Antimicrobial resistance (AMR) has emerged as one of the most serious global health crises, especially among pediatric populations. MRSA, VRE, and CRE are examples of multidrug-resistant organisms that pose significant challenges in infection management, especially among weak children in intensive care units. Increasing resistance among infections such as Escherichia coli and Klebsiella pneumoniae makes them more challenging to manage. Contributing factors to this problem are the misuse of antibiotics and the lack of pediatric-specific research, calling for comprehensive action. Root causes like the misuse of antibiotics and the lack of pediatric-relevant research are fueling the crisis, and that is why collective action is paramount. Interventions like implementing surveillance networks like the WHO’s Global Antimicrobial Resistance Surveillance System (GLASS) and facilitating antimicrobial stewardship programs (ASPs) age-specific for children, like the effective ASP model at Johns Hopkins Children’s Center, must be undertaken. Public health campaigns, for example, the CDC’s “Get Smart” program, show the power of education in averting the abuse of antibiotics. Treatment attempts are made more difficult by other serious multidrug-resistant pathogens that affect children, particularly in hospital settings, such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Clostridium difficile. To that end, multiple strategies are essential, such as establishing strong surveillance systems and antimicrobial stewardship programs (ASPs) that take into account the pediatric population. Understanding local resistance patterns is central to designing of region-specific public health interventions, especially in low-resource settings, where AMR burdens healthcare systems and threatens their fragile infrastructures. The discovery of genetic factors that cause resistance and the emergence of new drugs will play crucial roles in curbing this evolving threat while improving the well-being of children. A strategic approach to the challenge of AMR in the hospitalized child requires coordinated, multi-pronged efforts-education for health professionals and their families, public campaigns, and improved access to quality medical care. Prescription guidelines strengthened, and more effective surveillance systems should be put in place; targeted educational initiatives will ensure effective management of the rising tide of AMR within healthcare systems. Long-term solutions will only be achieved through collaboration among healthcare providers, policymakers, and researchers. Such collaboration will encourage over time, promote innovation, and ensure that better treatment options are developed. It is also crucial that evidence-based treatments are provided as well as healthcare systems are ready to address the pediatric patients because of the increase in multidrug-resistant like E. coli and Staphylococcus aureus. Commitment to vigilance, education, and innovation will be vital for mitigating AMR risks and protect the most vulnerable populations worldwide. This study focuses on the treatment challenges and adverse clinical outcomes associated with antimicrobial resistance (AMR) in pediatric populations. It highlights the role of resistance mechanisms, emerging pathogens, and the urgent need for targeted stewardship programs to protect child health.
Pediatric Healthcare, Emerging Pathogens, Antimicrobial Resistance
Risen to become the most pressing public health issue globally is antimicrobial resistance, which increases morbidity and mortality on a holistic level, from the neonate to the elderly patient. The current challenge of infectious disease management has been by the emergence of MDROs like MRSA, VRE, and CRE. WHO states further that antimicrobial resistant microorganisms kill some 700,000 individuals each year, while about 200,000 are among newborn children, and thus there is urgent need to curb AMR.1 The worldwide spread and appearance of resistant agents have been hastened by antibiotics overuse and misuse coupled with the ability of bacteria naturally to acquire the resistance mechanisms from gene mutations or horizontal gene exchange. AMR monitoring and control in children are of vital importance to avert long-term health consequences of excessive antibiotic use.2
In the past few years, there has been a shift in research towards the rising alarming proportion of AMR against Gram-negative and Gram-positive bacteria and they are responsible for a majority of nosocomial infections in children. General resistance to Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa against the first line of antibiotics primarily due to the rise of ESBL.3 The Brazilian University Hospital recently reported a finding that 24% of hospitalized toddlers were multidrug-resistant, 72% of whom were Gram-negative and nearly half of whom produced ESBL. The trend calls for swift provision of point-of-care, rapid, and accurate antimicrobial susceptibility testing for the guidance of therapy and resistance control through ongoing surveillance.4
Child antimicrobial resistance (AMR): which has been caused by economic disparity and inadequate access to quality health facilities, is a critical global health issue, particularly for middle- and low-income countries. AMR infections resulted in more than 3 million child deaths in 2022, most of which were newborns and Southeast Asian children. This new crisis is primarily caused by the abuse of antibiotics in humans and animals, inadequate healthcare facilities, and inadequate access to clean water and sanitation. Increased multidrug-resistant (MDR) infections are particularly alarming as it has been determined that bacteria resistant5 to two or more drugs account for as much as 30% of childhood infections in Southern Europe and Asia. Children’s AMR must be addressed by a multi-faceted approach consisting of fortifying surveillance systems worldwide to track resistance patterns, encouraging rational antibiotic use among medical professionals to avoid misuse, research and development funding for new pediatric-specific medicines, vaccines, and diagnostics, and international cooperation to pool resources, information, and innovation. Unless collective and urgent action, pediatric AMR burden will persist to increase and pose danger to the lives and health of children globally.6
Impact of AMR on public health and clinical outcomes
AMR is a major global health threat, and the WHO estimated that AMR was responsible for around 1.27 million deaths in 2019, aside from 5 million more deaths due to associated conditions. Pediatric populations are particularly vulnerable because infections from MDROs lead to increased severity, prolonged hospitalization, and higher fatality rates. For example, MDROs are reported to be the cause of an increase in children’s stay at hospitals by 20% as hospital-acquired mortality increases by up to 40%.7 MRSA and ESBL bacteria that can be seen producing strains within a pediatrician are among the worrisome types. Economic implications of AMR are a colossal burden since treatment costs reach a staggering figure of $2.39 billion yearly in the United States. In LMICs, where healthcare systems are already overstretched, the financial impact is even more deep-seated as these regions do not have available diagnostic resources or access to effective therapies. This is a need for AMS programs to counter the rising threat of AMR. AMS programs have also been successful in high-income countries, where it has been able to reduce the colonization and clinical infections of MDROs; however, similar strategies are required to be established in LMICs, which bear a more significant burden of AMR.8
Antibiotic misuse and overuse have been one of the major practices in pediatric care, particularly in the management of bacterial infections. Empirical prescribing of broad-spectrum antibiotics, usually without proper diagnostics, is a great contributor to the development of resistance. There have been increasing trends all around the world, for example in a study by luna9 at Santiago de los Caballeros, Dominican Republic reported that, Serratia marcescens isolates showed 95.9% resistance to third-generation cephalosporins and 58.4% of Staphylococcus aureus was MRSA. In pediatric populations, Southern India recorded high resistance rates for Acinetobacter baumannii and Pseudomonas aeruginosa, where 91% and 82% of isolates were resistant to carbapenems, respectively.10 The few available treatment options worsen the situation because drugs such as tetracyclines and fluoroquinolones are often not used in pediatrics because of safety reasons. Hospital-acquired infections (HAIs) are one of the significant concerns in neonatal and pediatric ICUs, and studies have shown high rates of MDROs in critically ill patients.
Blood stream infections, sepsis, and global impact
BSIs are the greatest global health issue, representing one of the primary causes of sepsis that remains fatal most of the time. In 2017 alone, there were 49 million episodes of sepsis worldwide that translated to 11 million deaths with 41% of them among children less than five years of age It is severe in India with approximately 11 million sepsis cases and 3 million deaths every year. Sepsis, an extreme overreaction of the body to infection, severely impacts the neonate and children population. It makes the treatment very difficult as multidrug-resistant organisms continue to grow and escalate the health costs. The surveillance systems, such as the Indian Council of Medical Research’s AMR Surveillance Network (IAMRSN): offer crucial information on AMR, thus facilitating the formulation of localized, data-driven treatment protocols.11 Such initiatives call for both global and localized strategies to address BSIs and reduce their catastrophic effects. Neonatal and Pediatric Sepsis: Diagnostic and Treatment Challenges.
Neonatal and pediatric sepsis has remained one of the major causes of morbidity and mortality, especially within low-resource economies.12 Neonatal sepsis, manifesting as conditions including septicemia and pneumonia is divided into two categories: early-onset or late-onset sepsis. Even though blood cultures provide the diagnostic benchmark, low-positive rates and resistance to MDROs have emerged as obstacles against effective treatment. Empirical antibiotic use, inevitably resulting from delays in diagnosis, is often the driver of accelerated AMR, and institution-specific guidelines that focus on microbial prevalence and associated patterns of susceptibility are therefore critical considerations. Pediatric sepsis similarly causes a million annual deaths, with Sub-Saharan African areas reporting higher mortality associated with restricted access to healthcare and infectious disease burdens. Strengthened diagnostic, treatment, and AMR surveillance frameworks are critical to reducing the incidence of sepsis and improving outcomes, especially in weak populations with inadequate healthcare infrastructure.
Diarrheal diseases and paediatric mortality
Diarrheal infections also form another major killer of children, especially those less than five years old. Diarrheal infections kill approximately 500,000 children per year, with an estimated 2 billion cases worldwide. Among the microorganisms that are most often responsible for these illnesses are Vibrio cholerae, Shigella spp., Enterotoxigenic E. coli, and Salmonella spp.13 In Bangladesh, for example, improper monitoring systems and wrong diagnoses have led to overuse of antibiotics in the treatment of diarrheal diseases, thereby increasing the problem of AMR. Only 5% of cases are correctly identified, thus the need for better diagnostic methods and more appropriate antibiotic usage to address diarrheal illnesses and the rising threat of resistance.
Common infections in children: viral, bacterial, and parasitic threats
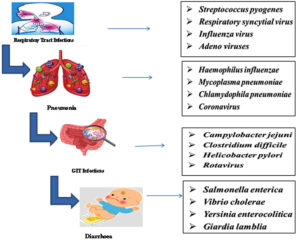
Bacterial, viral, and parasitic pathogens are the significant causes of infections in children as they can result in severe disease in children who have developing or compromised immune systems. Bacterial gastroenteritis by Escherichia coli, and respiratory infection by Streptococcus pyogenes are considered to be very common causes in children. On the other hand, foodborne pathogens like Salmonella and Shigella cause diseases in the intestines, majorly in immunocompromised children. Giardia and Cryptosporidium are some of the common parasitic infections, which are found in immunocompromised children, associated with malnutrition, contributing to developmental delay problems.14 Viral pathogens like Respiratory Syncytial Virus and Influenza Virus are among the causes of infection associated with respiratory disease, used to exemplify immunization utilization in reducing the burden of infectious diseases.15 It has greatly reduced infections caused by Streptococcus pneumoniae, such as pneumonia and meningitis, while others like methicillin-resistant Staphylococcus aureus show that antibiotic resistance is increasing. Others include Haemophilus influenzae type b, Klebsiella pneumoniae, and Staphylococcus aureus, which cause the more severe form of infections like pneumonia, epiglottitis, and skin diseases. Viral pathogens include adenovirus and parainfluenza viruses; these pathogens also contribute to respiratory and gastrointestinal illnesses, while fungal pathogens such as Pneumocystis jirovecii remain a threat to HIV-infected infants. Besides that, parasitic diseases such as prenatal infection of Toxoplasma gondii and helminthic diseases, especially in a poor sanitary condition, add further pressure on pediatric healthcare systems, highlighting the need for holistic prevention, diagnosis, and treatment to mitigate this multidimensional burden.16
Again, as with viral infections such as the novel coronavirus COVID-19, infections such as CNS complications can lead to disastrous neurological damage, which may have long-term consequences for developmental failures.17 Other viral pathogens that cause severe gastroenteritis, include rotavirus and norovirus, leading to significant morbidity among children under five years of age, often due to dehydration, which requires hospitalization. Chronic conditions like congenital heart defects and diabetes make infection management even more challenging and increase the likelihood of secondary complications. The pandemic revealed the weaknesses of the susceptible pediatric population, primarily children, who were hospitalized more frequently due to severe virally complicated cases. It also prompted the need for comprehensive strategies to lower childhood morbidity and mortality17(Figure 1).
Respiratory infections in pediatric patients
Common and prevalent health disorders in children range from mild and self-limiting conditions like a common cold, to severe illness such as pneumonia. Common cold is usually produced by rhinoviruses, and has symptoms that often include runny or stuffy nose, sneezing, coughing, sore throat, mild fever, reaches its peak between 3-6 days, and resolves in about 10-14 days mainly with rest and hydration. Sinusitis, which may be a complication, presents with facial pain, nasal congestion, cough, and postnasal drip. Most cases resolve within four weeks, but bacterial sinusitis may need antibiotic therapy in addition to decongestants and saline nasal rinses. In children, early diagnosis is important because delay in treatment may lead to complications. Pneumonia, whether viral or bacterial, is especially concerning in pediatric populations because of its potential severity. The symptoms include high fever, severe cough, and difficulty breathing. Early intervention is crucial, with bacterial pneumonia treated with antibiotics and viral cases managed through supportive care.18
Prevalence of Gram-negative Pathogens and Resistance Trends
Significantly, ESBL-producing Enterobacteriaceae and CRE are on the rise among children and pose significant challenges to the patient’s treatment guidelines. Other serious issues in a hospital environment are MRSA and VRSA.19 Actually, it is the best available public health microbiology knowledge that accounts for genetic determinants like horizontal gene transfer, efflux pumps, and target modifications that provided the ultimate antibiotic-resistance characteristic of bacteria to evade antibiotics.20 Misuse of antibiotics is further compounded by the limited availability of individual pediatric dosing and efficacy information. This and other factors highlight the pressing need to enhance antibiotic stewardship programs to ensure prudent use and forestall the development of resistance. If left unchecked in a timely manner, it can take us to the post-antibiotic era, where those infections that could be cured earlier can kill once more.
A recent research was done on 422 culture sensitivity reports analysis. It stated that Escherichia coli was the common Gram-negative pathogens, representing a total of 16.1% of isolates, after Acinetobacter, Klebsiella, and Pseudomonas; all of these organisms were sensitive to polymyxin B and colistin which are traditionally given as last-resort antibiotics due to multidrug-resistant infections. Ongoing surveillance of resistance patterns is important since these can affect the sustained use of widely available therapy for some Gram-negative infections. This necessitates the updating of antibiograms that assist clinicians in choosing the appropriate empiric antibiotics based on developing regional patterns of resistance. Such an active approach not only results in better patient outcomes but also adds a lot of value to global efforts to cut down on the transmission of resistant bacteria that add to the efforts against AMR.3 This implies that the use of antibiotics should become more judicious since the increasing issue of antimicrobial resistance necessitates judicious use, thereby highlighting the key role ASPs play in the objective of maximizing the utilization of antibiotics.
ASPs will thus enhance patient outcomes as well as prevent development and transmission of resistance, leading to reduced healthcare expenditures. These efforts thus advocate for the prescription of narrow-spectrum antibiotics, minimizing unnecessary prescribing, and assisting in resistance combat as well as enhancing quality of care. Nonetheless, an increase in resistance of Gram-negative bacteria, including ESBL-producing organisms, entails the need to create rapid diagnostics and timely and focused therapies for enhancing the ASPs and reversing the clinical dilemmas of AMR.21
Although ASPs have been exceedingly successful in adult populations, there has only now been a focus on pediatric-specific programs. Pediatrics are particularly problematic due to issues of pharmacokinetics, the safety profiles of drugs, and unusual presentations of disease, all of which create the need for individualized treatment and specialized treatment in pediatric ASPs. To make things worse, the late provision of effective treatment for recalcitrant infection in children serves to underscore the importance of universal systems that could address pediatric complexities. Herein, these programs must incorporate drug selection that is appropriate to age and disease control methods to produce maximum benefit without resistance among child patients.
These risks are ongoing with the AMR and have led to studies in pursuit of newer solutions such as AI forecasting of resistance patterns to inform the choice of treatments. Clinicians would be able to determine suitable alternatives, decrease the utilization of inappropriate broad-spectrum antibiotics, and increase the diagnostic precision of disease with AI-enabled tools.22 Such technologies, when blended with ASPs, can revolutionize the war against AMR by equipping healthcare providers with data-driven evidence. However, AI must be further tuned and adopted in standard clinical practice to fight developing trends of resistance and offer sustainable interventions for this international health emergency (Figure 2).
Impact of ABR on pediatric health outcomes
Resistance development is based on the mechanism by which bacteria adapt to antibiotics to evade treatment, thus causing infections that are no longer treatable. Overuse and misuse of antibiotics in human and veterinary medicine further contribute to this crisis by exerting selection pressure favouring the appearance and spread of resistant strains. Gram-negative bacteria, especially the ones that are ESBL producers,23 are a huge threat because of their intrinsic resistance to many drugs and their potential to break down most drugs, including penicillin and cephalosporins, commonly used for infections. Misuse of antibiotics in agriculture and human medicine and environmental exposure to these antibiotics increase the problems associated with this bacterium and raise the hospital-acquired infection mortality rates.24 This not only prolongs hospital stays but also increases healthcare costs and creates challenges in managing resistant infections.
The trend of ABR in pediatric populations is more worrisome since children are at higher risk because of their underdeveloped immunity and the increased need for antibiotics. The studies revealed that Staphylococcus aureus was found to be the most common (28.4%): followed by Escherichia coli, at 18.2%. In clinical practice, there was a very disturbing resistance pattern where 93% were resistant to ampicillin. The Gram-negative bacteria MDR E. coli and Klebsiella pneumoniae are more resistant to third-generation cephalosporins, whereas MRSA remains a prominent pathogen in pediatric wound infections and bacteremia. Overuse of antibiotics for viral infections along with poor compliance with pediatric guidelines leads to rising resistance trends in developing regions. AMR threatens the entire S. aureus as well as for other more commonly encountered pathogens, such as E. coli and K. pneumoniae, where clinical outcomes would be worse, hospital stays longer, and mortality greater, especially among the most susceptible populations, like children. The resistance rates, driven by geographic disparities and determined by local antibiotic prescribing practices and the healthcare infrastructure in Sub-Saharan Africa, thus need targeted interventions. In this respect, the most critical actions would be implementing targeted antimicrobial stewardship programs and continuous monitoring of resistance profiles for effective treatment options.25 Strengthening infection control measures and global antibiotic stewardship initiatives are important to mitigate the effects of ABR and protect vulnerable pediatric populations.
Factors contributing to the rise of AMR
A pressing concern among the global health-related concerns of late has emerged: antimicrobial resistance. In this regard, many researchers have pointed out that various factors are connected to its spread and subsequent effect on treatment processes of diseases. Antibiotic overuse, the productivity fall in pharmaceutical corporations related to generating new antibiotics are reported. They state that the appearance of gram-negative bacterial infections is a repeat of the pre-antibiotic era threatening to undermine the new medical therapies.26 Drugs called “salvage” therapies, colistin and tigecycline and others have already shown emergence of resistance, thereby highlighting an urgent requirement for pragmatic solutions to face this growing menace. The scarcity of viable antibiotics against resistant organisms underlines the need for renewed emphasis on antibiotic stewardship and development of new treatments.
A 2012 epidemiological investigation by showed that there is a clear link between delayed access to effective antibiotic therapies and higher fatality rates in infection patients.27 Regarding the study on patient care, there is a major underlying need-providing broad-spectrum empirical antibiotic treatment to the sick patients at an early time of infection so as to minimize the risk of mortality. The findings on this basis are such that it has been learnt that MDR gram-negative bacterial infections mostly develop among the immunocompetent persons who are admitted to the hospitals for critical care or those who have recently undergone broad-spectrum antibiotics. This is worsened by the lack of drugs that are efficient in the treatment of such conditions, as many drugs initially used have failed to protect against new strains that might be resistant.28
Implications of shifts in clonal complexes
The WHO has listed AMR among the main global health risks, notably ESKAPE pathogens including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These pathogens cause a huge quantity of human diseases, and epidemiological data exhibit a critical deficiency in high classes of antibiotics which remain effective against resistant gram-negative bacteria. Environmental factors too contribute to the spread of resistance gene, as hospitals and also communal settings function as reservoirs of resistant infections. Horizontal gene transfer (HGT) is significant in the development of antibiotic resistance among environmental bacteria because it facilitates the spread of resistance characteristics through processes such as coexistence and evolution. The interconnectivity of human health and the environment brings to the fore the necessity of a multidisciplinary approach to the AMR challenge.29
Molecular mechanisms of resistance
Bacterial resistance in juvenile patients is caused by different processes, which come in two types: intrinsic or acquired. Intrinsic resistance is that resistance found in Gram-negative bacteria, where it bases itself on structural defenses like the outer membrane. Acquired resistance is based on genetic mutations that enable the bacteria to degrade the antibiotics enzymatically, modify the target site (for example, modified PBPs): or use efflux pumps, such as Pseudomonas aeruginosa and Escherichia coli. Children are more susceptible because of the immature state of their immune system, increased hospitalizations, and anomalies in pharmacokinetics. Other such emerging diseases include MRSA, CRE, and others with colistin-resistant forms. Therefore, for the effective management of resistant infections in children, proper surveillance and management through antibiotic stewardship with continuing research for new medicines are important30 (Table).
Table:
Pathogens and its Resistance
| Grams stain | Organism | Drug-resistance | Reason for Resistance | References |
|---|---|---|---|---|
| Gram-positive | Staphylococcus aureus | Methicillin-resistant (MRSA) | Mutation of the penicillin-binding proteins (PBPs) | Abebe et al.33 |
| Streptococcus pneumoniae | Penicillin resistance | Alteration of PBPs and efflux pumps | Zahari et al.35 | |
| Enterococcus faecium | Vancomycin-resistant (VRE) | Acquisition of resistance genes (e.g., vanA) via plasmids or transposons | Li et al.19 | |
| Gram-negative | Escherichia coli | Extended-spectrum beta- lactamases (ESBLs) | Production of enzymes that hydrolyze extended-spectrum cephalosporins | Nagshetty et al.26 |
| Klebsiella pneumoniae | Carbapenem-resistant Enterobacteriaceae (CRE) | Production of carbapenemase enzymes, loss of outer membrane porins | Gogoi et al.36 | |
| Pseudomonas aeruginosa | Multidrug-resistance (MDR) | Efflux pumps, reduced permeability, and target modification | Lorusso et al.20 | |
| Neisseria meningitidis | Penicillin resistance | Alteration of PBPs and acquisition of resistance genes | Deghmane et al.23 |
Genetic determinants of antimicrobial resistance
The genetics of AMR is essential in establishing how bacteria thwart treatment. New research findings have shown that transposon insertional mutagenesis enhances carbapenem resistance in Klebsiella pneumoniae. The genetic function of this effect not only enables resistance against the carbapenems already established but also to the carbapenem-resistant ones newly emerging. Mutations in resistance genes, in this case, also cross-react against the enzyme activity on all carbapenems, thus complicating therapeutic strategy. Furthermore, the lack of CRISPR-Cas systems, which would otherwise restrain horizontal gene transfer, hastens the development of resistance genes, thereby increasing the difficulty of treating infections. Analogously, mutations in the Cutibacterium acnes gyrA gene facilitate fluoroquinolone resistance, whereas efflux-related genomic sequences give MLSB antibiotic resistance, which illustrates how genetic changes increase the survival chances of bacteria in antibiotic environments.31
Besides, the genetic evolution of Clostridioides difficile has resulted in an explosion of resistance mutations after 2000, particularly against primary therapies such as vancomycin and metronidazole. Although resistance genes in C. difficile are not prevalent, their growing prevalence is an indicator of a gigantic shift in the gene makeup of the pathogen, and thus there is a need for continuous monitoring and adaptive treatment strategies. Major determinants of resistance in Mycobacterium tuberculosis are mutations in genes whose activity is inhibited by first-line anti-tuberculosis drugs like isoniazid and ethambutol. It also complicates the treatment through changes in the coding sequences and also promoter regions that have drug efficacy implications. These studies collectively indicate the very complex interaction of AMR and genetic evolution, underlining the need for continued research and monitoring to stay ahead of bacterial evolutions.32
Horizontal gene transfer and its implications
Horizontal gene transfer (HGT) is one of the mechanisms of DNA acquisition and spread by bacteria with far-reaching implications in healthcare, especially in children hospitalized in hospitals. It encompasses transformation-the uptake of free DNA- and conjugation, or direct DNA transfer between bacteria via plasmids-transduction through bacteriophage. Such mechanisms enable quick horizontal spreading of antibiotic resistance and virulence factors, thereby complicating infection control within pediatric hospitals. The studies have shown that, in the hospital setting, MDROs usually transfer their MGEs across species, thus participating in antibiotic resistance and discarding standard treatment. Beyond mere resistance, HGT has the basis of spreading virulence factors, such as toxin-encoding genes, to increase the pathogenic potential of bacteria such as E. coli and Staphylococcus aureus.33 This might manifest in greater morbidity and a more vigorous treatment course in children. In addition, HGT alters the gut microbiome by means of bacteriophages, transferring resistance genes among gut bacteria, thus influencing both gastrointestinal and systemic health. The above effects require the reduction of such phenomena by increasing genomic surveillance in hospitals, the establishment of therapies aimed at MGEs, and the implementation of effective infection control practices. Such dynamics are critical for combating antibiotic resistance and safeguarding vulnerable pediatric patients.34
Emerging resistance mechanisms
The increasing trend in antimicrobial resistance in pediatric infections is a serious challenge worldwide. Inappropriate antibiotic use, predominantly in the hospital setting, accounts for most cases. Resistance mechanisms among pediatric pathogens, such as macrolide-resistant Streptococcus pneumoniae35 and carbapenem-resistant Enterobacteriaceae,36 are now increasingly making it difficult to treat, thereby resulting in higher morbidity and mortality in children. The underdeveloped immune system of children and their unique pharmacokinetic profile amplify the effects of multidrug-resistant infections. Effective antimicrobial stewardship programmes are critical to improving antibiotic use in children’s hospitals through monitoring prescription, peer benchmarking, continuous healthcare provider education, and the implementation of rapid diagnostic tests. The strategies reported on in research stress that the optimization of prescriptions via such interventions is critical to combating AMR, reducing avoidable prescriptions, and therefore decreasing healthcare costs. Ultimately, pediatric-specific data regarding resistance mechanisms as well as prescribing practices remain paramount for averting an approaching era of post-antibiotics when routine infections may quickly turn potentially lethal for young patients.37
Case studies highlighting emerging resistance
The hospital-acquired antibiotic resistance of children is a significant public health issue and one that has increased with much concern especially in the U.S., where multidrug-resistant Enterobacteriaceae infections have risen by 700% from 2007 through 2015, hospital stays are extended by nearly 20%. Most importantly, more than 75% of antibiotic-resistant infections are present on admission, making it a community problem rather than a nosocomial infection problem in the hospital. The most significant risk factor for prior antibiotic exposure, resistance rates for UTIs have reached 27% in children on prophylactic antibiotics compared to just 3% in those not on prophylaxis.
Bangladeshi research underlines the direst outcomes of antibiotic-resistant bacteremia in young children with pneumonia: the outcome leads to unacceptably high mortality rates in a resource-limited setting. The emergence of MRSA and carbapenem-resistant Enterobacteriaceae, with associated age- and healthcare-specific resistance patterns, mandate targeted antibiotic stewardship programs. However, an overuse and misuse of antibiotics, combined with a lack of pediatric-specific data and the complexity of children’s pharmacokinetics and immune systems, worsens the situation. Urgent measures are therefore necessary to counter this growing wave of antibiotic resistance.38
Barriers to effective antibiotic use in children
The Pediatric ASPs are very confronting barriers that complicate their effective implementation, especially in LMICs, where most often the infrastructural and healthcare system is strained. Diagnostic ambiguity in pediatric illnesses, wherein the symptoms of bacterial and viral infections are overlapped, leads to over prescription of antibiotics, particularly in emergency settings. This is worsened by the lack of clinical data specific to pediatrics because most treatment guidelines are adopted from adult studies, which may not be appropriate for children, particularly in specialized units such as PICUs and NICUs. In addition, the dearth of pediatric-trained infectious disease specialists and reliance on empirical therapy further worsen the problem. Economic and regulatory factors also play a role where low-cost antibiotics are abused in areas with poor medical regulation. LMICs lack quality drugs, and counterfeit drugs used in these regions reduce the efficacy of treatment and encourage resistance.
Nevertheless, as previously discussed, there is no age-specific pediatric formulation, which calls for a second-guessing of an adult dosage, thereby increasing the risk of resistance. An inappropriate use of antibiotics was attributed to poor healthcare infrastructure as patients were willing to spend long hours queuing for services, travel long distances, and lack money to seek healthcare in healthcare facilities but resort to pharmacies; hence, socio-economic factors such as gender inequality and poverty restrictions hinder treatment access. The issues are compounded by misconceptions surrounding antibiotics and high rates of self-medication, particularly in rural areas, worsen the situation.39 All these factors raise the imperative to develop context-sensitive strategies for enhanced stewardship of antibiotics and countering antimicrobial resistance in children.
Novel therapeutic approaches and alternative treatments
The pediatric ASP faces the most enormous challenges, mainly in LMICs, where conditions such as indeterminate diagnosis, infrastructural restraints, and scarce clinical evidence undermine the antibiotic stewardship. Distinguishing between bacterial and viral infections can be challenging among children, with antibiotic prescriptions remaining one of the biggest pressures in ERs.40 Moreover, pediatric guidelines are often borrowed from adult studies, thereby less relevant in specialized areas of pediatrics. The unavailability of infectious disease specialists trained in pediatrics, sole reliance on empirical therapy, and economic factors-the mass prescription of antibiotics that are inexpensive and available without regulatory supervision-worsen the scenario further.41
In LMICs, poor-quality medications are unavailable, and there is reliance on counterfeit drugs that cause ineffective treatments and resistance. The healthcare infrastructure problems are the long wait times and barriers in transportation. These cause individuals to look for antibiotics in pharmacies instead of the healthcare facilities, increasing misuse. Socioeconomic factors, including lack of finance and gender inequality, also impede access to proper care, while misconceptions and self-medication practices in rural areas contribute to resistance. These challenges combined point out the need for targeted, context-specific strategies that improve pediatric antibiotic stewardship and curb antimicrobial resistance.
Recommendations for future studies and interventions
Developing national surveillance programs, for instance, registries of AMR patterns in pediatric populations, such as the recent AGAR Kids Report in Australia, where bacteria resistant to several drugs caused 9.4% of bacteremia cases among children, makes it important for regionalized prescriptive recommendations of antimicrobials. Prescribers are also to be educated on effective antibiotic prescription habits and provided with regional recommendations obtained from local surveillance data. Rapid diagnostic technologies and molecular methods such as whole genome sequencing can further support the precise identification of infections and resistance mechanisms. Vulnerable pediatric populations, such as infants and immunocompromised children, will need special attention to their condition requiring bespoke interventions. There is, therefore, an urgent need to conduct research in novel treatments: new antibiotics, bacteriophage therapy, and immunomodulators, among others. There is a potential for public education on proper antibiotic use in children, combined with multicenter studies, which might help to shed light on AMR trends for national and international strategies. Stronger antibiotic prescribing guidelines, narrow-spectrum antibiotics, and stewardship programs must be implemented for the slowing of the spread of resistance, while building awareness among the healthcare providers would help in minimizing the overuse of antibiotics.42
Antimicrobial resistance in children is a rising international crisis, where drug-resistant microorganisms like MRSA, VRE, CRE, Escherichia coli, and Klebsiella pneumoniae are jeopardizing cure and increasing health risks, particularly in intensive care units. Despite progress in stewardship programs and monitoring, major challenges persist, including the lack of pediatric-specific clinical studies, failing to meet diagnostic capacity in low-resource environments, insufficient public health integration of resistance information, and inconsistent implementation of child-entered stewardship strategies. These loopholes reflect the pressing need to transition away from broad measures and, instead, construct focused solutions through narrowly tailored solutions that directly address children’s focused vulnerabilities’ uniformly coordinated, multi-faceted approach is needed to effectively fight pediatric AMR. Investment needs to particularly invest in pediatric-focused diagnostics, therapeutics, and clinical advice, with children being meaningfully involved in policy-making. Enrichment of health infrastructure, especially in needy areas, and the establishment of pediatric surveillance networks are necessary to develop region-specific treatment modalities. Ongoing education of the healthcare provider and awareness generation in the community will also check misuse of antibiotics. Long-term achievement will rest upon utilizing molecular technology to detect early resistance, fostering international collaboration in all aspects, and having in-depth knowledge about children’s specific medical needs. It is only by sustained, collective effort that we can ensure antimicrobials remain effective for generations to come.
ACKNOWLEDGMENTS
The authors are thankful to the faculties at the Department of Research, Meenakshi Academy of Higher Education and Research, Chennai, India, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
JPR performed assessment. YS, SSI and JPR wrote the initial draft. PB, YS, JPR, SSI and SSA reviewed the manuscript. SSI wrote the manuscript. PB, YS, JPR, SSI and SSA edited and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Romandini A, Pani A, Schenardi PA, Pattarino GA, De Giacomo GACP, Scaglione F. Antibiotic resistance in pediatric infections: global emerging threats, predicting the near future. Antibiotics. 2021; 10(4):393.
Crossref - Chanda W, Manyepa M, Chikwanda E, et al. Evaluation of antibiotic susceptibility patterns of pathogens isolated from routine laboratory specimens at Ndola Teaching Hospital, A retrospective study. Plos ONE. 2019;14(12):e0226676.
Crossref - Khalid N, Akbar Z, Mustafa N, Akbar J, Saeed S, SaleemZ. Trends in antimicrobial susceptibility patterns of bacterial isolates in Lahore, Pakistan. Front Antibiot. 2023; 2:21149408.
Crossref - Gajic I, Kabic J, Kekic D, et al. Antimicrobial susceptibility testing: a comprehensive review of currently used methods. Antibiotics. 2022; 11(4):427.
Crossref - Algammal AM, Mabrok M, Ezzat M, et al. Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture. 2022;548(Part 1):737643.
Crossref - Alenazi FS, Alzahrani KMS, Alhuwaiti MM, AlshahriAA. Antibiotic resistance in Saudi Arabia; review. International Journal of Medicine in Developing Countries. 2022;6(2):399-403.
Crossref - Abo YN, Freyne B, Kululanga D, Bryant PA. The impact of antimicrobial stewardship in children in low-and middle-income countries: a systematic review. Pediatr Infect Dis J. 2022; 41(3S):S10-S17.
Crossref - De Luna D, Sanchez JJ, Lopez M, et al. Antibiotic resistance profile in intrahospital pediatric services at third level centers in Dominican Republic. Infections. 2020; 24(2):66-70.
Crossref - Mogasale VV, Saldanha P, Pai V, Rekha PD, MogasaleV. A descriptive analysis of antimicrobial resistance patterns of WHO priority pathogens isolated in children from a tertiary care hospital in India. Sci Rep. 2021; 11(1):5116.
Crossref - Zhang C, Fu X, Liu Y, Zhao H, Wang G. Burden of infectious diseases and bacterial antimicrobial resistance in China: a systematic analysis for the global burden of disease study 2019. Lancet Reg Health West Pac. 2024; 43:100972.
Crossref - Karuna T, Gupta A, Vyas A, et al. Changing trends in antimicrobial susceptibility patterns of bloodstream infection (BSI) in secondary care hospitals of India. Cureus. 2023; 15(4):e37800.
Crossref - Sharif N, Parvez AK, Haque A, Talukder AA, Ushijima H, Dey SK. Molecular and epidemiological trends of human bocavirus and adenovirus in children with acute gastroenteritis in Bangladesh during 2015 to 2019. J Med Virol. 2020; 92(12):3194-3201.
Crossref - Zhu Y, Zhu X, Deng M, Wei H, Zhang M. Causes of death in hospitalized children younger than 12 years of age in a Chinese hospital: a 10 year study. BMC Pediatr. 2018;18(1):8.
Crossref - Stopyra L, Kowalik A, Stala J, et al. Characteristics of hospitalized pediatric patients in the first five waves of the COVID-19 pandemic in a single center in Poland-1407 cases. J Clin Med. 2022;11(22):6806.
Crossref - Deksne G, Krumins A, Mateusa M, et al. Occurrence of Cryptosporidium spp. and Giardia spp. infection in humans in Latvia: Evidence of underdiagnosed and underreported cases. Medicina. 2022;58(4):471.
Crossref - Ali-Alghamdi B, Al-Johani I, Al-Shamrani JM, et al. Antimicrobial resistance in methicillin- resistant Staphylococcus aureus. Saudi J Biol Sci. 2023; 30(4):103604.
Crossref - Chaudhry SA, Gad N, Koren G. Toxoplasmosis and pregnancy. Can Fam Physician. 2014;60(4):334-336.
- De Benedictis A, Lettieri E, Gastaldi L, Masella C, Urgu A, Tartaglini D. Electronic Medical Records implementation in hospital: An empirical investigation of individual and organizational determinants. PloS ONE. 2020;15(6):e0234108.
Crossref - Li G, Walker MJ, De Oliveira DM. Vancomycin resistance in Enterococcus and Staphylococcus aureus. Microorganisms. 2022; 11(1):24.
Crossref - Lorusso AB, Carrara JA, Barroso CD, Tuon FF, Faoro H. Role of efflux pumps on antimicrobial resistance in Pseudomonas aeruginosa. Int J Mol Sci. 2022; 23(24):15779.
Crossref - Soraas A, Olsen I, SundsȠord A, Handal T, Bjorang O, Jenum PA. Extended-spectrum beta-lactamase- producing bacteria are not detected in supragingival plaque samples from human fecal carriers of ESBL- producing Enterobacteriaceae. Journal of oral Microbiology, 2014;6(1):24026.
Crossref - Ventola CL. The antibiotic resistance crisis. Part 1:Causes and threats. Pharm Ther. 2015;40:277-283.
- Deghmane AE, Hong E, Taha MK. Recent evolution of susceptibility to beta-lactams in Neisseria meningitidis. Antibiotics. 2023;12(6):992.
Crossref - Emgוrd M, Mwangi R, Mayo C, et al. Antibiotic use in children under 5 years of age in Northern Tanzania: a qualitative study exploring the experiences of the caring mothers. Antimicrob Resist Infect Control. 2022;11(1):130.
Crossref - Kebbeh A, Dsane-Aidoo P, Sanyang K, et al. Antibiotics susceptibility patterns of uropathogenic bacteria: a cross-sectional analytic study at Kanifing General Hospital, The Gambia. BMC Infect Dis. 2023;23(1):723.
Crossref - Nagshetty K, Shilpa BM, Patil SA, Shivannavar CT, Manjula NG. An overview of extended spectrum beta lactamases and metallo beta lactamases. Adv Microbiol. 2021;11(01):37.
Crossref - Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Micro Revi. 2012;25:450-470.
Crossref - Sharma R, Hammerschlag MR. Treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in children: a reappraisal of vancomycin. Curr Infect Dis Rep. 2019;21(10):37.
Crossref - Cambaco O, Alonso Menendez Y, Kinsman J, et al, Community knowledge and practices regarding antibiotic use in rural Mozambique: where is the starting point for prevention of antibiotic resistance? BMC Public Health. 2020;20(1):1183.
Crossref - Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482-501.
Crossref - Beirne C, McCann E, McDowell A, Miliotis G. Genetic determinants of antimicrobial resistance in three multi-drug resistant strains of Cutibacterium acnes isolated from patients with acne: a predictive in silico study. Access Microbiol. 2022;4(8):000404.
Crossref - CRyPTIC Consortium, Carter JJ. Quantitative measurement of antibiotic resistance in Mycobacterium tuberculosis reveals genetic determinants of resistance and susceptibility in a target gene approach. bioRxiv. 2021:09.
- Abebe AA, Birhanu AG. Methicillin resistant Staphylococcus aureus: molecular mechanisms underlying drug resistance development and novel strategies to combat. Infect Drug Resist. 2021;16:7641-62.
Crossref - Evans DR, Griffith MP, Sundermann AJ, et al. Systematic detection of horizontal gene transfer across genera among multidrug-resistant bacteria in a single hospital. Elife. 2020;9:e53886.
Crossref - Zahari NI, Engku Abd Rahman EN, Irekeola AA, et al. A review of the resistance mechanisms for b-lactams, macrolides and fluoroquinolones among Streptococcus pneumoniae. Medicina. 2023;59(11):1927.
Crossref - Gogoi I, Puzari M, Chetia P. Porin-Mediated Carbapenem Resistance in Klebsiella pneumoniae: an Alarming Threat to Global Health. Curr Clin Microbiol Rep. 2023;10(4):255-265.
Crossref - Yu D, Zheng Y, Shen A, et al. Antimicrobial resistance in pediatric infectious diseases: antimicrobial resistance, resistance mechanisms and antimicrobial use. Front Cell Infect Microbiol. 2023;13:1287051.
Crossref - Sivasankar S, Goldman JL, Hoffman MA. Variation in antibiotic resistance patterns for children and adults treated at 166 non-affiliated US facilities using EHR data. JAC-Antimicrobial Resistance. 2023;5(1):dlac128.
Crossref - Probst V, Islamovic F, Mirza A. Antimicrobial stewardship program in pediatric medicine. Pediatr Investig. 2021;5(3):229-238.
Crossref - Branstetter JW, Barker L, Yarbrough A, Ross S, Stultz JS. Challenges of antibiotic stewardship in the pediatric and neonatal intensive care units. J Pediatr Pharmacol Ther. 2021;26(7):659-668.
Crossref - Fanelli U, Chine V, Pappalardo M, Gismondi P, EspositoS. Improving the quality of hospital antibiotic use: Impact on multidrug-resistant bacterial infections in children. Front Pharmacol. 2020;11:745.
Crossref - Lipworth S, Vihta KD, Davies T, et al. Molecular epidemiology and antimicrobial resistance phenotype of paediatric bloodstream infections caused by Gram- negative bacteria. Commun Med. 2022;2(1):101.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.