ISSN: 0973-7510
E-ISSN: 2581-690X
Vibrio alginolyticus, an opportunistic pathogen in humans, obtains iron from various iron-containing compounds, including hemin, a heme derivative. This process may be an important virulence-associated adaptation for evolution into a human pathogen. The easiest way for a pathogen to acquire iron from a host is through erythrocyte lysis by hemolysins, which increases the available iron pool in vivo. Hemolysins are important virulence factors in many bacterial pathogens. In this study, a DNA fragment containing the 1281 bp ORF of the hemolysin gene from V. alginolyticus was identified by isolating a hemolytic-negative mutant from a random mutagenesis library. The external DNA sequence from the transposon inserted during random mutagenesis was identified by DNA sequencing. The hemolysin gene was amplified and cloned into the expression vector pET28a. The expression vector containing the hemolysin gene was transformed into Escherichia coli BL21(DE3), and the overexpressed protein had approximately 47 kDa molecular weight, as predicted by SDS-PAGE. The hemolytic activity of whole-cell lysate with overexpressed protein AGV17462.1 was tested, and it shows hemolysis slightly less than the positive control, which means 100% hemolysis. No hemolysis was observed after heat treatment at 100 °C for 10 min. Therefore, it is considered a thermolabile hemolysin. In conclusion, transposon-induced gene disruption leads to the loss of the hemolytic phenotype in the isolated mutant, in which the DNA sequence has been identified and characterized as a new hemolysin gene.
Hemolysin Gene, Random Mutagenesis, Hemolysis, Vibrio Alginolyticus, Transposon Insertion
Marine aquatic animals suffer from high mortality due to vibriosis, and Vibrio alginolyticus, a gram-negative halophilic bacterium prevalent in marine ecologies such as estuaries and coastal regions, is one of the causative agents.1 It is ubiquitous in seawater, can cause ear and wound infections, has been identified as the most prevalent pathogen in infected fish, and displays signs of bacterial septicemia. It is also responsible for ulcer diseases in marine animals including fish.1 In 1973, it was isolated from a patient with gastroenteritis, calf wound, and purulent discharge from the conjunctivae. It is an opportunistic pathogen in humans.2-4 V. alginolyticus infection is more common in summer than in winter.5-7 Present knowledge of vibriosis is mostly based on the role of toxins in pathogenesis after extracellular protease (ECP), lipopolysaccharide (LPS), and outer membrane protein (OMP) isolation and purification.8-10 The V. alginolyticus infection and transmission mechanism remains unclear, but indicates transmission through seawater.11 Previous studies have strongly recommended that V. alginolyticus has a pool of virulence genes that are well-known in other species of Vibrio.12 These genes may play important roles in establishing infections in host organisms including humans.13-15 The first virulence gene, similar to trh, was identified in V. alginolyticus from the Alaskan region and Tunisia.16,17 The scientific communities in China, Europe, and the United States of America have also highlighted V. alginolyticus virulence.18-21 According to some studies, V. alginolyticus infection is associated with fatalities in immunocompromised patients.22
V. alginolyticus uses hemin, a heme derivative, as its sole iron source, which strongly suggests that V. alginolyticus produces hemolysin.23-25 Hemolysin is a pore-forming exotoxin that lyses the membranes of erythrocytes and other mammalian cells. In addition to hemolysis, it induces various cytotoxic effects that often result in viability loss.26,27 The main role of hemolysin is to liberate hemoglobin from erythrocytes for the utilization of hemoglobin iron, which plays an important role in establishing the infection process in Vibrio spp.28 Thermostable direct hemolysin (TDH), HlyA (El Tor), TLH (Thermolabile hemolysin), δ-VPH (Thermostable hemolysin), and novel hemolysin gene (HLX), are the representative hemolysins present in the Vibrios.29 Two of these, HlyA and TDH, have been investigated extensively, and are closely related to virulence.29,30 However, TLH, δ-VPH, and HLX hemolysins have not been fully understood, and require further research.31 In V. alginolyticus, the thermostable TDH-related hemolysin TRH has been reported, but has not characterized till now.16,30 These hemolysins have been identified as human virulence factors that can cause septicemia, gastrointestinal illnesses, and even death.32-34 TDH and other hemolysins of Vibrio spp. and their virulence mechanisms in diarrhea have been studied, and detailed information on the structural and functional characteristics of TDH-encoding genes is well represented in the international DNA and amino acid databases of Vibrio parahaemolyticus. However, very few reports have focused on V. alginolyticus-encoded hemolysin, which is lethal to mice and aquatic animals.30,35-38 In this study, we identified a major thermolabile hemolysin gene with nucleotide and amino acid sequences different from those of previously reported hemolysins. The hemolysin gene identified in V. alginolyticus was heterologously overexpressed in Escherichia coli BL21(DE3) and its hemolytic activity was studied. The hemolytic-negative V. alginolyticus mutants did not lyse erythrocytes and may not be pathogenic or less pathogenic than the wild type. This finding may help to develop tools to regulate or block hemolysin expression, which ultimately inhibits erythrocyte lysis and reduces V. alginolyticus pathogenicity. This finding will also help in designing new drugs that can be used as antibiotics.39
Chemicals and reagents
Agarose, ethanol, 25:24:1 phenol-chloroform-isoamyl alcohol (PCI), and potassium acetate were purchased from Sigma-Aldrich. Tris-base, hydrochloric acid, sodium hydroxide, phenol, chloroform, isopropanol, proteinase K, RNase, ethidium bromide, EDTA, calcium chloride, magnesium chloride, glucose, and sodium chloride were purchased from HiMedia Life Sciences. Defibrinated sheep blood was obtained from KDT Biotechnologies (India). Chemicals and enzymes for molecular biology, including Taq polymerase, dNTPs, nuclease-free water, restriction enzymes, 10× standard buffer for PCR, and T4-DNA ligase were purchased from New England Biolabs (NEB) Ltd.
Bacterial strains, plasmids, and media
V. alginolyticus was grown in Luria Bertani medium (LB) containing 2% sodium chloride for routine culture and genomic DNA isolation. E. coli containing plasmid pET-28a was grown in LB containing 20 µg/mL kanamycin (Km), while E. coli DH5a and E. coli BL21(DE3) without plasmid were grown in LB without antibiotics. After transformation, the cells were grown on LBA plates containing 20 µg/mL Km for 24 h. The transformed colonies appeared on kanamycin plates and were grown in LB containing Km.
Transposon mutagenesis and mutant isolation
A streptomycin-resistant V. alginolyticus ATCC17749 colony was isolated as the recipient strain for conjugation mating. Donor strain SM10 l pir pSC189 and recipient strain V. alginolyticus ATCC 17749 StrR were directly inoculated from a glycerol stock on LB containing Km and LB containing streptomycin (Sm) + 2% sodium chloride, respectively. After overnight growth of donor and recipient cells at 37 °C and 200 rpm, cells from each tube were freshly inoculated in 10 mL respective broth with antibiotics and allowed to grow at 37 °C and 200 rpm for 3 h. Both donor and recipient cells were centrifuged at 4000 rpm for 3 min, washed two times with LB, and suspended in 200 µL LB. Both cells were mixed gently and spread on 0.45-µm filter paper placed on an LB agar plate without antibiotics and again grown for 4 h at 37 °C. The mating was then scraped up and resuspended in 10 ml LB containing 2% sodium chloride and 15% glycerol. The glycerol stock was stored at -80 °C and further used as a mutant library. Briefly, 100 µL mutant library was plated on LB agar containing 2% sodium chloride and 20 µg/mL Km and Sm and incubated at 37 °C overnight; the next day 200-230 colonies were observed. Every colony was picked and transferred to selective LB sheep blood agar plates containing 1% sodium chloride, 20 µg/mL Km, 20 µg/mL Sm, and 5% RBC suspension. Nearly 10,000 hemolytic-negative colonies were observed, and six hemolytic-negative phenotype mutants were further studied. Selected hemolytic-negative mutants were further confirmed by a hemolytic assay in a liquid broth.
Genomic DNA isolation
The bacterial cells were harvested from overnight grown cultures in LB containing 2% sodium chloride. Cell pellets were collected from 3.0 mL culture by centrifugation at 4000 rpm for 1 min, dissolved in 400 µL TE (pH-8.0) by vortex mixing, and incubated at 37 °C for 1 h after adding 50 µL 10% SDS and 50 µL 20 mg/mL proteinase K in TE (pH-7.5). Then, 500 µL 25:24:1 PCI was added to the reaction tube and the two phases were mixed by gentle inversion. The aqueous phase was extracted with 500 µL chloroform three times after centrifugation at 13000 rpm at 4 °C. The DNA-containing aqueous supernatant was transferred into new 1.5 mL microcentrifuge tubes and 0.8 volume chilled isopropanol was added. The tube was kept on ice for 30 min for DNA precipitation and centrifuged at 13000 rpm for 30 min at 4 °C. The supernatant was discarded; the DNA pellet was washed twice with 70% chilled ethanol, air-dried, re-precipitated after RNase treatment at 37 °C for 30 min, centrifuged at 13000 rpm, air-dried, and finally dissolved in 40 µL 10 mM Tris buffer.
Mutant DNA sequencing
The genomic DNA isolated from hemolysin-negative mutants was digested with BamHI for 6 h at 37 °C. The digested product was precipitated with 0.8 volume chilled isopropanol and dissolved in 50 µL 10 mM Tris-HCl. The BamHI-digested genomic DNA product was ligated with T4-DNA ligase overnight at room temperature to create a circular DNA-like plasmid with R6K ɤ ori, containing the inserted sequence of the transposon. The designed primers sequenced the region outward from where the transposon was inserted. The sequencing reaction was run at the BRIPL (India) using primers P1: 5′-TAATCACCGTCATGGTCTTTGTA-3′ and P2: 5′-GCCTTCTTGACGAGTTCTTCTGA-3′. DNA sequences were investigated individually and alignment and sequence similarity were determined using NCBI nucleotide BLAST tools.
Bioinformatic analysis
The NCBI BLAST tool was used for DNA sequence search and alignment. For the protein, AGV17462.1 bioinformatic characterization was done using the ProtParam tool hosted by Expasy. The SignalP server (version 5.0) was used to identify the signal peptide for secretion. PSORTb version 3.0.3 was used for cellular localization.
Hemolysin gene amplification by PCR
The gene ORF identified by the V. alginolyticus ATCC17749 mutant DNA sequence analysis was amplified by PCR using specific primers AGV17462F:
5′-AACCATATGGACGACATCTCTA-3′ and AGV17462R: 5′-CTAACTCGAGATTCTGTTGAGA-3′. PCR was done in 50 µL reaction mixture, containing 25 ng genomic DNA, 0.2 µM each primer, 200 µM each dNTP, 2.5 U Taq DNA polymerase, 10 mM Tris-HCl, 50 mM KCl, and 1.5 mM MgCl2. The amplification protocol of the hemolysin gene consisted of an initial denaturation at 96 °C for 5 min; followed by 30 cycles of amplification with denaturation at 96 °C for 20 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 2 min; and a final elongation at 72 °C for 10 min. The PCR product was purified using Monarch® PCR Cleanup Kit (NEB).
Hemolysin gene cloning into E. coli
Amplified DNA sequences of the gene of interest and pET28a were digested with NdeI and XhoI for 3 h. The digested PCR-amplified DNA was mixed with the digested plasmid, precipitated with 0.8 vol chilled isopropanol, and dissolved in 50 µL 10 mM Tris-HCl. T4 DNA ligase was used to ligate the DNA mixture into a plasmid during overnight incubation at room temperature. The resultant ligated product was used to transform E. coli DH5a. LB agar plate supplemented with 20 µg/mL Km was used to select the transformed colonies.
The protocol used for the purification of genomic DNA, plasmid DNA, and agarose gel electrophoresis has been described previously, with some modifications.40-42 E. coli DH5a and E. coli BL21(DE3) was transformed as described by Lederberg and Cohen, with some modifications.43
Cloned gene overexpression in E. coli
The pET28a expression vector was used to clone the hemolysin gene and transform it into E. coli BL21(DE3) strain to express it. The transformed cells were inoculated in 10 mL LB containing 20 mg/mL Km and incubated at 37 °C and 200 rpm overnight. This culture was inoculated in 1000 mL LB containing Km and allowed to grow for 2.5 h or until the OD600 value reached 0.6. Hemolysin expression was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 16 °C overnight. The cells were harvested from 250 mL culture by centrifugation at 4000 rpm for 10 min after overexpression. The cell pellet was washed twice with 1× PBS and the cells were collected by centrifugation at 4000 rpm for 10 min. The pellet was resuspended in 10 mM Tris-Cl buffer at three times the cell pellet volume. SDS was added to the resuspended cells at 2% (w/v) final concentration and 1 mM PMSF final concentration was also added. The samples were sonicated for 15 rounds of sonication, each consisting of 30 s exposure at 40% energy, separated by 30 s cooling between sonication cycles. The sample containing overexpressed protein was centrifuged at 12000 rpm for 20 min and protein-containing supernatant was collected in new microcentrifuge tubes and stored at -20 °C for further utilization.
Hemolytic assay
AGV17462 cloned under the T7 polymerase promoter-transformed E. coli BL21(DE3) was grown overnight in 10 ml LB broth. The supernatant was collected by centrifugation; then, 800 µL supernatant was added to 200 µL washed sheep erythrocytes, incubated at 37 °C for 2 h, and then centrifuged to remove all unlysed erythrocytes, according to a protocol developed by modifying that reported by Mercurio and Manning.44 The supernatant absorbance (A540) was recorded to determine the number of lysed erythrocytes. Adding 800 µL sterile deionized water to 200 µL washed sheep erythrocytes lysed all erythrocytes (100%) and taken as a positive control. E. coli BL21(DE3) cultures grown in an LB medium were used as negative controls.
Random mutant library and hemolysin-negative mutant isolation
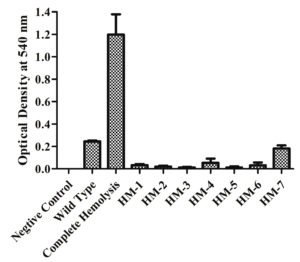
A 20 µg/mL Sm-resistant spontaneous V. alginolyticus ATCC17749 mutant was isolated by plating on LB agar medium containing 2% NaCl and 20 µg/ml Sm. Hemolytic V. alginolyticus ATCC 17749 StrR strain was subjected to random mutagenesis by transposon insertion via conjugal mating with SM10 lpir pSC189. A pool of transposon insertion mutants in a library with approximately 6× coverage of V. alginolyticus ATCC 17749 functional genes (23000 mutants) was obtained. Approximately 10000 mutants were screened for an apparent hemolytic activity loss on blood agar plates. Six phenotypically non-hemolytic mutants were isolated for further hemolysis characterization in V. alginolyticus ATCC 17749. Mutants HM-1-6 had hemolytic activity on sheep blood agar plates (Figure 1). In the broth assay experiments, adding deionized water achieved total lysis and a sterile medium was used as the negative control. HM-1-6 did not lyse the erythrocytes present in the reaction mixture (Figure 2).
Figure 1. Phenotypically hemolysis negative mutant of Vibrio alginolyticus ATCC 17749 and wild type Vibrio alginolyticus ATCC 17749 on Blood Agar Plate
Figure 2. Hemolytic activity is expressed as the A540 of supernatant after incubation with 5% Red Blood Cells. All values shown represent the mean and standard deviation of triplicate
Hemolysin gene identification by DNA sequencing
The plasmids with R6K g ori isolated from mutant genomic DNA were isolated and sequenced away from an inserted transposon. The nucleotide sequences in the hemolytic mutant DNA sequences were analyzed using the online tool nucleotide BLAST (NCBI). This confirmed that the mutant strain carrying the transposon insertion into a 1281 bp ORF (accession number CP006718.1) encodes a 426 amino acid protein (accession number AGV17462.1). The protein sequence was subjected to pBLAST against a non-redundant database. The best hit was the CNNM domain-containing protein (accession number WP_005379948.1) from Vibrio with 100% identity and 100% query coverage. In V. alginolyticus, it showed the best hit against the DUF21 domain-containing protein (accession number EGQ9715304.1), with 99.77% identity at 100% query coverage. It also showed 99.77% identity at 100% coverage with Vibrio sp. JCM 18904 protein hemolysin and related proteins containing the CBS domain (accession number GAJ70819.1). This is a novel thermolabile hemolysin gene found in V. alginolyticus. The nucleotide and amino acid sequences differed from those previously reported for hemolysins. The protein is 47.6 kDa with a theoretical pI of 5.73.
V. alginolyticus hemolysin gene cloning into E. coli and overexpression
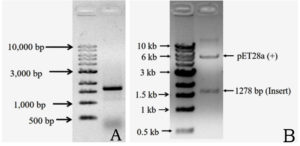
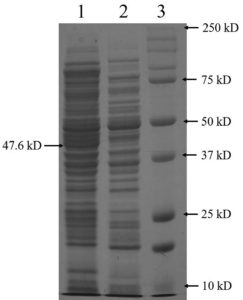
The complete DNA sequence of the hemolysin gene is a 1281 base pair that encodes a 426 amino acid protein. The primers amplified a 1.28 kb DNA sequence containing the complete hemolysin gene sequence (Figure 3a). The amplified product from V. alginolyticus was cloned into E. coli BL21(DE3) cells using the pET28a vector. Plasmids were isolated from the transformed colonies and digested with the restriction enzymes NdeI and XhoI, followed by agarose gel electrophoresis. The presence of a double band (Figure 3b) confirmed the presence of an insert in the clone E. coli. Final confirmation was performed by sequencing. The hemolysin gene could overexpress by the induction with IPTG in E. coli BL21(DE3) at 25 °C. Total protein was extracted using the SDS whole-cell lysate method, and SDS-polyacrylamide gel electrophoresis analysis showed that the molecular weight of the overexpressed hemolysin was 48 kDa (Figure 4). We employed SignalP 5.0, which failed to detect the Sec or Tat system signal peptide for secretion. A slight signal was detected for lipoprotein signal peptides, and a strong signal was detected for other signal peptides. Therefore, a novel mechanism may underlie its secretion. PSORTb CMSVM, ModHMM, and SCL-BLAST suggested that it was a cytoplasmic membrane protein. No secretory signalling peptides were detected. Hemolysis was observed on a plate away from the colony.
Figure 3. Amplification of hemolysin gene from V. alginolyticus ATCC 17749 with the primer P1 and P2. Lane 1: DNA marker and lane 2: amplified product of the gene (3A). Double digestion of cloned plasmid confirmed gene of desired insert cloned successfully. Lane 1: DNA marker and lane 2: pET28a (+) and desired gene (3B)
Figure 4. SDS-PAGE analysis of the overexpressed hemolysin protein in E. coli BL21(DE3). Lane 1: Whole protein from cloned cells including hemolysin; lane 2: proteins from wild type BL21(DE3); lane 3: molecular weight protein marker
Hemolytic activity of heterologously expressed hemolysin gene
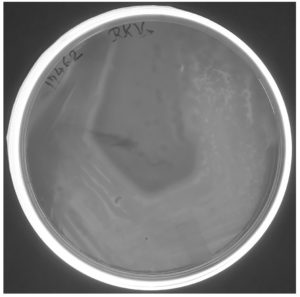
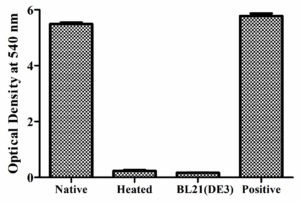
E. coli BL21(DE3) cells with pET28a -AGV17462.1 were induced with IPTG and whole-cell protein was extracted by sonication. Hemolytic activity of the protein was detected in whole-cell lysate crude extract with overexpressed hemolysin, but not E. coli BL21(DE3) proteins used as a negative control; E. coli BL21(DE3) cells with PET28a-AGV17462.1 were plated on a blood agar plate that showed hemolysis (Figure 5); E. coli BL21(DE3) pET28a was used as a negative control. The hemolytic activity of protein AGV17462.1 was 5.49 in terms of the optical density at 540 nm, whereas that of the positive control was 5.78. The thermostability of the overexpressing hemolysin was tested at 100 °C for 20 min. The hemolytic activity of AGV17462.1 crude extract was abolished after heat treatment, rendering it thermolabile (Figure 6).
Figure 5. Overexpression of cloned in E. coli BL21(DE3) hemolysin gene AGV17462.1 on sheep blood agar plate. A clear hemolysis zone visualized around the growing colonies
Figure 6. Detection of hemolytic activity of overexpressed hemolysin before and after heating. Proteins were incubated with washed sheep erythrocytes for 2 hours at 37 °C. Hemolysis were determined by optical density at 540 nm. Native is non heat treated, heated is heat treated at 100 °C for 20 minutes, and BL21(DE3) is from E. coli taken as negative, and complete hemolysis was achieved by deionized water taken as positive
Present knowledge of the role of hemolysin in Vibrio spp. mainly focuses on TLH, VHA, Vp-TDH, HlyIII, VLLY, VHH, HlyA purification, characterization, and pathogenicity.29,37,45-55 The predicted molecular weight of the hemolysin was 47.6 kDa. The protein AGV17462.1 contains 426 amino acids encoded by a 1281 bp DNA sequence present on chromosome 1 of V. alginolyticus. The amino acid sequence of the protein was highly similar to that of Vibrio proteins. Vvh from Vibrio sp. JCM was 99.77% identical with 100% coverage with AGV17462.1 from V. alginolyticus ATCC17749. The hemolysin gene was cloned into a vector plasmid in E. coli DH5a and further cloned in E. coli BL21(DE3) for expression.
The amount of hemolysin secreted in the medium from E. coli BL21(DE3) recombinant plasmid was lesser than that released into V. alginolyticus ATCC 17749 supernatant. Similar to V. cholerae enterotoxin synthesis in E. coli, it accumulates in the periplasmic space.56 Another study also recommended that the V. fluvialis hemolysin is secreted into the extracellular environment after N-terminal region cleavage.55 No secretion signals for the Sec or Tat system in AGV17462.1 hemolysin protein was observed. Pure TLH showed strong phospholipase activity against egg yolk emulsions. Thus, TLH protein may be a phospholipase similar to the VHH hemolysin identified in V. harveyi.57,58 Both hemolysin from V. cholerae O139 and the lecithin-dependent hemolysin from V. parahemolyticus have phospholipase activity.59,60 Extracellular phospholipase A2 and lysophospholipase from V. vulnificus demonstrated hemolytic activity in sheep and mouse erythrocytes, whereas PhlA from V. mimicus showed strong hemolytic activity in rainbow trout and tilapia erythrocytes.61,62 The hemolysin gene was overexpressed in E. coli BL21(DE3) cells. SDS-PAGE displayed that the molecular mass of the overexpressed protein was 48 kDa. The hemolytic activity of protein was approximately equal to that of the positive control (100% sheep erythrocyte lysis). Heating the protein at 100 °C for 10 min abolished the hemolytic activity, making it thermolabile. The mechanism by which this helps in V. alginolyticus virulence has not been determined yet.
In conclusion, we report the identification, molecular cloning, and overexpression of a new hemolysin gene from V. alginolyticus ATCC 17749. The nucleotide and amino acid sequences of this hemolysin differed from those of known hemolysins. The protein with accession number AGV17462.1 is the major hemolysin protein present in V. alginolyticus ATCC17749. The transposon was inserted into a gene, and this disruption led to the loss of hemolytic activity of the isolated mutant; the nucleotide sequence of the disrupted gene was identified and characterized as a hemolytic gene. This study aids in understanding the relationship between hemolysin and virulence factors. These findings may ultimately aid in the diagnosis and treatment of infections caused by V. alginolyticus and other Vibrio species.
ACKNOWLEDGMENTS
The authors are grateful to the Department of Microbiology, Central University of Haryana, for providing the necessary infrastructure.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Egidius E. Vibriosis: Pathogenicity and Pathology. A Review. Aquaculture. 1987;67(1-2):15-28.
Crossref - Zen-Yoji H, Hitokoto H, Morozumi S, Le Clair RA. Purification and characterization of a hemolysin produced by vibrio parahaemolyticus. J Infect Dis. 1971;123(6):665-667.
Crossref - Rubin SJ, Tilton RC. Isolation of Vibrio alginolyticus from wound infections. J Clin Microbiol. 1975;2(6):556-558.
Crossref - Schmidt U, Chmel H, Cobbs C. Vibrio alginolyticus infections in humans. J Clin Microbiol. 1979;10(5):666-668.
Crossref - Blake PA, Weaver RE, Hollis DG. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341-367.
Crossref - Pinto ADI, Ciccarese G, Tantillo G, Catalano D, Forte VT. A Collagenase-Targeted Multiplex PCR Assay for Identification of Vibrio alginolyticus, Vibrio cholerae, and Vibrio parahaemolyticus. J Food Prot. 2005;68(1):150-153.
Crossref - Zhang W, Chen K, Zhang L, et al. The impact of global warming on the signature virulence gene, thermolabile hemolysin, of Vibrio parahaemolyticus. Microbiol Spectr. 2023;11(6):e0150223.
Crossref - Neilands JB. Microbial Envelope Proteins Related to Iron. Annu Rev Microbiol. 1982;36(1):285-309.
Crossref - Wong HC, Liu CC, Yu CM, Lee YS. Utilization of iron sources and its possible roles in the pathogenesis of Vibrio parahaemolyticus. Microbiol Immunol. 1996;40(11):791-798.
Crossref - Lv T, Dai F, Zhuang Q, et al. Outer membrane protein OmpU is related to iron balance in Vibrio alginolyticus. Microbiol Res. 2020;230:126350.
Crossref - Ben Kahla-Nakbi A, Besbes A, Chaieb K, Rouabhia M, Bakhrouf A. Survival of Vibrio alginolyticus in seawater and retention of virulence of its starved cells. Mar Environ Res. 2007;64(4):469-478.
Crossref - Sheikh HI, Alhamadin NII, Liew HJ, et al. Virulence Factors of the Zoonotic Pathogen Vibrio alginolyticus: A Review and Bibliometric Analysis. Appl Biochem Microbiol. 2024;60(3):514-531.
Crossref - Lafisca A, Pereira CS, Giaccone V, Rodrigues DDP. Enzymatic characterization of Vibrio alginolyticus strains isolated from bivalves harvested at Venice Lagoon (Italy) and Guanabara Bay (Brazil). Rev Inst Med Trop Sao Paulo. 2008;50(4):199-202.
Crossref - Masini L, De Grandis G, Principi F, Mengarelli C, Ottaviani D. Research and characterization of pathogenic vibrios from bathing water along the Conero Riviera (Central Italy). Water Res. 2007;41(18):4031-4040.
Crossref - Wang R, Hu X, Deng Y, Gooneratne R. Effect of food matrix type on growth characteristics of and hemolysin production by Vibrio alginolyticus. J Food Prot. 2021;84(8):1411-1420.
Crossref - Gonzalez-Escalona N, Blackstone GM, DePaola A. Characterization of a Vibrio alginolyticus strain, isolated from Alaskan oysters, carrying a hemolysin gene similar to the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus. Appl Environ Microbiol. 2006;72(12):7925-7929.
Crossref - Kahla-Nakbi A Ben, Chaieb K, Besbes A, Zmantar T, Bakhrouf A. Virulence and enterobacterial repetitive intergenic consensus PCR of Vibrio alginolyticus strains isolated from Tunisian cultured gilthead sea bream and sea bass outbreaks. Vet Microbiol. 2006;117(2-4):321-327.
Crossref - Xie ZY, Hu CQ, Chen C, Zhang LP, Ren CH. Investigation of seven Vibrio virulence genes among Vibrio alginolyticus and Vibrio parahaemolyticus strains from the coastal mariculture systems in Guangdong, China. Lett Appl Microbiol. 2005;41(2):202-207.
Crossref - Barbieri E, Falzano L, Fiorentini C, et al. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl Environ Microbiol. 1999;65(6):2748-2753.
Crossref - Hervio-Heath D, Colwell RR, Derrien A, Robert-Pillot A, Fournier JM, Pommepuy M. Occurrence of pathogenic vibrios in coastal areas of France. J Appl Microbiol. 2002;92(6):1123-1135.
Crossref - Matte GR, Matte MH, Sato MIZ, Sanchez PS, Rivera IG, Martins MT. Potentially pathogenic vibrios associated with mussels from a tropical region on the Atlantic coast of Brazil. J Appl Bacteriol. 1994;77(3):281-287.
Crossref - Campanelli A, Sanchez-Politta S, Saurat JH. Cutaneous ulceration after an octopus bite: Infection due to Vibrio alginolyticus, an emerging pathogen. Annales de Dermatologie et de Vénéréologie. 2008; 135(3): 225-227.
Crossref - Vibhuti RK, Pramanik A. Investigations of hemolytic activity and iron utilization sources of Vibrio alginolyticus ATCC 17749. Res J Biotech. 2023;18(1):100-106.
Crossref - Kamal VR, Kumar Y, Avijit P. In silico analysis of putative hemolysin proteins in the genome of Vibrio alginolyticus ATCC 17749 and their structure prediction. Res J Biotech. 2024;19(1):55-58.
Crossref - Bari SM, Marma M, Reza NB, et al. In Silico Identification of Emblica officinalis Compounds Inhibiting Thermolabile Hemolysin from Vibrio alginolyticus in Shrimp. Curr Comput Aided Drug Des. 2025;21.
Crossref - Fabbri A, Falzano L, Frank C, et al. Vibrio parahaemolyticus thermostable direct hemolysin modulates cytoskeletal organization and calcium homeostasis in intestinal cultured cells. Infect Immun. 1999;67(3):1139-1148.
Crossref - Zhao X, He J, Liu J, Deng H, Pan Y, Ye S. Arbutin interacts with Vibrio harveyi hemolysin to alleviate damage from associated infection. Aquaculture. 2024;584:740633.
Crossref - Shinoda S. Protein toxins produced by pathogenic vibrios. J Nat Toxins. 1999;8(2):259-269.
- Zhang XH, Austin B. Haemolysins in Vibrio species. J Appl Microbiol. 2005;98(5):1011-1019.
Crossref - Cai SH, Wu ZH, Jian JC, Lu YS. Cloning and expression of gene encoding the thermostable direct hemolysin from Vibrio alginolyticus strain HY9901, the causative agent of vibriosis of crimson snapper (Lutjanus erythopterus). J Appl Microbiol. 2007;103(2):289-296.
Crossref - Singh V, Somvanshi P, Rathore G, Kapoor D, Mishra BN. Gene cloning, expression and homology modeling of hemolysin gene from Aeromonas hydrophila. Protein Expr Purif. 2009;65(1):1-7.
Crossref - Fukui T, Shiraki K, Hamada D, et al. Thermostable direct hemolysin of Vibrio parahaemolyticus is a bacterial reversible amyloid toxin. Biochemistry. 2005;44(29):9825-9832.
Crossref - Ju CH, Yeung PSM, Oesterling J, Seigerman DA, Boor KJ. Vibrio parahaemolyticus growth under low-iron conditions and survival under high-magnesium conditions. J Food Prot. 2006;69(5):1040-1045.
Crossref - Baffone W, Casaroli A, Campana R, et al. “In vivo” studies on the pathophysiological mechanism of Vibrio parahaemolyticus TDH+ – induced secretion. Microb Pathog. 2005;38(2-3):133-137.
Crossref - Takahashi A, Sato Y, Shiomi Y, et al. Mechanisms of chloride secretion induced by thermostable direct haemolysin of Vibrio parahaemolyticus in human colonic tissue and a human intestinal epithelial cell line. J Med Microbiol. 2000;49(9):801-810.
Crossref - Taniguchi Y, Kubomura S, Hirano H, Mizue K, Ogawa M, Mizuguchi Y. Cloning and charecterization of a gene encoding a new thermostable hemolysin from from Vibrio parahemolyticus. FEMS Microbiol Lett. 1990;67(3):339-346.
Crossref - Hall RH, Drasar BS. Vibrio cholerae HlyA hemolysin is processed by proteolysis. Infect Immun. 1990;58(10):3375-3379.
Crossref - Wang Y, Luo J, Zhao Y, Zhang J, Guan X, Sun L. Haemolysins are essential to the pathogenicity of deep-sea Vibrio fluvialis. iScience. 2024;27(5):109558.
Crossref - Zhao X, Guo Y, He J, Liu J, Ye S. Gastrodin relieves Vibrio harveyi infection by blocking hemolysin active centers. Aquaculture. 2021;544:737056.
Crossref - Humphreys GO, Willshaw GA, Anderson ES. A simple method for the preparation of large quantities of pure plasmid DNA. BBA Sect Nucleic Acids Protein Synth. 1975;383(4):457-463.
Crossref - Green MR, Sambrook J. Preparation of Plasmid DNA by Alkaline Lysis with Sodium Dodecyl Sulfate: Minipreps. Cold Spring Harb Protoc; 2016;2016(10):prot093344.
Crossref - Summerton J, Atkins T, Bestwick R. A rapid method for preparation of bacterial plasmids. Anal Biochem. 1983;133(1):79-84.
Crossref - Lederberg EM, Cohen SN. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974;119(3):1072-1074.
Crossref - Mercurio A, Manning PA. Cellular localization and export of the soluble haemolysin of Vibrio cbolerae El Tor. Mol Gen Genet. 1985;(200):472-475.
Crossref - Cai Q, Zhang Y. Structure, function and regulation of the thermostable direct hemolysin (TDH) in pandemic Vibrio parahaemolyticus. Microb Pathog. 2018;123:242-245.
Crossref - Marolda CL, Valvano MA, Lawlor KM, Payne SM, Crosa JH. Flanking and internal regions of chromosomal genes mediating aerobactin iron uptake systems in enteroinvasive Escherichia coli and Shigella flexneri. J Gen Microbiol. 1987;133(8):2269-2278.
Crossref - Nagamune K, Yamamoto K, Honda T. Intramolecular Chaperone Activity of the Pro-region of Vibrio cholerae El Tor Cytolysin*. J Biol Chem. 1997;272(2):1338-1343.
Crossref - Miyoshi SI, Sasahara K, Akamatsu S, et al. Purification and characterization of a hemolysin produced by Vibrio mimicus. Infect Immun. 1997;65(5):1830-1835.
Crossref - Nishibuchi M, Kaper JB. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: A virulence gene acquired by a marine bacterium. Infect Immun. 1995;63(6):2093-2099.
Crossref - Yu-chung C, M-chung C, Y-ching C, C-ling J. Characterization and Virulence of Hemolysin III from Vibrio vulnificus. Curr Microbiol. 2004;49(3):175-179.
Crossref - Baida GE, Kuzmin NP. Cloning and primary structure of a new hemolysin gene from Bacillus cereus. BBA – Gene Struct Expr. 1995;1264(2):151-154.
Crossref - Chang TM, Chuang YC, Su JH, Chang MC. Cloning and sequence analysis of a novel hemolysin gene (vllY) from Vibrio vulnificus. Appl Environ Microbiol. 1997;63(10):3851-3857.
Crossref - Yoh M, Honda T, Miwatani T. Purification and partial characterization of a non-O1 Vibrio cholerae hemolysin that cross-reacts with thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1986;52(1):319-322.
Crossref - Yoshida H, Honda T, Miwatani T. Purification and characterization of a hemolysin of Vibrio mimicus that relates to the thermostable direct hemolysin of Vibrio parahaemolyticus. FEMS Microbiol Lett. 1991;84(3):249-254.
Crossref - Han JH, Lee JH, Choi YH, Park JH, Choi TJ, Kong IS. Purification, characterization and molecular cloning of Vibrio fluvialis hemolysin. Biochim Biophys Acta – Proteins Proteomics. 2002;1599(1-2):106-114.
Crossref - Gennaro ML, Greenaway PJ, Broadbent DA. The expression of biologically active cholera toxin in Escherichia coli. Nucleic Acids Res. 1982;10(16):4883-4890.
Crossref - Zhong Y, Zhang X hua, Chen J, et al. Overexpression, Purification, Characterization, and Pathogenicity of Vibrio harveyi Hemolysin VHH. Infect Immun. 2006;74(10):6001-6005.
Crossref - Sun B, Zhang XH, Tang X, et al. A single residue change in Vibrio harveyi hemolysin results in the loss of phospholipase and hemolytic activities and pathogenicity for turbot (Scophthalmus maximus). J Bacteriol. 2007;189(6):2575-2579.
Crossref - Shinoda S, Matsuoka H, Tsuchie T, et al. Purification and characterization of a lecithin-dependent haemolysin from Escherichia coli transformed by a Vibrio parahaemolyticus gene. J Gen Microbiol. 1991;137(12):2705-2711.
Crossref - Lohner S, Walev I, Boukhallouk F, Palmer M, Bhakdi S, Angela V. Pore formation by Vibrio cholerae cytolysin follows the same archetypical mode as b barrel toxins from gram positive organisms. FASEB J. 2009;23(8):2521-2528.
Crossref - Lee JH, Ahn SH, Kim SH, Choi YH, Park KJ, Kong IS. Characterization of Vibrio mimicus phospholipase A (PhlA) and cytotoxicity on fish cell. Biochem Biophys Res Commun. 2002;298(2):269-276.
Crossref - Testa J, Daniel LW, Kreger AS. Extracellular phospholipase A2 and lysophospholipase produced by Vibrio vulnificus. Infect Immun. 1984;45(2):458-463.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.