ISSN: 0973-7510
E-ISSN: 2581-690X
This study investigates the therapeutic potency of endophytic fungi, Schizophyllum commune (AVNK2) isolated from Nigella sativa seeds. As endophytes are promising sources of bioactive compounds that mimic the host plant, research in this area leads to novel drug discovery with minimal side effects. 8 Fungal endophytes (AVNK1-AVNK8) were isolated from N. sativa seeds with the potent endophyte (AVNK2) molecularly identified as Schizophyllum commune using 18S rRNA sequencing and phylogenetic analysis and submitted in the GenBank with the accession number. Antimicrobial screening against human pathogens (Candida albicans, Salmonella typhimurium, Pseudomonas aeruginosa, Staphylococcus aureus, and Aspergillus brasiliensis) was done using dual-culture and the agar-well diffusion methods. S. commune confirms its highly potent antimicrobial activity with a MIC value of 25 µg/ml and IC50 value of 2.05 millimolar (mM). The bioactivity of S. commune was enhanced using one-factor-at-a-time (OFAT) optimization of physio-chemical parameters of the culture medium. The optimal physicochemical parameters of culture media for enhancing bioactivity were glucose as carbon, beef extract as nitrogen sources, pH 7.0, and temperature 30 °C with 21 days of incubation. Characterisation of S. commune shows extracellular enzymes and potent bioactive secondary metabolites (flavonoids, terpenoids, phenols, steroids, tannins, and cardiac glycosides) responsible for its therapeutic efficacy. The anticancer efficacy of methanolic extract was evaluated using GC-MS analysis and molecular docking studies (Software-AutoDock Vina). In silico studies show binding affinity of -5.9 kcal/mol against colon cancer target protein (PDB ID 2HQ6) and -22.0 kcal/mol against tumour-homing peptide (PDB ID 7W8O), confirming S. commune as a promising source for anticancer treatment.
Secondary Metabolite, Molecular Docking, Human Pathogens, Phylogenetic Analysis, Metabolomic Profiling
Infectious diseases and cancer are global health issues. Increased relapsing of earlier known diseases and cumulative after effects of the existing non-natural and manmade chemical drugs urges the emergence and evolution of current drugs into safe, novel, and potent drugs with minimal side effects.1 In rural majority communities, people use traditional medicine of ethnomedical plant extracts due to their accessibility and affordability, as they serve as reservoirs of novel bioactive constituents.2,3 Nigella sativa, a nonindigenous ethnomedicinal plant, has been used for centuries for the treatment of dreadful diseases and wide-spreading microbial infections. Its odor, spicy taste, and medicinal properties make it a popular ingredient in cooking.4 Its bioactive compounds possess antibacterial, anticancer, and antioxidant properties by scavenging free radicals and preventing cell damage.5-7
Endophytes are endomutualistic microorganisms that colonize the intercellular or intracellular plant cells, produce phytohormones and bioactive metabolites to enhance the host’s growth, tolerate stress, and resist pathogens/pests/insects.8 They are of biotechnological interest to humankind as they are the treasure trove of therapeutically active constituents, which are of more importance in the field of biomedicine and of industrial use. The present study explores the bioactivity of S. commune isolated from N. sativa seeds. Schizophyllum commune, a basidiomycete, produces lignocellulolytic enzymes such as cellulases, pectinases, and laccases responsible for the degradation and decolorization of synthetic dyes,9 and can directly produce bioethanol using lignocellulosic biomass. S. commune is important in oil recovery, the food industry, and the pharmaceutical industry for its therapeutic efficacy and non-specific stimulator of the immune system.
In the recent decade, the most pressing issue faced by humankind is infectious diseases as they take less time to spread and more time to diagnose. Perhaps the COVID-19 contagion spread all over the world in a minimal time, by the outburst of coronavirus and taken out the lives of more than 600 million people all over the globe and stands as the greater example.10 The inappropriate frequent and extensive use of synthetic antibiotics compelled human pathogens to develop resistance to the existing man-made medicine. In Consequence, the hunt for natural antimicrobial substances with advanced novel structures and bioactivities and designing them into commercial therapeutic drugs has been elevated as a research hotspot in the field of biomedicine.11
GC-MS technique for investigating bioactive metabolomes of endophytes made the evaluation easy and accurate.12 It is considered the gold standard for understanding fungal endophyte mycochemical profiles, aiding in drug development through virtual screening and low-cost computational methods.
The present study focuses on the isolation of endophytic fungi from the seeds of Nigella sativa, evaluates the production of Bioactive metabolites, and optimizes the culture conditions of S. commune for enhancing bioactive metabolome production and analyses. The therapeutic potency in terms of antimicrobial and anticancer efficacy using GC-MS, in vitro screening, and in silico studies.
Isolation of endophytic fungi from Nigella sativa seeds
Healthy dried seeds of Nigella sativa are collected from the market in Saudi Arabia and preserved in the aseptic environment for the future purpose of research. 20 grams of the Nigella sativa seeds were measured and washed thoroughly under the tap water with consistent force to remove epiphytic filth and contaminants. Then the seeds were thoroughly surface sterilized with 20% hydrogen peroxide for 20 minutes, 95% ethanol for 5 minutes, 4% sodium hypochlorite for 3 minutes, 0.1% mercuric chloride solution for 1 minute, and 20% formaldehyde for 2 minutes. The surface sterilized seeds were rinsed multiple times with double-distilled water. An aliquot of the last rinse was used as a control to assess surface sterilization effectiveness. Then the sterile seeds were placed in the sterile mortar and pestle and macerated well into a fine paste and then added with 5 ml of double-distilled water, diluted for 10 serial dilutions, and 100 µl of each dilution was inoculated on Sabouraud’s media using the pour plate method. Triplicates of the inoculated plates were incubated for a period of 7-14 days at room temperature, and the emerging isolated endophytic fungal colonies were subcultured separately based on the morphological differences to obtain pure cultures.13
Molecular identification of AVNK2
The molecular level of identification of AVNK2 was done through PCR and 18S rRNA sequencing (Sanger dideoxy sequencing) using Location/Qualifiers source 1..1302 /organism=”Schizophyllum commune”/ mol_type=”genomic DNA” /isolate=”AVNK2″ /db_xref=”taxon:5334″ /geo_loc_name=”Saudi Arabia” /collection_date=”24-Mar-2023″ rRNA <1..>1302 /product=”small subunit ribosomal RNA” by Macrogen, a commercial company in Seoul, South Korea, followed by phylogenetic analysis using the neighbor-joining method, and the identified strain (AVNK2) was deposited at the NCBI gene bank in India.
Morphological characterisation of Schizophyllum commune
S. commune is examined microscopically using the slide culture technique. The slide culture technique is done using 2 × 2 cm of solidified agar block was transferred onto a sterile glass slide was inoculated with the actively growing S. commune in a sterile environment, covered up with a sterilized cover slip to allow the growth of the mycelium onto the coverslip, and it was enclosed within a sterile Petri plate.14 S. commune is incubated till the complete growth. Then the coverslip was stained with lactophenol cotton blue placed on a clean and sterile slide and examined under the microscope.15 Morphological characterization was done by observing the surface and texture of the colony, mycelium, position, and structure of the conidia, and spore morphology.
Preliminary screening of antibacterial activity using the dual-culture method
Spot inoculation of actively growing S. commune and the pathogenic fungus Aspergillus brasiliensis (NCIM1196) was done on the opposite ends of the Petri plate. The plate without endophytic fungi serves as the negative control for the growth of the pathogenic fungi.16 For a period of about 12-14 days, triplicates of the dual-culture plates were allowed to get incubated at ambient room temperature. The antagonistic activity was analyzed in terms of growth inhibition of pathogenic fungi when compared with that of negative control plates.17 The antimicrobial efficacy of the potent S. commune was further quantitatively analyzed using the agar well-diffusion assay or disk diffusion test.
Preparation of Inoculum for Antimicrobial Efficacy Using Agar Well-Diffusion Assay/Disc Diffusion Test
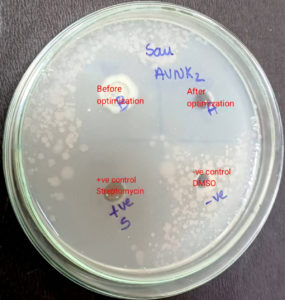
The antimicrobial potentiality of S. commune was examined against Staphylococcus aureus (NCIM2079), Clostridium sporogenes (NCIM5113) which were gram-positive bacteria, gram-negative bacteria (In this research we have used Salmonella typhimurium (NCIM2501), Pseudomonas aeruginosa (NCIM2200), and the pathogenic yeast Candida albicans (NCIM3471).18 The concentration of the Mueller-Hinton Broth in which pathogens were pre-cultured was made into 108 cells/mL by adjusting it to the turbidity/ concentration of a 0.5 McFarland standard solution.19 Three-day-old culture of S. commune was used as inoculum in the Sabouraud’s broth and incubated for 21 days. After the incubation period, the fully grown culture of S. commune was filtered with the help of Whatman paper, and the filtrate which was collected after centrifugation.20 The pellet was discarded, and the supernatant was used for antimicrobial activity. 1 ml of freshly grown pathogenic bacterial culture was added to Mueller-Hinton Agar medium, mixed well, and poured. Upon solidification, 6 mm wells were made using a cork borer which was sterilized by heating it to red hot, 100 µl of Inoculum, and 100 µl of standard streptomycin solution (the positive control) were added into the well with the empty well as a negative control.21 The bioactivity was evaluated according to the clearance zone or the zone of inhibition (ZOI) after 18-24 hours of incubation.22
Isolation of secondary metabolites and metabolic profiling of Schizophyllum commune
Schizophyllum commune was cultured in Sabouraud’s broth and incubated for an incubation period of 21 days. Then the broth was filtered using Whatman No. 1 filter paper and centrifuged and the pellet collected was discarded, and the supernatant was collected and used for metabolomic profiling.23 The primary metabolites of S. commune were analyzed by various enzymatic assays (amylase, cellulase, protease, laccase, and pectinase),24 while the bioactive constituents derived from the methanolic extract of endophytic fungi, S. commune, were evaluated qualitatively by different myco-chemical studies.25
Optimisation of Physical and chemical culture parameters and solvent selection for extraction of bioactive metabolomes from the Schizophyllum commune
Firstly, the growth curve of the endophytic fungus Schizophyllum commune was analyzed. The fungal inoculum was added into 300 ml of the Sabouraud’s broth and harvested at different points of the incubation period (7, 14, 21, 28, and 35 days); the wet weight and dry weight of S. commune were noted, and its antimicrobial activity against human pathogens was tested. The optimal incubation period was determined by the highest ZOI.
In the second study, different physical and chemical culture conditions like temperature, pH, carbon source, and nitrogen source were tested by inoculating S. commune in various ranges of physio-chemical parameters, respectively, and harvested after 21 days. The wet weight and dry weight of S. commune were noted, and its antimicrobial activity against human pathogens was tested. The culture with the highest ZOI is the optimal culture condition for enhanced antimicrobial metabolite production.
In the third study, secondary metabolites were extracted with different solvents and tested for their antimicrobial activity after incubating in the above-optimized conditions.26,27 The highest ZOI confirms the most suitable solvent for the extraction of bioactive metabolites. Further, optimization efficiency was checked by comparing the antimicrobial activity of the media before and after optimization.28
Determination of antimicrobial susceptibility or Minimum Inhibitory Concentrations (MIC)
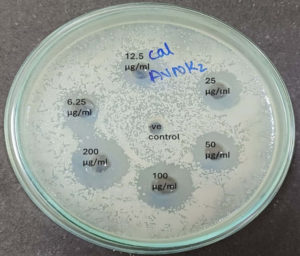
The Antimicrobial susceptibility is usually evaluated by analyzing the minimum inhibitory concentration of S. commune crude extract against human pathogens. The minimum inhibitory concentration of S. commune crude extract can be determined by any of the standard and reliable techniques, i.e. Broth Micro dilution method/Agar dilution methods.29 Agar dilution method offers advantages such as the ability to test multiple concentrations of the drug simultaneously and reduce the risk of drug precipitation, exposure to pathogens resulting in more biosafety. In our study, Agar diffusion method is done with minor modifications by the addition of doubling concentrations of extract (6.25 µg/ml, 12.5 µg/ml, 25 µg/ml, 50 µg/ml, 100 µg/ml, and 200 µg/ml) into the wells of agar plate supplemented with known concentration of pathogens (adjusted using McFarland’s standard suspension).14 The lowest concentration required for the inhibition of the visible growth of pathogens gives the MIC of S. commune crude extract.30
GC-MS evaluation of extracts obtained from S. commune and Nigella sativa seeds
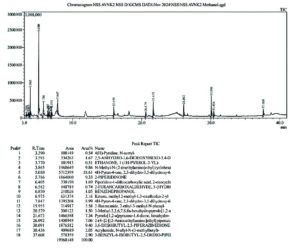
GC-MS technique and the analysis of its report were done to assess the Mycoconstituent profile of ethyl acetate extracts from Schizophyllum commune and Nigella sativa seeds. By comparing the retention duration, mass peak patterns, and spectrum acquired concerning the (NIST) database and also from the Wiley Registry of Mass Spectral Data, New York,31 the therapeutically active constituents within the extracts were analyzed. Ethyl acetate crude extracts of the endophytic fungi, Schizophyllum commune, and Nigella sativa seeds were compared and assessed to confirm that the endophyte was isolated from the host plant and to verify that the common bioactive compounds were present due to the horizontal transfer of therapeutic genes. The methanolic extract of S. commune was subsequently examined for the wide variety of bioactive components using GC-MS analysis as it had the largest zone of inhibition and the lowest MIC value thereby confirming the high range of Antimicrobial susceptibility 32
Molecular docking investigations
Using in silico studies, key elements of the methanolic extract’s mycochemical profile were chosen and their potential as an anticancer agent was examined. The main bioactive secondary metabolites of S. commune were docked as ligands into the target proteins’ receptor’s active site using AutoDock Vina.33 By downloading the PDB ID from the protein data bank34,35 the metabolites’ SMILE ID from PubChem was matched with the target proteins’ residual site. The stick model representation of the ligands includes interacting residues33 and variously colored H-bonds, which were utilized as input data for the Seam Dock application using AutoDock Vina as a software within it to perform the docking stimulation.33,36,37 The grid box for the docking simulations was constructed using a graphical user interface tool, and the best-docked conformation between the ligand and the target receptor protein with the most advantageous least free binding energy was selected using the docking algorithm.38
Isolation of fungal endophytes from Nigella sativa seeds
A total of 8 fungal endophytes (AVNK1-AVNK8) were isolated from the N. sativa seeds and subcultured into pure cultures. AVNK2 was chosen for further study due to its bioactive potential.
Molecular identification of AVNK2
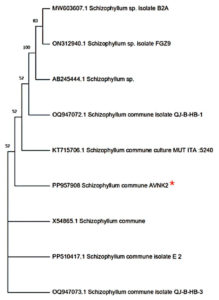
The 18S rRNA gene sequence from AVNK2 was amplified by PCR technique and Sanger dideoxy sequencing. The blast sequence of the AVNK2 strain in ex-taxon was subjected to the pairwise sequence similarity search and showed that the 18S rRNA gene sequence from AVNK2 has shown 100% similarity with the partial 18S rRNA sequence of Schizophyllum species. Phylogenetic studies based on 18S rRNA partial gene sequence revealed that AVNK2 is closely related to Schizophyllum commune and illustrated using the dendrogram in Figure 1 and deposited in NCBI as Schizophyllum commune with Gene Bank accession number PP957908.
Figure 1. Dendrogram constructed by neighbor-joining method showing that AVNK2 has 100% sequence similarity with Schizophyllum commune (PP957908)
Morphological characteristics of Schizophyllum commune
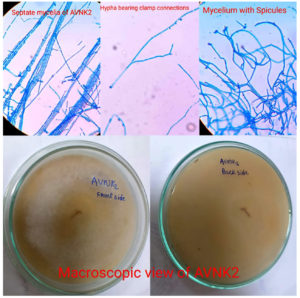
Microscopically, the lactophenol cotton blue mount of S. commune revealed hyaline, septate, non-dichotomously branched hyphae with clamp connections and spicules, as well as an aerial, spreading cottony, whitish mass growing linearly and apically on Sabouraud’s agar medium. The morphological characteristics are illustrated in Figure 2.
Preliminary screening of antimicrobial activity using the dual culture method
Figure 3 illustrates the antimicrobial activity of S. commune, which was confirmed by comparing the growth radius of pathogenic fungi in a control plate (pathogenic fungi alone) with that of endophytic fungi in a dual culture plate (Pathogenic fungi along with Endophytic Fungi, S. commune). After 21 days, S. commune showed high growth inhibition on Aspergillus brasiliensis, confirming its antimicrobial activity.
The radius of Pathogenic fungi alone in the control plate = 80 mm
The radius of Pathogenic fungi along with S. commune in Dual culture plate = 0.4 mm
Radius of growth inhibition (RGI)
Radius of pathogenic fungal colony without S. commune (in mm) – Radius of pathogenic fungal colony with S. commune (in mm).39
RGI of S. commune = 80-0.4 = 49.6 mm
% of Growth inhibition (GI) of S. commune = (RGI / Radius of Fungal Colony without S. commune) × 100
% of GI of S. commune = 49.6/50 × 100 = 0.992 × 100 = 99.2%
The percentage of Growth inhibition of pathogenic fungi by S. commune is 99.2%. The Schizophyllum commune is isolated from the seeds of Nigella sativa exhibited the highest growth inhibition of 99.2%, which is greater than the Growth Inhibition of S. commune isolated from the soil exhibited 86.47% growth Inhibition as reported by Ricky B. Acantlo.
Antimicrobial efficacy of S. commune using Agar well–diffusion assay or disc diffusion method
The antimicrobial efficacy of S. commune against various human pathogens was determined in terms of Zone of Inhibition (clearance zone in mm) using the Agar Well-Diffusion assay or disc diffusion method, as shown in Table 1. According to the American Society of Microbiology, the zone of inhibition <14 mm shows resistance, >15-18 mm shows intermediate, and >19 mm shows Susceptibility to the pathogenic bacteria. Table 1 illustrates that S. commune extracts exhibited considerable antimicrobial efficacy against a few Gram-positive bacteria that were utilized for this study (Staphylococcus aureus and Clostridium sporogenes) than the Gram-negative bacteria (Salmonella Typhimurium and Pseudomonas aeruginosa). Gram-positive bacteria have a less complex structure of the rigid peptidoglycan cell wall and so are more likely to be more sensitive to antimicrobial agents than Gram-negative bacteria probably due to the inhibition of cell wall synthesis.40 As illustrated in Table 1, Endophytic fungi, Schizophyllum commune shows significant antimicrobial activity against pathogenic bacteria with the highest clearance zone of 48 mm which is comparatively greater than the clearance zone of 11 mm by S. commune reported by Mirfat et al., against Staphylococcus aureus.41
Table (1):
Antimicrobial activity of S. commune against Human Pathogens
| Antimicrobial activity of S. commune done using the Agar Well Diffusion method | ||
|---|---|---|
| No. | Human Pathogen | Zone of Inhibition (in mm) |
| 1 | Pseudomonas aeruginosa | 18 |
| 2 | Salmonella typhimurium | 18 |
| 3 | Staphylococcus aureus | 48 |
| 4 | Candida albicans | 24 |
| 5 | Clostridium sporogenes | 42 |
Zone of Inhibition (mm) <14 mm (Resistant); >15-18 mm (Intermediate); >19 mm (Susceptible)
Physiological characterization of S. commune
Table 2 illustrates the physiological characterization confirming the production of extracellular enzymes and the bioactive constituents contributing to their establishment inside the host and their therapeutic efficacy. Table 2 illustrates the presence of extracellular enzymes like cellulases, and pectinases which help the endophyte to enter the cell by disrupting the plant cell wall and colonizing inside the host cell by counteracting its chemical defenses. Amylases produced by S. commune are useful for the breakdown of the starch content within the host plant and thereby indirectly involved in the metabolism of production of different potent bioactive metabolites.42 Table 2 also shows the presence of proteases and laccases which help in the biodegradation of pollutants, wastewater treatment, and Biofuel production used in the Pharmaceutical, cosmetic, detergent, paper, and leather industries apart from their ecological applications.43 The mycochemical profile (Table 2) illustrates that S. commune has a higher amount of Phenols, terpenoids, sterols, and cardiac glycosides, followed by tannins and saponins, followed by Flavonoids. Phenols have been in the spotlight in recent times due to their antitumor and antioxidant activities, terpenoids have a wider range of biological activity in the pharma industry ranging from Antiinflammatory, and anticancer to antimicrobial activity, certain Tannins were reported to have antiviral activity and can even inhibit HIV replication.
Table (2):
Metabolomic profiling of S. commune
| Primary/Secondary Metabolites | Result | |
|---|---|---|
| Extracellular Enzymatic Assays Cellulase | Amylase | +++ |
| Laccase | ++ | |
| Cellulase | +++ | |
| Protease | ++ | |
| Pectinase | +++ | |
| Myco-chemical Screening | Phenols | +++ |
| Flavonoids | + | |
| Terpenoids | +++ | |
| Sterols | +++ | |
| Tannins | ++ | |
| Saponins | ++ | |
| Cardiac Glycosides | +++ |
+++ (Indicates) Maximal Presence; ++ (Indicates) Moderate Presence; + Presence
Optimization of the physical and chemical culture parameters for the growth and yield of bioactive compounds from S. commune
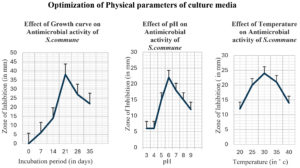
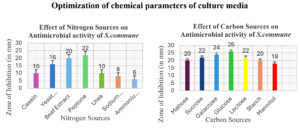
The optimal culture conditions for enhanced production of antimicrobial compounds from S. commune were Sabouraud’s media with glucose (40 g/litre) as carbon and peptone (10 g/litre) as nitrogen sources, 30 °C in the case of Temperature, 6.0 as optimal pH, and 21 days incubation period. Figure 4 and Figure 5 illustrate the effect of different physical and chemical culture parameters on the production of bioactive secondary metabolites.
Figure 4. Effect of Optimization of Culture Conditions (Physical parameters) on Antimicrobial activity of S. commune
Figure 5. Effect of Optimization of culture conditions (chemical parameters) on Antimicrobial activity of S. commune
The methanolic extract and the ethyl acetate extract of S. commune were proven to extract the major proportion of bioactive compounds possessing antimicrobial activity compared to the aqueous, chloroform, and hexane extract. The efficiency of different solvents on the extraction of secondary metabolites and enhancement of the antimicrobial activity of S. commune was illustrated in Figure 6. As the methanolic extract and the ethyl acetate extract of S. commune show the highest antimicrobial activity, they were selected for further studies analyzed by GC-MS analysis and evaluated by Docking.44,45
The ZOI of S. commune after optimization is greater than that of the prior one, demonstrating the enhancement of the antimicrobial activity of the bioactive compounds of S. commune after utilizing the optimal conditions is clearly illustrated in Figure 7.
Antimicrobial susceptibility or Minimum Inhibitory Concentration (MIC) and IC50 value of S. commune
As shown in Figure 8, the MIC of S. commune crude extract is the concentration at which no pathogen growth takes place in the medium.46 The half-maximal inhibitory concentration (IC50 value) was determined using a sigmoid (logistic) dose-responsive curve where ZOI is plotted against the Concentration of S. commune extract (plotting the linear relation between the inhibitory probability and the concentration logarithm), which indicates the amount of drug required to inhibit the growth by half (50%), as illustrated in Table 3. A non-linear regression analysis equation is used to calculate the IC50 value by using the data of ZOI of antimicrobial susceptibility (MIC) activity. According to the American Society of Microbiology, the zone of inhibition <14 mm shows resistance, Though 6.25 µg/ml and 12.5 µg/ml show ZOI of 10 mm and 14 mm the pathogen may develop resistance towards the extract and may not be effectively controlled. The MIC is 25 µg/ml, while the half-maximal inhibitory concentration (IC50) is 2.05 mM, serving as a benchmark for the antimicrobial efficacy of bioactive secondary metabolites.
Table (3):
IC50 value of S. commune extract against Candida albicans
| Half-maximal inhibitory concentration of S. commune | |||
|---|---|---|---|
| No. | Concen. of Methanolic Extract of S. commune (in µg/ml) | Zone of Inhibition (in mm) | Half-maximal concen. (IC50 value) |
| 1 | 00 | 00 | 2.05 mM |
| 2 | 6.25 | 10 | |
| 3 | 12.5 | 14 | |
| 4 | 25 | 18 | |
| 5 | 50 | 20 | |
| 6 | 100 | 22 | |
| 7 | 200 | 24 | |
GC-MS evaluation of extracts of S. commune and Nigella sativa seeds
The GC-MS examination of S. commune and Nigella sativa ethyl acetate extracts were analysed and the highest components of the metabolomic profile of extracts were reported in Table 4 illustrating the common Bioactive constituents. The present study confirms the horizontal transfer of the bioactive genes to the endophytic fungi from the host plant Nigella sativa. Sharing of the therapeutically active compounds in their metabolomic profile of the Nigella sativa and Schizophyllum commune was first reported in this study.
Table (4):
GC-MS analysis of S. commune and Nigella sativa seed extracts
No. |
Area % |
Bioactive Metabolite in Nigella sativa ethyl acetate extract |
Area % |
Bioactive Metabolite in S. commune ethyl acetate extract |
|---|---|---|---|---|
1 |
39.68 |
(Z)-18-Octadec-9-enolide |
23.72 |
9,12-Octadecadienoic acid (Z,Z)- |
2 |
16.89 |
Linoleic acid ethyl ester |
14.39 |
Linoleic acid ethyl ester |
3 |
9.49 |
Ethyl Oleate |
12.53 |
Oleic Acid |
4 |
6.23 |
9,12-Octadecadienoic acid (Z,Z)-, methyl ester |
9.92 |
Ethyl Oleate |
5 |
4.38 |
n-Hexadecanoic acid |
7.48 |
n-Hexadecanoic acid |
6 |
3.81 |
9-Octadecenoic acid, methyl ester, (E)- |
5.29 |
9,12-Octadecadienoic acid (Z,Z)-, methyl ester |
7 |
3.78 |
Hexadecanoic acid, ethyl ester |
3.65 |
Hexadecanoic acid, ethyl ester |
8 |
2.08 |
Octadecanoic acid, ethyl ester |
3.23 |
9-Octadecenoic acid, methyl ester, (E)- |
9 |
0.96 |
Hexadecanoic acid, methyl ester |
1.51 |
Octadecanoic acid, ethyl ester |
10 |
0.85 |
Ethyl (9Z,12Z)-9,12-Octadecadieno |
1.02 |
Hexadecanoic acid, methyl ester |
Molecular docking studies
The report of GC-MS analysis of the methanolic extract of S. commune shows very potent antimicrobial activity and is further analyzed for anticancer activity using computational methods. Figure 9 illustrates different bioactive molecules out of which 4H-pyran-4-one-3,5-Dihydroxy-2,3-Dihydro-6-Methyl. (DDMP) (37.62%) is the end product of the Millard reaction and reported to possess Anticancer,47 Anti-diarrhoeal,48 Nephro protective,49 Anti-obesity,50 Antidiabetic,50 Anti Tubercular,51 Antimicrobial and Hepatoprotective properties followed by several potent Therapeutically active metabolites.
The bioactive metabolites analyzed from the methanolic extract of the S. commune were already known to possess antioxidant activity52,53; anti-inflammatory, anti-helminthic, and anti-diarrhoeal activities; antidiabetic activity; antimicrobial activity against different human pathogens; anti-sickling activity54; nephroprotective50; and antiproliferative activity against different human cancer cell lines.49
In silico’s molecular docking analysis of DDMP and 5HMF was done using the software AutoDock Vina. The binding affinity of the bioactive compounds of S. commune with target proteins, which computationally evaluates the therapeutic potencies as anticancer drugs, is listed in Table 5.
Table (5):
Docking analysis of S. commune
| No. | Bioactive Compound | Ligand/ SMILE ID | Receptor/ target protein (PDB ID) | Disease | Binding Affinity | Residue involved in Hydrogen Interactions (Ligand Atom-Receptor) | Residue Involved in Hydrophobic Interactions (Ligand Atom-Receptor) |
|---|---|---|---|---|---|---|---|
| 1 | 3,5-Dihydroxy-2,3- Dihydro-6-Methyl-4H-pyran-4-one(DDMP) | CC1=C(C(=O) C(CO1)O)O | 2HQ6 | Colon Cancer | -5.9 | O1-G74(A)O | No Interactions |
| O1-G75(A)O | |||||||
| O4-G74(A)O | |||||||
| O4-G75(A)O | |||||||
| O3-A1029A)O | |||||||
| O1-S111(A)N | |||||||
| O1-Q112(A)N | |||||||
| 2 | DDMP | CC1=C(C(=O) C(CO1)O)O | 7W8O | Different types of Cancer | -22.0 | O1-Y18(B)O | C1-W19(F)CZ3 C1-W19(H)CZ2 |
| O1-Y18(H)O | |||||||
| O4-Y18(B)O | |||||||
| O3-W19(A)O | |||||||
| O3-W19(B)O | |||||||
| O3-W19(F)O | |||||||
| O3-W19(J)O | |||||||
| O3-W19(L)O | |||||||
| O3-W19(M)O | |||||||
| O3-E20(D)O | |||||||
| O3-E20(I)O | |||||||
| O3-E20(K)OE2 |
The antimicrobial activities of Schizophyllum commune were proved by the dual-culture and the agar well-diffusion methods against human pathogens. The efficiency of antimicrobial activity was illustrated by growth inhibition (99.2%), MIC value (25 µg/ml), and IC50 value (2.05 µg/ml). The major compound responsible for its bioactivity was analyzed by GC-MS as 4H-pyran-4-one-3,5-dihydroxy-2,3-dihydro-6-methyl. (DDMP). The therapeutic efficiency of DDMP as antimicrobial and anticancer drugs was analyzed by AutoDock Vina software and showed the strong binding affinity of -5.9 kcal/mol against colon cancer target proteins (PDB ID 2HQ6), and -22.0 kcal/mol against (PDB ID W8O) Disulfide-rich tumor-homing peptides-binding protein that can bind to mdm-2 (oncoprotein) using P53-mediated tumor suppression. According to our study, S. commune was confirmed to be a promising source of novel antimicrobial and anticancer drugs based on in vitro and in silico studies.
ACKNOWLEDGMENTS
The authors are thankful for the financial assistance from the Seed grant and from the RUSA-ANU Research Project of Acharya Nagarjuna University, Guntur, Andhra Pradesh, India. Authors are also thankful to the University Research Fellowship for providing Financial support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by Seed Money Grant vide reference number ANU/CIIPR/Project Proposals/Sanction of Finance Assistance/2023, University Research Fellowship vide reference number ANU/UCS/URF/Research Fellowship-2021-22 and 2022-23 and RUSA (2.0) Research Project Fellowship with Lr. No. ANU/RUSA 2.0/R.P/Mrs.S.M.K./P.F/Dept. of Bot.µ./Appt.order/2024.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Jones KE, Patel NG, Levy MA, et al. Global Trends in Emerging Infectious Diseases. Nature. 2008;451(7181):990-993.
Crossref - Schenone M, Dancik V, Wagner BK, Clemons PA. Target identification and mechanism of action in chemical biology and drug discovery. Nat Chem Biol. 2013;9(4):232-240.
Crossref - Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H. Medicinal plants: History and future perspective. J Herbmed Pharmacol. 2018;7(1):1-7.
Crossref - Al Attas SA, Zahran FM, Turkistany SA. Nigella sativa and its active constituent thymoquinone in oral health. Saudi Med J. 2016;37(3):235-244.
Crossref - Hassanien MFR, Assiri AMA, Alzohairy AM, Oraby HF. Health-promoting value and food applications of black cumin essential oil: an overview. J Food Sci Technol. 2015;52(10):6136-6142.
Crossref - Kassab RB, El-Hennamy RE. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rats. Egypt J Basic Appl Sci. 2017;4(3):160-167.
Crossref - Gnanasekaran P, Roy A, Natesh NS, Raman V, Ganapathy P, Arumugam MK. Removal of microbial pathogens and anticancer activity of synthesized nano-thymoquinone from Nigella sativa seeds. Environmental Technology & Innovation. 2021;24:102068.
Crossref - Singh R, Dubey AK. Endophytic Actinomycetes as Emerging Source for Therapeutic Compounds. Indo Global Journal of Pharmaceutical Sciences. 2015;05(02):106-116.
Crossref - Hermann P, Heinze T. Renewable Thermoplastics – Starch Fatty acid Esters as Alternatives to Synthetics. BioResources. 2022;17(3): 3871-3874.
Crossref - Caruso DJ, Palombo EA, Moulton SE, Zaferanloo B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms. 2022;10(10):1990.
Crossref - Mao Z, Zhang W, Wu C, et al. Diversity and antibacterial activity of fungal endophytes from Eucalyptus exserta. BMC Microbiol. 2021;21(1):155.
Crossref - Ramadan MF. Black Cumin (Nigella Sativa) Seeds: Chemistry, Technology, Functionality, and Applications. Springer, Cham. 2021.
Crossref - Emitaro WO, Kawaka F, Musyimi DM, Asenath Adienge. Diversity of endophytic bacteria isolated from leguminous agroforestry trees in western Kenya. AMB Express. 2024;14(1):18.
Crossref - Sharma A, Rashid M, Chauhan P, Kaur S, Kaur A. In vitro antibacterial and anti-biofilm potential of an endophytic Schizophyllum commune. AMB Express. 2024;14(1):10.
Crossref - Rosana Y, Matsuzawa T, Gonoi T, Karuniawati A. Modified Slide Culture Method for Faster and Easier Identification of Dermatophytes. Microbiology Indonesia. 2014;8(3):135-139.
Crossref - Azuddin NF, Mohd MH, Rosely NFN, Mansor A, Zakaria L. Molecular Phylogeny of Endophytic Fungi from Rattan (Calamus castaneus Griff.) Spines and Their Antagonistic Activities against Plant Pathogenic Fungi. J Fungi. 2021;7(4):301.
Crossref - Mishra A, Singh SP, Mahfooz S, et al. Bacterial endophytes modulate the withanolide biosynthetic pathway and physiological performance in Withania somnifera under biotic stress. Microbiol Res. 2018;212-213:17-28.
Crossref - Chew SY, Teoh SY, Sim YY, Nyam KL. Optimization of ultrasonic extraction condition for maximal antioxidant, antimicrobial, and antityrosinase activity from Hibiscus cannabinus L. leaves by using the single factor experiment. J Appl Res Med Aromat Plants. 2021;25:100321.
Crossref - Bhalodia NR, Shukla V. Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: An ethnomedicinal plant. J Adv Pharm Technol Res. 2011;2(2):104-109.
Crossref - Kadaikunnan S, Rejiniemon TS, Khaled JM, Alharbi NS, Mothana R. In vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann Clin Microbiol Antimicrob. 2015;14:9. Published 2015 Feb 22.
Crossref - Bodke YD, Shankerrao S, Kenchappa R, Telkar S. Synthesis, antibacterial and antitubercular activity of novel Schiff bases of 2-(1-benzofuran-2-yl)quinoline-4-carboxylic acid derivatives. Russ J Gen Chem. 2017;87(8):1843-1849.
Crossref - Gonelimali FD, Lin J, Miao W, et al. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front Microbiol. 2018;9(1639):1639.
Crossref - Wang H, Liu Z, Duan F, et al. Isolation, identification, and antibacterial evaluation of endophytic fungi from Gannan navel orange. Front Microbiol. 2023;14:1172629.
Crossref - Udayan E, Gnanadoss JJ. Isolation and Characterization of L-Asparaginase Producing Endophytic Fungi from Medicinal Plants of Rutaceae Family. Biosci Biotech Res Asia. 2023;20(1):241-253.
Crossref - Kancherla N, Dhakshinamoothi A, Chitra K, Komaram RB. Preliminary Analysis of Phytoconstituents and Evaluation of Anthelminthic Property of Cayratia auriculata (In Vitro). Maedica. 2019;14(4):350-356.
Crossref - Jiang C, Li J, Zhang J, et al. Isolation, Identification, and Activity Evaluation of Chemical Constituents from Soil Fungus Fusarium avenaceum SF-1502 and Endophytic Fungus Fusarium proliferatum AF-04. J Agricu Food Chem. 2019;67(7):1839-1846.
Crossref - Kumar S, Agarwal RP, Singh D, et al. Isolation and Chemical Structural Elucidation of Antibacterial Bioactive Compounds from Endophytic Fungal Strain Phoma sp. D1. Curr Pharmacol Rep. 2023;9(3):128-143.
Crossref - Taritla S, Kumari M, Kamat S, Bhat SG, Jayabaskaran C. Optimization of PhysicoChemical Parameters for Production of Cytotoxic Secondary Metabolites and Apoptosis Induction Activities in the Culture Extract of a Marine Algal-Derived Endophytic Fungus Aspergillus sp. Front Pharmacol. 2021;12.
Crossref - Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 2008;3(2):163-175.
Crossref - Aykul S, Martinez-Hackert E. Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal Biochem. 2016;508(1):97-103.
Crossref - Akshata P, Chandrakala S. Physicochemical, phytochemical, and GC-MS analysis of leaf and fruit of Pouteria campechiana (Kunth) Baehni. J Appl Biol Biotechnol. 2020;8(04):90-97.
Crossref - Lall N. (Ed.). Aquatic Plants: Pharmaceutical and Cosmetic Applications (1st ed.). CRC Press; 2020.
Crossref - Anza M, Endale M, Cardona L, et al. Antimicrobial Activity, in silico Molecular Docking, ADMET and DFT Analysis of Secondary Metabolites from Roots of Three Ethiopian Medicinal Plants. Adv Appl Bioinform Chem. 2021;2021(14):117-132.
Crossref - Weyesa A, Eswaramoorthy R, Melaku Y, Mulugeta E. Antibacterial, Docking, DFT and ADMET Properties Evaluation of Chalcone-Sulfonamide Derivatives Prepared Using ZnO Nanoparticle Catalysis. Adv Appl Bioinform Chem. 2021;2021(14):133-144.
Crossref - Degfie T, Ombito JO, Demissie TB, Eswaramoorthy R, Dekebo A, Endale M. Antibacterial and Antioxidant Activities, in silico Molecular Docking, ADMET and DFT Analysis of Compounds from Roots of Cyphostemma cyphopetalum. Adv Appl Bioinform Chem. 2022;2022(15):79-97.
Crossref - Sindhe MA, Bodke YD, Kenchappa R, Telkar S, Chandrashekar A. Synthesis of a series of novel 2,5-disubstituted-1,3,4-oxadiazole derivatives as potential antioxidant and antibacterial agents. J Chem Biol. 2016;9(3):79-90.
Crossref - Matin MM, Hasan MS, Uzzaman M, et al. Synthesis, spectroscopic characterization, molecular docking, and ADMET studies of mannopyranoside esters as antimicrobial agents. J Mol Struct. 2020;1222:128821.
Crossref - Dixit D, Bhandari VM, Mane MB, Balapure K. Studies in instant water disinfection using natural oils. Biochem Eng J. 2022;187:108631.
Crossref - Chun J, Yoon HR, Lee SJ, Kim DH. Co-infection with two novel mycoviruses affects the biocontrol activity of Trichoderma polysporum. Biological Control. 2024;188:105440-105440.
Crossref - Mcotshana ZKS, McGaw LJ, Kemboi D, et al. Cytotoxicity and antimicrobial activity of isolated compounds from Monsonia angustifolia and Dodonaea angustifolia. J Ethnopharmacol. 2023;301:115170.
Crossref - Rajesh PS, Rai VR. Hydrolytic enzymes and quorum sensing inhibitors from endophytic fungi of Ventilago madraspatana Gaertn. Biocatal Agric Biotechnol. 2013;2(2):120-124.
Crossref - Khatami SH, Vakili O, Movahedpour A, Ghesmati Z, Ghasemi H, Taheri-Anganeh M. Laccase: Various types and applications. Biotechnol Appl Biochem. 2022;69(6):2658-2672.
Crossref - Asemoloye MD, Sunmola N, Jonathan G, Chikwem J. Mycochemical screening reveals exopolysaccharide secretion, antioxidant and larvicidal activities of three oyster mushrooms. J Sci Food Agric. 2021;102(5):2120-2126.
Crossref - Kalusalingam A, Rozario AHJ, Subramanian P, et al. Preliminary Exploration of Bioactive Compounds and Anthelmintic Activity in Diospyros kaki Fruits. Malaysian Applied Biology. 2024;53(6):123-129.
Crossref - Chatterjee SN, Ghosh S, Mandal NC. Potential of an endophytic fungus Alternaria tenuissima PE2 isolated from Psidium guajava L. for the production of bioactive compounds. SAJ Bot. 2022;150:658-670.
Crossref - Davidson PM, Sofos JN, Branen AL, eds. Antimicrobials in Food;(3rd Ed), CRC Press. 2005.
Crossref - Guchhait KC, Manna T, Barai M, et al. Antibiofilm and anticancer activities of unripe and ripe Azadirachta indica (neem) seed extracts. BMC Complement Med Ther. 2022;22(1):42.
Crossref - Nemkul CM, Bajracharya GB, Maeda H, Shrestha I. Ethnomedicinal Knowledge Verification for the Antidiarrheal and Antioxidant Effects of Rhus chinensis Mill. Fruits with Identification of Thirty Constituents. Pharmacogn J. 2021;13(1):37-43.
Crossref - Shukla R, Banerjee S, Tripathi YB. Antioxidant and Antiapoptotic effect of aqueous extract of Pueraria tuberosa (Roxb. Ex Willd.) DC. On streptozotocin-induced diabetic nephropathy in rats. BMC Complement Altern Med. 2018;18(1):156.
Crossref - Mopuri R, Ganjayi M, Banavathy KS, Parim BN, Meriga B. Evaluation of anti-obesity activities of ethanolic extract of Terminalia paniculata bark on high fat diet-induced obese rats. BMC Complemen Altern Med. 2015;15:76.
Crossref - Arora G, Gagandeep, Behura A, et al. NSC 18725, a Pyrazole Derivative Inhibits Growth of Intracellular Mycobacterium tuberculosis by Induction of Autophagy. Front Microbiol. 2020;10:3051.
Crossref - Kanzler C, Haase PT, Schestkowa H, Kroh LW. Antioxidant Properties of Heterocyclic Intermediates of the Maillard Reaction and Structurally Related Compounds. J Agricu Food Chem. 2016;64(41):7829-7837.
Crossref - Chen Z, Liu Q, Zhao Z, et al. Effect of hydroxyl on antioxidant properties of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one to scavenge free radicals. RSC Adv. 2021;11(55):34456-34461.
Crossref - Hannemann A, Cytlak UM, Rees DC, Tewari S, Gibson JS. Effects of 5-hydroxymethyl-2-furfural on the volume and membrane permeability of red blood cells from patients with sickle cell disease. J Physiol. 2014;592(18):4039-4049.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.