ISSN: 0973-7510
E-ISSN: 2581-690X
With their ability to produce antibiotics, influence drug transport, and serve as vehicles or adjuvants for drug delivery, microbial signatures may provide new information on the pathophysiology of different lung illnesses. Most investigations of lung microbiome signatures were previously conducted using bronchoalveolar lavage (BAL) fluid and usually required bronchoscopy, a technique that involves passing an optical device through the airways to visualize the tracheobronchial tree. In the context of lung illnesses, this method is a multipurpose modality with diagnostic and therapeutic potential. To diagnose lung illness using bronchoscopy samples, we conducted a comprehensive literature search to identify clinical trials that evaluated the use of microbial signature analysis using polymerase chain reaction (PCR). Only 17 of the 1,784 studies met the inclusion criteria. The effect of pulmonary microbiota on the outcome of lung disease has been the subject of few studies. The data and results indicated that microbial signatures are significantly associated with lung disease. Despite conflicting findings, bronchoscopy-based analysis of lung microbiome signatures for lung disease diagnosis and prognosis remains a promising new area of treatment. Analysis of lung microbial signatures opens the door to the possibility of restoring native microorganisms and treating dysbiosis by manipulating the composition of the lung microenvironment.
Microbiota, Lung Disease, Bronchoscopy, Diagnosis, Prognosis
The microbiome, also known as the microbial signature, is an exhaustive inventory of all microbes that inhabit the human body and impact health. The multipurpose microbial signature may produce antibiotics, alter the rate and direction of drug transport, serve as a drug delivery method, or act as an adjuvant to other drugs.1 Probiotics and prebiotics are examples of microbial signatures that are also thought of as medications. Probiotics are essential for preserving the microbial equilibrium in the respiratory system, and there has been a recent increase in interest in the link between lung microbiota and respiratory disorders.2 Probiotics are living microorganisms that, when consumed in adequate amounts, have beneficial effects on host health. While probiotics are widely recognized for their role in maintaining gut health, emerging research suggests that they also play a crucial role in preserving the microbial balance within the respiratory system. By supporting a healthy microbiota in the upper respiratory tract, probiotics help reduce the risk of infections, such as upper respiratory tract infections (URIs), acting as a defense against viral and bacterial invasions. Certain probiotic strains have demonstrated the ability to inhibit the growth of harmful pathogens, produce antimicrobial substances, and enhance the integrity of the epithelial cell barrier in the respiratory tract. These functions are vital for maintaining a balanced and resilient respiratory microbiota. Moreover, probiotics regulate immune system activity, influencing both innate and adaptive immune responses. This regulatory effect helps the host immune system recognize and combat potential threats more effectively, contributing to the prevention and management of respiratory diseases.3 The role of microbial signatures in the pathophysiology of human illnesses, especially lung diseases, has been extensively studied.4 Researchers have shown that the shape and content of lung microbial signatures may predict the outcomes of chronic respiratory disorders.5 Previous studies have shown that the lung microbiome influences immunological modulation and disease progression and prognosis.6 Growing evidence suggests that lung microorganisms play a crucial role in the development of lung diseases.7
Lung illnesses are better understood through research on lung microbial signatures. Currently, the 16S rRNA gene is used in molecular biochemical procedures, such as polymerase chain reaction (PCR), for bacterial identification in microbial signature analysis.8 Bacterial species and genera can be identified using these small, conserved regions of the genome. Until recently, bronchoscopy was the gold standard for analyzing bronchoalveolar lavage (BAL) fluid for microbial signatures in the lungs.9
A bronchoscope is an optical device inserted into the airways to visually inspect the tracheobronchial tree. This method has several diagnostic applications in medical fields. Sampling procedures may include bronchial brushing, bronchial cleaning, transbronchial needle aspiration (TBNA), and BAL.10
The primary objective of this study was to evaluate the potential of microbial profiling as a diagnostic and prognostic tool for the management of lung diseases.
This systematic literature review aimed to identify and evaluate studies that met predefined inclusion and exclusion criteria, using a qualitative descriptive analysis approach. The purpose of this methodology was to support the development of robust clinical inquiries through comprehensive evidence synthesis. This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency and methodological rigor.11 The initial step was to identify and merge research papers from all search sources. In the second step, we used the criteria to filter the titles and abstracts of the papers and chose the ones for inclusion. The final step was to determine whether all research publications met the inclusion criteria. Finally, in the fourth step, the pertinent material was extracted and processed according to the title and subject.12 The systematic review protocol was formally registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42024579893.
Literature search
A systematic literature search was performed across three major databases (PubMed, ProQuest, and Science Direct). For each database, tailored search strategies were applied to account for variations in indexing and search functionalities. Boolean operators (AND, OR, NOT) were adjusted accordingly and not generalized across all platforms to maintain the specificity and sensitivity of each search. A literature search was conducted electronically in December 2022.
Using a combination of Boolean operators (AND and OR) and the medical subject headings (MeSH) equivalent, we used terms relevant to the study issue for Pubmed: (“bronchoscopy” OR “bronchoscopic” OR “bronchoscopies” OR “BAL” OR “bronchial washing” OR “bronchial lavage” OR “bronchoalveolar lavage” OR “lung lavage” OR “bronchopulmonary lavage”) AND (“microbiome” OR “microbiota” OR “microbial”). No filters or constraints were implemented during the search.
For Proquest: TI, AB, SU (“bronchoscopy” OR “bronchoscopic” OR bronchoscopies” OR “BAL” OR “bronchial washing” OR “bronchial lavage” OR “bronchoalveolar lavage” OR “lung lavage” OR “bronchopulmonary lavage”) AND (“microbiome” OR “microbiota” OR “microbial”).
For Science Direct: (“bronchoscopy” OR “bronchoscopic” OR “bronchoscopies” OR “BAL” OR “bronchial washing” OR “bronchial lavage” OR “bronchoalveolar lavage” OR “lung lavage” OR “bronchopulmonary lavage”) AND (“microbiome” OR “microbiota” OR “microbial”).
Selection criteria
The following eight factors were considered for inclusion in this literature review: (1) Research designs that included randomized control trials, cross-sectional studies, case-control studies, or cohort studies. (2) Pulmonology’s most common diseases should be discussed. (3) Bronchoscopy should be used as the sampling tool. (4) The study population consisted of adults. (5) The microbial signature should be identified as a diagnostic and prognostic factor. (6) PCR can be used to analyze microbial signatures. (7) Significant probability values (p < 0.05) and diagnostic and prognostic values should be reported. (8) The research should be written in English. Four criteria were used as exclusion criteria in this systematic literature review: (1) Use of non-pulmonary samples for microbial signature analysis. (2) Microbiological signature analysis using microbial culture. (3) Redundant literature. (4) Case reports, literature reviews, case series, meta-analyses, and systematic review research methods.
Data selection and extraction
All records retrieved from the databases were compiled, and duplicates were removed using Rayyan software. The total number of articles retrieved from each database, along with the date and time of each search, was recorded for reproducibility using the Rayyan website. Rayyan facilitates systematic literature reviews, allowing us to choose and retrieve the necessary data. After the data were extracted from a predefined database, unnecessary materials were removed. Data were retrieved using a pre-designed table once the appropriate literature was collected.
Literature quality assessment
The quality of observational studies was evaluated separately using the Newcastle-Ottawa Scale (NOS). Selection, comparability, and exposure/outcome were the three criteria used to evaluate the bias. While cross-sectional studies could only obtain a maximum score of 8, case-control and cohort studies achieved a maximum score of 9. Excellent quality research was defined as a total score of at least 7 for cohort and case-control studies and at least 6 for cross-sectional studies.13
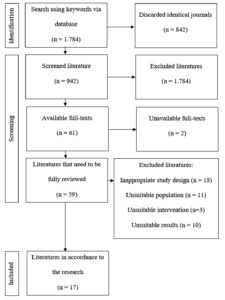
Figure shows a flow diagram of the book selection process. By searching the aforementioned databases for relevant terms, 1,784 articles were identified; however, 842 were deemed unnecessary and were eliminated. Additionally, 881 articles were deemed irrelevant based on their titles and abstracts. Subsequently, 59 full-text publications were evaluated to determine their suitability. The qualitative synthesis for the systematic review ultimately included 17 publications.
Table 1 lists the features reported in the literature. Nine studies used case-control research design, six used cohort study design, and two relied on cross-sectional study design. The USA, Italy, South Korea, Japan, and the UK are among the many nations that hosted these research projects. Five nations- the UK, Italy, Poland, Germany, and Hungary- conducted joint multicenter research for one study. The publications of the papers ranged from 2014 to 2022. Out of the 1,421 cases, 376 were derived from case-control studies. Ten articles mostly dealt with lung cancer, which is the most frequent type of lung illness. Idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD), bronchial asthma, and infectious lung disorders such as bacterial and Coronavirus disease 2019 (COVID-19) pneumonia were also discussed in other articles.
Table (1):
Summary of the literature search
Authors |
Country |
Study Design |
Number of Cases |
Type of Disease |
Control |
|---|---|---|---|---|---|
Bello et al.14 |
Spain |
Case control |
24 cases, 10 controls |
Lung cancer |
Patients with no history of lung cancer |
Boesch et al.15 |
Switzerland |
Case control |
35 cases, 10 controls |
Immunotherapy in advanced-stage NSCLC |
NSCLC undergoing surgery |
Cheng et al.16 |
China |
Case control |
32 cases, 22 controls |
Lung cancer |
Benign lung disease |
An et al.17 |
China |
Prospective cohort |
80 cases |
Immunotherapy in stage 4 NSCLC |
N/A |
Denner et al.18 |
United States of America |
Cross sectional retrospective |
39 cases, 19 controls |
Bronchial asthma |
Healthy patients |
Of et al.19 |
China |
Prospective cohort |
67 cases |
Severe pneumonia |
N/A |
Gaibani et al.20 |
Italy |
Case control |
24 cases, 24 controls |
COVID-19 |
Non-COVID-19 pneumonia |
Jang et al.21 |
South Korea |
Prospective cohort |
84 cases |
NSCLC |
N/A |
Kyo et al.22 |
Japan |
Case control |
40 cases, 7 controls |
Intubated ARDS |
Intubated non-ARDS |
Lee et al.23 |
South Korea |
Prospective cohort |
28 cases |
Lung cancer |
Benign mass |
Liu et al.24 |
China |
Case control |
24 cases, 18 controls |
Lung cancer with unilateral lobar mass |
Healthy patients |
Liu et al.25 |
China |
Prospective cohort |
9 cases |
Lung cancer |
N/A |
Molyneaux et al.26 |
United Kingdom |
Case control |
20 cases, 15 controls |
Acute exacerbation of IPF |
Stable IPF |
Molyneaux et al.27 |
United Kingdom |
Case control |
65 cases, 44 controls |
IPF |
Healthy patients |
Ramsheh et al.28 |
United Kingdom, Germany, Italy, Poland, and Hungary |
Case control |
339 cases, 207 controls |
COPD |
Healthy patients |
Tsay et al.29 |
United States of America |
Cross sectional prospective |
85 cases |
Lung cancer |
N/A |
Zhuo et al.30 |
China |
Prospective cohort |
50 cases |
One-side lung cancer and metastases |
N/A |
*NSCLC = Non-Small Cell Lung Cancer, N/A = Not Applicable or Not Available, COVID-19 = Coronavirus Disease 2019, ARDS = Acute Respiratory Distress Syndrome, IPF = Idiopathic Pulmonary Fibrosis, COPD = Chronic Obstructive Pulmonary Disease
Table 2 shows the inclusion criteria for the literature evaluation, including the use of bronchoscopy to collect lung samples for PCR-based microbial signature analysis. The majority of the samples collected using bronchoscopy were from BAL fluid; this method was described in 10 papers. Alternatively, other studies used brushing or tissue biopsies to collect samples.
Table (2):
Analysis methods and outcomes of microbiota determined by 16S rRNA gene based on literature
Authors |
Sampling Method |
Type of Microbial Signature |
Significant Outcome |
NOS Score |
|---|---|---|---|---|
Bello et al.14 |
TBLB |
Streptococcus genus |
Streptococcus served as a diagnostic factor in lung cancer patients (sensitivity = 93%, specificity = 83.3%). |
7 |
Boesch et al.15 |
TBLB |
Gammaproteobacteria class |
The Gammaproteobacteria class was associated with low expression of PD-L1 (p = 0.006) and low progression-free survival (p = 0.003). |
8 |
Cheng et al.16 |
BAL |
TM7-3 class, TM7 phylum, Pseudomonadaceae phylum, Capnocytophaga, Sediminibacterium, Gemmiger, Blautia, Oscillospira, Stenotrophomonas, Microbacterium, Blautia, and Lautropia genera |
The TM7 phylum, TM7-3 class, as well as Capnocytophaga, Gemmiger, Sediminibacterium, Blautia, and Oscillospira genera were more common in lung cancer than in benign lung disease (p
| 7 |
An et al.17 |
BAL |
Fusobacterium genus |
Fusobacterium was associated with poor response to anti-PD-L1 treatment (p
| 7 |
Denner et al.31 |
BAL |
Lactobacillus, Pseudomonas, and Rickettsia genera |
Lactobacillus, Pseudomonas, and Rickettsia. were more common in patients with bronchial asthma (p
| 6 |
Of et al.32 |
BAL |
Prevotellaceae and Actinomycetaceae families |
The Prevotellaceae and Actinomycetaceae families increased clinical improvement by 14% and 10% in severe pneumonia (95% CI [1.04, 1.25], p = 0.006; 95% CI [1.02, 1.18, p = 0.01). |
9 |
Gaibani et al.20 |
BAL |
Pseudomonas spp. species |
Pseudomonas spp. were more commonly found in COVID-19 than non-COVID-19 pneumonia (p = 0.021) |
8 |
Jang et al.21 |
BAL then TBLB |
Neisseria genus, Veillonella dispar, and Haemophilus influenzae species |
Neisseria bacteria were more commonly found in NSCLC with low PD-L1 levels than in NSCLC with high PD-L1 levels (p = 0.037).21 In addition, Veillonella dispar was more commonly found in NSCLC with high PD-L1 levels (p = 0.028) and in the NSCLC group in comparison with the non-responder group (p = 0.041). Finally, Haemophilus influenzae & Neisseria perflava highly found in the non-responder NSCLC group (p = 0.041). |
8 |
Kyo et al.22 |
BAL |
Betaproteobacteria class, Enterobacteriaceae family, Staphylococcus, and Streptococcus genera |
The Betaproteobacteria class was less common in ARDS patients who did not survive than in ARDS patients who survive (p = 0.012). Staphylococcus, Enterobacteriaceae, and Streptococcus were significantly associated with the increase of IL-6 levels in ARDS patients who did not survive (p
| 8 |
Lee et al.23 |
BAL |
Firmicutes phylum, TM7, Veillonella, and Megasphaera genera |
The Firmicutes and TM7 phyla were more commonly found in lung cancer than in benign mass (p = 0.037 and p = 0.035, respectively). Megasphaera and Veillonella were more common in lung cancer than in benign mass (p = 0.003 and p = 0.022, respectively; AUC = 0.888; sensitivity = 95.0%, specificity = 75.0% and sensitivity = 70.0%, specificity = 100.0%; p = 0.002). |
7 |
Liu et al.24 |
PSB |
Streptococcus genus |
Streptococcus could predict the presence of lung cancer (AUC = 0.693, sensitivity = 87.5%, specificity = 55.6%). |
8 |
Liu et al.25 |
BAL |
Oscillospirales order, Christensenellaceae family, Lactobacillus, Marseilles, and Lactococcus genera |
The Oscillospirales order, Christensenellaceae family, as well as Lactobacillus, Marseille, and Lactococcus genera were more common in lung cancer (p
| 8 |
Molyneaux et al.26 |
BAL |
Campylobacter sp., Stenotrophomonas sp., and Veillonella sp. species |
Campylobacter sp. and Stenotrophomonas sp. were commonly found in acute exacerbation IPF than stable IPF (p=0.02 and p=0.03), while Veillonella sp. were commonly found in stable than IPF acute exacerbation IPF (p
| 7 |
Molyneaux et al.27 |
BAL |
Haemophilus, Streptococcus, and Neisseria genera and Veillonella spp. species |
The Haemophilus, Streptococcus, and Neisseria genera as well as Veillonella spp. species were more common in IPF than healthy patients (p
| 8 |
Ramsheh et al.28 |
Brushing |
Prevotella, Streptococcus, and Moraxella genera |
Prevotella was found to be lower in COPD patients than in healthy patients (p Streptococcus was more prevalent among COPD patients compared to healthy individuals. (p Moxarella was more common in COPD patients than in healthy patients (p Prevotella was more commonly found in COPD patients without inhaled steroid therapy compared to COPD patients with inhaled steroid therapy (p = 0.021). |
8 |
Tsay et al.29 |
Brushing
|
Streptococcus and Veillonella genera |
Streptococcus and Veillonella were more common in lung cancer (p = 0.026). |
7 |
Zhuo et al.30 |
BAL |
Spiroplasma and Weissella genera |
Spiroplasma and Weissella were more common in lung cancer in cancerous lesions than in noncancerous lesions (p = 0.003 and p = 0.009, respectively). |
7 |
Jang et al.21 |
BAL then TBLB |
Neisseria genus, Veillonella dispar, and Haemophilus influenzae species |
Neisseria bacteria were more commonly found in NSCLC with low PD-L1 levels than in NSCLC with high PD-L1 levels (p = 0.037).21 In addition, the Veillonella dispar species was more commonly found in NSCLC with high PD-L1 levels (p = 0.028) and in the NSCLC responder group in comparison with the non-responder group (p = 0.041). Finally, Haemophilus influenzae and Neisseria perflava were more commonly found in the NSCLC non-responder group (p = 0.041). |
8 |
Acute Respiratory Distress Syndrome (ARDS), Phosphate Solubilizing Bacteria (PSB), Idiopathic Pulmonary Fibrosis (IPF), Chronic Obstructive Pulmonary Disease (COPD), Bronchoalveolar Lavage (BAL), Programmed Death-Ligand 1 (PD-L1), Coronavirus Disease 2019 (COVID-19), Area Under the Curve (AUC), PSB, IPF, NSCLC, ARDS, IPF, and COPD are acronyms
Literature quality assessment results
The NOS was used to evaluate the potential for bias in case-control, cross-sectional, and cohort studies. The NOS ratings for each study are shown in Table 2. A score of 8 indicated good quality and little risk of bias, which was achieved in most studies.
Outcome results and microbial signature analysis
Lung Diseases in Oncology
Microbial signature analysis has a dual use in diagnosing and predicting outcomes in patients with lung cancer. Streptococcus bacteria showed promising diagnostic results in a study by Bello et al. in patients with lung cancer (sensitivity, 93%; specificity, 83.3%).14 Liu et al. found that Streptococcus is a good predictor of lung cancer (AUC = 0.693, sensitivity = 87.5%, specificity = 55.6%), lending credence to a previous claim.24 Nonetheless, a study conducted by Lee et al. revealed that lung cancer had higher levels of Veillonella and Megasphaera bacteria than benign masses (p = 0.003 and p = 0.022, respectively; AUC = 0.888).23 Nonetheless, the findings from microbial signature analyses of immunotherapy-treated lung cancer were contradictory. Low PD-L1 expression (p = 0.006) and progression-free survival (p = 0.003) were related to the Gammaproteobacteria class, according to Boesch et al.15 Chu et al. found a strong association (p < 0.001) between Fusobacterium and a subpar reaction to anti-PD-L1 medication.17 Jang et al. also showed that compared to Non-Small Cell Lung Cancer (NSCLC) with high PD-L1 levels, NSCLC with low PD-L1 levels had a much higher prevalence of Neisseria bacteria (p = 0.037). According to other findings, the non-responder group had a lower frequency of Veillonella dispar than the NSCLC responder group (p = 0.041) and the patients with NSCLC and elevated PD-L1 levels (p = 0.028). Neisseria perflava and Haemophilus influenzae were more common in the NSCLC non-responder group than in the other groups (p = 0.041 and p = 0.041, respectively).21
This study revealed a wide range of microbial signature communities. The TM7 phylum was more common in lung cancer cases than in benign masses, according to Cheng et al. and Lee et al. (p = 0.035 and p < 0.05, respectively).16,23 Moreover, according to Cheng et al., there was a greater incidence of the TM7-3 class in instances of lung cancer in comparison with cases of benign lung illness (p < 0.05), along with the genera Capnocytophaga, Sediminibacterium, Gemmiger, Blautia, and Oscillospira.13 Conversely, Liu et al. revealed that there was an increased prevalence of the Oscillospirales order, Christensenellaceae family, Lactobacillus, Marseille, and Lactococcus genera in lung cancer (p < 0.05).25 Tsay et al. found that lung cancer was most often caused by bacteria from the genera Veillonella and Streptococcus (P = 0.026).29 A study conducted by Zhuo et al. revealed an interesting finding: Spiroplasma and Weissella genera were more abundant in malignant lung lesions than in noncancerous lesions (p = 0.003 and p = 0.009, respectively).30
Infectious lung disease
Microbial signature analysis has the potential to be a useful predictor of clinical improvement in infectious lung diseases. Patients with severe pneumonia had 14% and 10% better prognoses (p = 0.006 and p = 0.001, respectively) when exposed to microbial signatures from the Prevotellaceae and Actinomycetaceae families, respectively.32 Pseudomonas spp. was more often detected in COVID-19, according to Gaibani et al. (p = 0.021).20 Acute respiratory distress syndrome (ARDS) may arise as a result of serious infectious illnesses. The Betaproteobacteria class was found to be less prevalent in patients with ARDS who did not survive than in those who did (p = 0.012), according to research conducted by Kyo et al. In the group that did not make it, members of Staphylococcus, Streptococcus, and Enterobacteriaceae families were associated with higher levels of IL-6. This may be a possible indicator of the severity of inflammation-induced illness in this group (p < 0.005).22
Obstructive lung disease
Patients suffering from asthma and chronic obstructive pulmonary disease (COPD) were shown to have higher prevalence of certain microbial signatures when in comparison with healthy individuals. Individuals with asthma bronchiale had considerably higher levels of Lactobacillus, Pseudomonas, and Rickettsia compared to healthy individuals, according to a study by Denner et al. (p < 0.01).31 According to Ramsheh et al., a significant difference was observed (p < 0.0001) in the prevalence of the Streptococcus and Moxarella genera between healthy individuals and patients with COPD. At the same time, a considerably smaller number of members of the Prevotella genus was found in patients with COPD compared to healthy individuals (p < 0.0001). Individuals with COPD who did not use inhaled steroids had a greater prevalence of Prevotella infection than those who did (p = 0.021). The severity of COPD symptoms was inversely associated with Prevotella prevalence, but lung function and physical activity were favorably associated.28
Idiopathic pulmonary fibrosis
A significant increase in the abundance of Haemophilus, Streptococcus, Neisseria, and Veillonella species was observed in patients with IPF compared to healthy individuals (p < 0.001, p < 0.01, p < 0.05, and p < 0.001, respectively).27 Campylobacter sp. and Stenotrophomonas sp. were more prevalent in patients with acute IPF exacerbation than in those with stable IPF. Additionally, compared with stable IPF, acute exacerbation of IPF was less likely to have Veillonella sp. (p < 0.01).26
Researchers in the field of microbiology have long assumed that the lungs are completely germ-free.33 Culture samples obtained from patients with acute or chronic illnesses are the gold standard for identifying and detecting microorganisms in the human body. However, modern technology allows the detection and identification of many bacteria through molecular biochemical analyses, bypassing the need for culture procedures. Researchers can detect and categorize various microorganisms in ecological communities using technologies, such as genomic techniques for molecular biochemical analyses. To reproduce bacterial DNA sequences, this approach employs quantitative PCR to identify 16S rRNA.4 Crucially, the molecular methods for bacterial identification described earlier can only detect DNA in the material under study and cannot distinguish between live and dead bacteria. Culture methods and other more conventional approaches, on the other hand, need the presence of actual live organisms.
According to the findings of these studies, the lungs may not be completely sterile. According to Dickson et al., the state of microbial signatures in the lungs is affected by three factors: (1) the entry of microbes into the airways, (2) the expulsion of microbes from the respiratory system, and (3) the development of microbes in certain habitats.6 Oxygen tension, pH, and immunological state are only a few of the lung microenvironmental factors that might change the microbial spectrum.9 Thus, changes in the dynamic state of lung microbes may lead to the development of lung illness.6,31
Lung microbial fingerprints were substantially linked to lung disorders, including asthma, cystic fibrosis (CF), COPD, IPF, and respiratory infections, according to this meta-analysis that gathered data from many investigations. We identified these disorders by collecting samples that included microbes and analyzing them using PCR, which entails sequencing the genomes of the microbes. Prior to classification using the current taxonomy database, the sequences are aligned based on predefined degrees of homology.9,34,35
Several studies have compared the lung microbial signatures in healthy individuals with those in illness states, and the findings show that the two groups vary significantly in composition.27,33,36,37 Lower bacterial diversity, or dominance by a single or small group of taxa, is linked with disease conditions, according to the research.38 Information gained from genetic and clinical studies has improved our understanding of disease causation within the complex microbiome milieu of healthy individuals and patients with specific lung illnesses.28,39-43 Nowadays, most people agree that a diverse community of bacteria called the lung microbiota is fundamental for maintaining lung health.44 Several lung disorders have been linked to dysbiosis, which is characterized by alterations in lung microbiota composition.34,45,46
Based on sputum samples, Taylor and Simpson et al. postulated that airway microbial makeup is related to the asthma phenotype. In contrast, patients with eosinophilic asthma show a greater diversity in bacterial load, with relative enrichment in Moraxella and Haemophilus spp., and a relative decrease in the presence of Streptococcus, Gemella, and Porphyromonas, when treated with high doses of inhaled corticosteroids (ICSs).47,48 Acute exacerbation of COPD can be prevented by keeping the lung and gut microbial signatures intact, as the gut-lung axis may influence the severity of COPD. Research has shown that, during an acute exacerbation episode, Bacteroidetes and Proteobacteria are more abundant in the fecal microbial profile, whereas Firmicutes and Actinobacteria are less abundant, lending credence to this notion.42,49 Huang et al. found that the lung microbial profile is related to histology and risk of disease progression.50 Metastatic adenocarcinoma had far lower Streptococcus levels than non-metastatic adenocarcinoma, according to bronchial washing fluid samples. Metastatic SCC, on the other hand, had higher levels of Veillonella and Rothia.51 Because this could affect the microbial signature composition, it may be important to consider the types of samples that are tested. Durack et al. showed notable differences between sputum and bronchoalveolar lavage fluid (BALF) samples.15
Sputum analysis of lung microbial fingerprints remains the gold standard for studying healthy individuals. Given the combination of substances originating from the upper, lower, and oral tracts, the function of sputum as a lung representation is still up for dispute. Because of its exceptional capacity to record the topographical distribution of microbes, the lung tissue is, in theory, the best material for microbial signature analysis of the airway and lungs. Only patients who undergo lung resections, cancer surgeries, or biopsies have been able to benefit from it because of the difficulty in obtaining lung tissue in most therapeutic settings.51
Another option for collecting lung disease samples for microbial signature analysis is non-invasive techniques such as bronchoscopy. Currently, BAL fluid is used for most lung microbial signature analyses. Another option is to employ bronchoscopy for bronchial cleaning, biopsies, transbronchial needle aspiration (TBNA), and bronchial brushing.10 It is possible to introduce oral microbial signatures into the sputum and saliva. As it may reduce the impact of oral contamination, some scientists believe that BAL fluid is a good choice for studying lung microbiomes.16
Finally, bronchoscopy-based microbial signature analysis of the lungs to diagnose and predict the prognosis of lung illnesses has yielded inconsistent findings. Therefore, to understand their possible function in lung illness and to characterize the prognosis and reaction of individuals to immunomodulatory treatments, microbial signatures must be understood. Local microenvironments are formed by microbes and/or their metabolism, which affect the immune response and cancer assault mechanisms. According to Bello et al., microbes may control the equilibrium between tumor-induced inflammation and antitumor immunity in different microenvironments.14
Microbial signature analysis is a potential method for identifying novel therapeutic targets among lung microbes. The analysis of lung microbial signatures opens the door to the possibility of treating dysbiosis and restoring native bacteria by manipulating the composition of the lung microenvironment. This objective may be improved through the use of antibiotics, quorum-sensing inhibitory compounds, probiotics (health-promoting extrinsic microorganisms), and prebiotics (specific bacterial growth-promoting, non-absorbable chemicals). In addition, treatment interventions based on lung microbial signature analysis may target the most pathogenic microbes, while avoiding other potentially harmful microbes.9 Some studies have used systemic antibiotics to control respiratory microbiomes. The impact of oral ciprofloxacin on clinical pulmonary endpoints in patients with IPF was first unclear,52,53 although one trial indicated a possible benefit in terms of mortality.50 Lung microbial signature results have been observed in several studies of systemic antibiotic use, which has improved our knowledge of the processes driving clinical findings.54,55 According to the BLESS study, all patients with bronchiectasis who did not have CF showed a decrease in exacerbation rates and changes in the sputum microbiota after receiving long-term erythromycin therapy.19 Notably, the clinical and microbiological effects of erythromycin treatment differed depending on whether P. aeruginosa was the predominant species in the airway secretions.19 Studies have shown that erythromycin amplifies resistance genes56 and may reduce the disease-causing potential of P. aeruginosa by disrupting cell-to-cell communication.57
As a new sampling method for diagnosing and predicting the prognosis of lung disorders, bronchoscopy offers alternatives and references for microbial signature analysis. However, this comprehensive literature review has a few limitations. One limitation of this study is that the function of microbial signatures in diagnostic and prognostic statistics was not examined using a meta-analysis method. Second, no analysis has been conducted on the impact of microbial diversity on diagnosis and prognosis. Finally, this study only considered publications written in English; papers written in other languages that fulfilled the study requirements were not reviewed.
Through the use of PCR for quantitative microbial signature analysis, scientists can detect and categorize a wide range of microbes in ecological communities by focusing on the 16S rRNA gene. Patients with lung disorders may also have samples taken for microbial signature analysis using non-invasive techniques such as bronchoscopy. Therefore, microbial detection is a promising avenue for future treatment strategies. The analysis of lung microbial signatures opens the door to the possibility of treating dysbiosis and restoring native bacteria by manipulating the composition of the lung microenvironment.
ACKNOWLEDGMENTS
The authors would like to show their gratitude to Dr. Soetomo General Academic Hospital for the place of this study. The authors are also grateful to Aisyah Tsabita Zaki Ihsani for providing editing support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
IS and IAM conceptualized the study. IS, RYS, and SW wrote the original draft. IS, RYS, SW, IAM, and MM wrote, reviewed, and edited the manuscript. IAM and MM supervised the study. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Blum HE. The human microbiome. Adv Med Sci. 2017;62(2):414-420.
Crossref - Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013;7(3):245-257.
Crossref - Beane J, Mazzilli SA, Tassinari AM, et al. Detecting the Presence and Progression of Premalignant Lung Lesions via Airway Gene Expression. Clin Cancer Res. 2017;23(17):5091-5100.
Crossref - Amir-Behghadami M, Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020;37(6):387.
Crossref - Page MJ, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372.
Crossref - Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed April 13, 2023.
- Bello S, Vengoechea JJ, Ponce-Alonso M, et al. Core Microbiota in Central Lung Cancer With Streptococcal Enrichment as a Possible Diagnostic Marker. Arch Bronconeumol. 2021;57(11):681-689.
Crossref - Liu HX, Tao LL, Zhang J, et al. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int J Cancer. 2018;142(4):769-778.
Crossref - Lee SH, Sung JY, Yong D, et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer. 2016;102:89-95.
Crossref - Boesch M, Baty F, Albrich WC, et al. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology. 2021;10(1):1988403.
Crossref - Chu S, Cheng Z, Yin Z, et al. Airway Fusobacterium is Associated with Poor Response to Immunotherapy in Lung Cancer. Onco Targets Ther. 2022;15:201-213.
Crossref - Jang HJ, Choi JY, Kim K, et al. Relationship of the lung microbiome with PD-L1 expression and immunotherapy response in lung cancer. Respir Res. 2021;22(1):322.
Crossref - Cheng C, Wang Z, Wang J, et al. Characterization of the lung microbiome and exploration of potential bacterial biomarkers for lung cancer. Transl Lung Cancer Res. 2020;9(3):693.
Crossref - Liu B, Li Y, Suo L, et al. Characterizing microbiota and metabolomics analysis to identify candidate biomarkers in lung cancer. Front Oncol. 2022;12:108456.
Crossref - Tsay JCJ, Wu BG, Badri MH, et al. Airway Microbiota Is Associated with Upregulation of the PI3K Pathway in Lung Cancer. Am J Respir Crit Care Med. 2018;198(9):1188-1198.
Crossref - Zhuo M, An T, Zhang C, Wang Z. Characterization of Microbiota in Cancerous Lung and the Contralateral Non-Cancerous Lung Within Lung Cancer Patients. Front Oncol. 2020;10:01584.
Crossref - Du S, Wu X, Li B, et al. Clinical factors associated with composition of lung microbiota and important taxa predicting clinical prognosis in patients with severe community-acquired pneumonia. Front Med. 2022;16(3):389-402.
Crossref - Gaibani P, Viciani E, Bartoletti M, et al. The lower respiratory tract microbiome of critically ill patients with COVID-19. Sci Rep. 2021;11(1):10103.
Crossref - Kyo M, Nishioka K, Nakaya T, et al. Unique patterns of lower respiratory tract microbiota are associated with inflammation and hospital mortality in acute respiratory distress syndrome. Respir Res. 2019;20(1):246.
Crossref - Denner DR, Sangwan N, Becker JB, et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol. 2016;137(5):1398.
Crossref - Ramsheh MY, Haldar K, Esteve-Codina A, et al. Lung microbiome composition and bronchial epithelial gene expression in patients with COPD versus healthy individuals: a bacterial 16S rRNA gene sequencing and host transcriptomic analysis. Lancet Microbe. 2021;2(7):e300-e310.
Crossref - Molyneaux PL, Cox MJ, Willis-Owen SAG, et al. The Role of Bacteria in the Pathogenesis and Progression of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2014;190(8):906-913.
Crossref - Molyneaux PL, Cox MJ, Wells AU, et al. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res. 2017;18(1):29.
Crossref - Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4(1):59-72.
Crossref - Dickson RP, Erb-Downward JR, Martines FJ, Huffnagle GB. The Microbiome and the Respiratory Tract. 2016;78(1):481-504.
Crossref - Kim G, Park C, Yoon YK, et al. Prediction of lung cancer using novel biomarkers based on microbiome profiling of bronchoalveolar lavage fluid. Sci Rep. 2024;14(1):1691.
Crossref - Zhang Y, Chen X, Wang Y, et al. Alterations of lower respiratory tract microbiome and short-chain fatty acids in different segments in lung cancer: A multiomics analysis. Front Cell Infect Microbiol. 2023;13:1261284.
Crossref - Muhlebach MS, Zorn BT, Esther CR, et al. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog. 2018;14:e1006798.
Crossref - Philley JV, Kannan A, Olusola P, et al. Microbiome Diversity in Sputum of Nontuberculous Mycobacteria Infected Women with a History of Breast Cancer. Cell Physiol Biochem. 2019;52(2):263-279.
Crossref - Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med. 2013;187(10):1118-1126.
Crossref - Huang YJ, Nariya S, Harris JM, et al. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874-884.
Crossref - Arrieta MC, Arevalo A, Stiemsma L, et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol. 2018;142(2):424-434.e10.
Crossref - Begley L, Madapoosi S, Opron K, et al. Gut microbiota relationships to lung function and adult asthma phenotype: A pilot study. BMJ Open Respir Res. 2018;5(1):e000324.
Crossref - Haldar K, George L, Wang Z, et al. The sputum microbiome is distinct between COPD and health, independent of smoking history. Respir Res. 2020;21(1):47.
Crossref - Jang YO, Lee SH, Choi JJ, et al. Fecal microbial transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp Mol Med. 2020;52(7):1128-1139.
Crossref - Gomes S, Cavadas B, Ferreira JC, et al. Profiling of lung microbiota discloses differences in adenocarcinoma and squamous cell carcinoma. Sci Rep. 2019;9(1):12838.
Crossref - Jin J, Gan Y, Liu H, et al. Diminishing microbiome richness and distinction in the lower respiratory tract of lung cancer patients: A multiple comparative study design with independent validation. Lung Cancer. 2019;136:129-135.
Crossref - Wang D, Cheng J, Zhang J, et al. The Role of Respiratory Microbiota in Lung Cancer. Int J Biol Sci. 2021;17(13):3646-3658.
Crossref - Yang D, Xing Y, Song X, Qian Y. The impact of lung microbiota dysbiosis on inflammation. Immunology. 2020;159(2):156-166.
Crossref - Cho JY, Kim MY, Kim JH, et al. Characteristics and intrasubject variation in the respiratory microbiome in interstitial lung disease. Medicine. 2023;102(14):e33402.
Crossref - Taylor SL, Leong LEX, Mobegi FM, et al. Long-Term Azithromycin Reduces Haemophilus influenzae and Increases Antibiotic Resistance in Severe Asthma. Am J Respir Crit Care Med. 2019;200(3):309-317.
Crossref - Simpson JL, Daly J, Baines KJ, et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47(3):792-800.
Crossref - Wu Y, Luo Z, Liu C. Variations in fecal microbial profiles of acute exacerbations and stable chronic obstructive pulmonary disease. Life Sci. 2021;265:118738.
Crossref - Huang D, Su X, Yuan M, et al. The characterization of lung microbiome in lung cancer patients with different clinicopathology. Am J Cancer Res. 2019;9(9):2047-2063.
- Durack J, Lynch SV, Nariya S, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol. 2017;140(1):63-75.
Crossref - Yi X, Gao J, Wang Z. The human lung microbiome-A hidden link between microbes and human health and diseases. iMeta. 2022;1(3):e33.
Crossref - Varney VA, Parnell HM, Salisbury DT, Ratnatheepan S, Tayar RB. A double blind randomised placebo controlled pilot study of oral co-trimoxazole in advanced fibrotic lung disease. Pulm Pharmacol Ther. 2008;21(1):178-187.
Crossref - Shulgina L, Cahn AP, Chilvers ER, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. 2013;68(2):155-162.
Crossref - Segal LN, Clemente JC, Wu BG, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax. 2017;72(1):13-22.
Crossref - Brill SE, Law M, El-Emir E, et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial. Thorax. 2015;70(10):930-938.
Crossref - Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013;309(12):1260-1267.
Crossref - Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir Med. 2014;2(12):988-996.
Crossref - Choo JM, Abell GCJ, Thomson R, et al. Impact of long-term erythromycin therapy on the oropharyngeal microbiome and resistance gene reservoir in non-cystic fibrosis bronchiectasis. MSphere. 2018;3(2):e00103-18.
Crossref - Burr LD, Rogers GB, Chen AC, et al. Macrolide treatment inhibits Pseudomonas aeruginosa quorum sensing in non-cystic fibrosis bronchiectasis: an analysis from the Bronchiectasis and Low-Dose Erythromycin Study trial. Ann Am Thorac Soc. 2016;13(10):1697-1703.
- Liu C, Wu K, Sun T, et al. Effect of invasive mechanical ventilation on the diversity of the pulmonary microbiota. Critical Care. 2022;26(1):252.
Crossref - Wang H, Wang Y. What makes the gut-lung axis working? from the perspective of microbiota and traditional chinese medicine. Canadian Journal of Infectious Diseases and Medical Microbiology. 2024;2024(1):8640014.
Crossref - Herivaux A, Willis JR, Mercier T, et al. Lung microbiota predict invasive pulmonary aspergillosis and its outcome in immunocompromised patients. Thorax. 2021;77(3):283-291.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.