with high diabetes prevalence.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Microbiology, School of Life Sciences, Vels University, Pallavaram, Chennai, Tamil Nadu, India, for providing necessary facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
KY conceptualized the study and performed literature review. AKK performed analysis, data interpretation and data validation. KY wrote the manuscript. AKK reviewed the manuscript. AKK and KY revised the manuscript. AKK approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Chan JCN, Lim LL, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives [published correction appears in Lancet. 2021;396(10267):2019-2082.
Crossref - Sun H, Saeedi P, Karuranga S, et al. Erratum to ”IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045”. Diabetes Res Clin Pract. 2023;204:110945.
Crossref - Afroz A, Alramadan MJ, Hossain MN, et al. Cost-of-illness of type 2 diabetes mellitus in low and lower-middle income countries: a systematic review. BMC Health Serv Res. 2018;18(1):972.
Crossref - GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021 [published correction appears in Lancet. 2023;402(10408):1132.
Crossref - Anjana RM, Unnikrishnan R, Deepa M, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474-489.
Crossref - American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43-S48.
Crossref - Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of Clinical Outcomes and Adverse Events Associated with Glucose-Lowering Drugs in Patients with Type 2 Diabetes: A Meta-analysis. JAMA. 2016;316(3):313-324.
Crossref - DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism. 2016;65(2):20-29.
Crossref - Banting FG, Best CH. Pancreatic extracts. J. Lab. Clin. Med. 1990;115:254-272. https://pubmed.ncbi.nlm.nih.gov/2405086/
- Laing R, Waning B, Gray A, Ford N, ‘t Hoen E. 25 years of the WHO essential medicines lists: progress and challenges. Lancet. 2003;361(9370):1723-1729.
Crossref - Beran D, Ewen M, Laing R. Constraints and challenges in access to insulin: a global perspective. Lancet Diabetes Endocrinol. 2016;4(3):275-285.
Crossref - Sharma A, Bhandari PM, Neupane D, Kaplan WA, Mishra SR. Challenges constraining insulin access in Nepal-a country with no local insulin production. Int Health. 2018;10(3):182-190.
Crossref - WHO Essential Medicines and health products information portal [Internet]. India: National list of essential medicines. 2015. http://apps.who.int/medicinedocs/en/d/Js23088en/. Accessed 16 June 2018.
- La Forgia G, Nagpal S. Government-sponsored health insurance in India: are you covered? Washington, DC: World Bank Publications. 2012.
Crossref - Ravi S, Ahluwalia R, Bergkvist S. Health and morbidity in India (2004-2014). Brookings India. Research Paper No. 092016. 2016.
- Government of India, Department of Pharmaceuticals. Jan Aushadhi. Delhi: Bureau of Pharma PSUs of India. http://janaushadhi.gov.in/. Accessed April 19, 2017.
- Singhal GL, Anita K, Nanda A. Jan Aushadhi store in India and quality of medicines therein. Int J Pharm Pharm Sci. 2011;3(1):204-207.
- Hozayen WG, Mahmoud AM, Soliman HA, Mostafa SR. Spirulina versicolor improves insulin sensitivity and attenuates hyperglycemia-mediated oxidative stress in fructose-fed rats. J Intercult Ethnopharmacol. 2016;5(1):57-64.
Crossref - Yuvarani K, Kathireshan AK. Genomic exploration of insulin-like proteins in Arthrospira platensis SPKY1. Curr Res Cmpl Alt Med. 2024;8:231.
Crossref - Zarrouk C. Contribution to the study of a cyanobacterium: influence of various physical and chemical factors on the growth and photosynthesis of Spirulina maxima (Setchell and Gardner) Geitler. [PhD thesis]. University of Paris; France: 1966.
- Donmez GC, Aksu Z, Ozturk A, Kutsal T. A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem. 1999;34(9):885-892.
Crossref - National Center for Biotechnology Information. PubChem Patent Summary for US-3945988-A, Process for isolation of insulin from plant source. https://pubchem.ncbi.nlm.nih.gov/patent/US-3945988-A. Accessed Jan. 31, 2025.
- Ozcan M, Canpolat S, Bulmus O, et al. Agomelatine pretreatment prevents development of hyperglycemia and hypoinsulinemia in streptozotocin-induced diabetes in mice. Fundam Clin Pharmacol. 2019;33(2):170-180.
Crossref - Sharma A, Kaplan WA. Challenges constraining access to insulin in the private-sector market of Delhi, India. BMJ Glob Health. 2016;1(3):e000112.
Crossref - Walsh G. Therapeutic insulins and their large-scale manufacture. Appl Microbiol Biotechnol. 2005;67(2):151-159.
Crossref - Nilsson J, Jonasson P, Samuelsson E, Stוhl S, Uhlen M. Integrated production of human insulin and its C-peptide. J Biotechnol. 1996;48(3):241-250.
Crossref - Ferrer-Miralles N, Villaverde A. Bacterial cell factories for recombinant protein production; expanding the catalogue. Microb Cell Fact. 2013;12:113.
Crossref - AlFadhly NKZ, Alhelfi N, Altemimi AB, Verma DK, Cacciola F, Narayanankutty A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules. 2022;27(17):5584.
Crossref - Soni AR, Sudhakar K, Rana RSA. Spirulina – From growth to nutritional product: A review. Trends Food Sci Technol. 2017;69(Part A):157-171.
Crossref - Layam A, Reddy CLK. Antidiabetic property of Spirulina. Diabetol Croat. 2007;35(2):29-33.
- Guldas M, Ziyanok S, Sahan Y, Yildiz E, Gurbuz O. Antioxidant and anti-diabetic properties of Spirulina platensis produced in Turkey. Food Sci Technol. 2021;41(3):615-625.
Crossref - Simon JP, Baskaran UL, Shallauddin KB, Ramalingam G, Evan Prince S. Evidence of antidiabetic activity of Spirulina fusiformis against streptozotocin-induced diabetic Wistar albino rats. 3 Biotech. 2018;8(2):129.
Crossref - Anwer R, Alam M, Khursheed S, Shaikh MK, Kabir H, Fatma T. Spirulina: Possible pharmacological evaluation for insulin-like protein. J Appl Phycol. 2012;25(3).
Crossref - Hozayen WG, Mahmoud AM, Soliman HA, Mostafa SR. Spirulina versicolor improves insulin sensitivity and attenuates hyperglycemia-mediated oxidative stress in fructose-fed rats. J Intercult Ethnopharmacol. 2016;5(1):57-64.
Crossref - Pandey JP, Tiwari A, Mishra G, Mishra R. Role of Spirulina maxima in the control of blood glucose levels and body weight in streptozotocin-induced diabetic male Wistar rats. J Algal Biomass Util. 2011;2(4):35-37.
- Silva LB, Santos SSS, Azevedo CR, et al. The leaves of green plants as well as a cyanobacterium, a red alga, and fungi contain insulin-like antigens. Braz J Med Biol Res. 2002;35(3):297-303.
Crossref - Anwer R, Khursheed S, Fatma T. Detection of immunoactive insulin in Spirulina. J Appl Phycol. 2011;24(3).
Crossref - Kaushik BD, Sharma RK. Influence of salinity on selected enzymes in cyanobacteria. Indian J Microbiol. 1997;37:99-100.
- El-Monem A, Gharieb M, Hussian AE, Doman K. Effect of pH on phytochemical and antibacterial activities of Spirulina platensis. Int J Appl Environ Sci. 2018;13(4):339-351.
- Abd El-Baky H, El-Baz FK, El-Baroty GS. Spirulina species as a source of carotenoids and a-tocopherol and its anticarcinoma factors. Biotechnol. 2003;2(3):222-240.
Crossref - Rafiqul IM, Jalal KCA, Alam MZ. Environmental factors for optimisation of Spirulina biomass in laboratory culture. Biotechnol. 2005;4(1):19-22.
Crossref - Thirumala M. Optimization of growth of Spirulina platensis for production of carotenoids. Int J Life Sci Biotechnol Pharm Res. 2012;1(2):152-157.
- Hsieh-Lo M, Castillo G, Ochoa-Becerra MA, Mojica L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019;42:101600.
Crossref - Masojidek J, Torzillo G, Koblizek M. Photosynthesis in microalgae. Handbook of Microalgal Culture: Biotechnology and Applied Phycology. 2013:20-39.
Crossref - de Mooij T, Janssen M, Cerezo O, et al. Antenna size reduction as a strategy to increase biomass productivity: A great potential not yet realized. J Appl Phycol. 2015;27(3):1063-1077.
Crossref - Liu Q, Huang Y, Zhang R, Cai T, Cai Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid Based Complement Alternat Med. 2016;2016(1):7803846.
Crossref - Goksan T, Zekeriyaoglu A, Ilknur Ak . The growth of Spirulina platensis in different culture systems under greenhouse condition. Turk J Biol. 2007;31:47-52.
- Andrade MR, Costa JA. Outdoor and indoor cultivation of Spirulina platensis in the extreme south of Brazil. Z Naturforsch C J Biosci. 2008;63(1-2):85-90.
Crossref - Wu B, Tseng CK, Xiang W. Large-scale cultivation of Spirulina in seawater-based culture medium. Botanica Marina. 1993;36(2):99-102.
Crossref - Dineshkumar R, Sampathkumar P, Rajendran N. Cultivation of Spirulina platensis in different selective media. Indian J Mar Sci. 2016;45(12).
- Harutyunyan A, Gabrielyan L, Aghajanyan A, et al. Comparative Study of Physicochemical Properties and Antibacterial Potential of Cyanobacteria Spirulina platensis-Derived and Chemically Synthesized Silver Nanoparticles. ACS Omega. 2024;9(27):29410-29421.
Crossref - Shi X, Zhang X, Chen F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme Microb Technol. 2000;27(3-5):312-318.
Crossref - Isleten-Hosoglu M, Gultepe I, Elibol M. Optimization of carbon and nitrogen sources for biomass and lipid production by Chlorella saccharophila under heterotrophic conditions and development of Nile red fluorescence-based method for quantification of its neutral lipid content. Biochem Eng J. 2012;61:11-19.
Crossref - Tan CK, Johns MR. Fatty acid production by heterotrophic Chlorella saccharophila. Hydrobiologia. 1991;215:13-19.
Crossref - Qiao H, Wang G. Effect of carbon source on growth and lipid accumulation in Chlorella sorokiniana GXNN01. Chinese J Oceanol Limnol. 2009;27(4):762-768.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.