ISSN: 0973-7510
E-ISSN: 2581-690X
Medical device-related infections are deep-seated infections that are complex to treat owing to the emergence of antibiotic-resistant organisms. Bacteriophages are non-antibiotic tools that act as either an alternative or complementary option to antibiotics in managing bacterial diseases. The host specificity of bacteriophages restricts their clinical application to specific bacterial infections. This systematic review aims to summarize the application of bacteriophage as an anti-biofilm agent and their efficacy and safety in preventing or controlling device-associated bacterial infections by analyzing research findings from the last 10 years. We conducted a systematic search of four electronic databases to identify articles, and 30 eligible articles were included in this review. During the follow-up period specified in the articles, 93.75% of patients achieved complete microbiological recovery from the target infection and 6.2% experienced a relapse. Therefore, through this systematic review, we emphasize that it is necessary to establish standardized and reproducible methods for coating indwelling devices with bacteriophages, ensuring their long-lasting and effective functionality for the benefit of patients.
Bacteriophage, Indwelling Device, Biofilm, Phage Therapy
Implants and medical indwelling devices are crucial components in revolutionary medicine. They involve rapid technologies that benefit patient’s health. Catheters, endotracheal tubes, pacemakers, ventricular assist devices, and hip and joint implants are examples of implantable medical devices.1 While these advancements have extended and enhanced quality of life, the introduction of foreign materials into patients inevitably creates conditions conducive to microbial colonization and infection. The rate of infections associated with indwelling devices is steadily increasing, provided that the sterility of medical procedures is not maintained. They are responsible for 50-70% of the nearly 2 million healthcare-associated infections reported by the Centers for Disease Control. The rising rates and varieties of device utilization, coupled with the aging population and the growing prevalence of comorbid conditions resulting in compromised immune systems, are common reasons for medical device-related infections.1 Bacteriophages represent one of the most promising alternatives to antibiotics in clinical applications. Before antibiotics were discovered and widely used, it was suggested that bacterial infections could be prevented or treated by bacteriophage administration. Later, there was a rapid increase in interest in phage therapy, as evidenced by the significantly higher number of case reports detailing patients undergoing treatment.
This systematic review provides a brief overview of the medical indwelling device-associated infections, biofilm development and hurdles in its treatment, various antibiofilm strategies, and clinical as well as some of the in vitro studies related to phage therapy.
Burden of bacterial biofilm
Biofilms are structured and clustered communities of microorganisms that are encased in a self-produced polymeric matrix. These extracellular substances are complex matrices of organic polymers made up of different biomolecules such as carbohydrates, proteins, and DNA. They play a crucial role in facilitating microbial adhesion to surfaces and in mediating interactions between microbial cells and their surrounding environment. The biofilm matrix accounts for over 90% of the dry mass in most biofilms, with microbial cells representing less than 10%.2 The property of biofilm can be observed in various groups of microorganisms, including single-celled eukaryotes like yeast.3 Biofilm serves as a survival mechanism by acting as a barrier that isolates bacterial cells from the host environment. Hence, it displays phenomenal features like innate resistance to host immune defence, increased resilience to mechanical and physiological stress, and antimicrobial agents.4
Approximately 65% of bacterial infections are linked to the presence of bacterial biofilms, and device-associated biofilm infections are common in the healthcare setup. Biofilms on medical devices serve as a reservoir for bacteria to trigger recurrent infections, inflammation, and tissue damage. Endotracheal tubes frequently lead to the formation of biofilms, which can harbor pathogens including Methicillin–resistant Staphylococcus aureus (MRSA) as well as Gram-negative bacilli such as Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii.5 Common microbial contaminants known to form biofilms on urinary catheter devices include Escherichia coli, Enterococcus faecalis, Staphylococcus epidermidis, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, and other Gram-negative bacteria.6
Antimicrobial resistance is one of the emerging battlegrounds of the 21st century, which poses a threat to the future generation of therapeutic options left behind and difficulty in the discovery of new drugs. The burden of antibiotic resistance on global health is enormous and has been described as a slow-motion pandemic.7 A recent publication by the United Nations Environmental Programme states that in 2019, the global death count was approximately 1.27 million and that they are directly connected to drug-resistant infections. It is also estimated that there will be approximately 10 million deaths annually by 2050 due to antibiotic inefficiency. This affects the annual GDP and socioeconomic status of people.8
Mechanism of biofilm formation
Biofilms exhibit diverse pathological presentations and are ubiquitous, colonizing medical implants, biological tissues, water conduits, pipelines, hospital environments, food processing facilities, and a range of other living and non-living surfaces. Biofilm-associated microorganisms display alterations in phenotype and gene expression, leading to resistance against established antibiotics, decreased metabolic activity and growth rates, and production of virulence-related factors. In a cross-sectional study conducted in 2022, lasR-deficient Pseudomonas aeruginosa isolates upregulated the expression of quorum sensing regulator lasR gene. In the case of lasR-deficient P. aeruginosa, without any environmental trigger, the mutant develops a biofilm around it.9
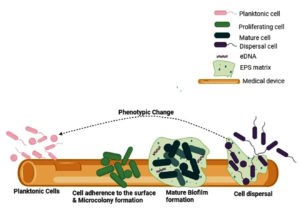
Figure 1. Stages of Biofilm formation on medical devices. Free-flowing organisms adhere loosely and reversibly to selected biotic and abiotic surfaces. They multiply and encase within a self-produced EPS matrix. Multiple layers of cells accumulate on the surface to form mature biofilms. Later, it ruptures and disperses to start a new life cycle (Source: created by Biorender.com)
Biofilm formation is a multi-step process. The process of biofilm formation has been explained elsewhere10 and is shown in Figure 1. Briefly, the four stages of development of biofilm were as follows:
- Attachment: Biofilm formation is initiated when planktonic microorganisms adhere to the surfaces. In the early phase of biofilm formation, microorganisms attach loosely and reversibly to develop a poor connection with the surface and later change their orientation and attach irreversibly to form biofilms.
- Microcolony formation: The formation of a biofilm matrix is facilitated by the production of extracellular polymeric substances (EPS), which are primarily composed of polysaccharides, proteins, and DNA. They form the first layer of cells that covers the surface.
- Maturation: This is a mushroom or tower-shaped microbial structure consisting of three layers: the inner regulatory layer, the middle microbial basement layer, and the outer layer of planktonic cells, which are ready to exit the biofilm. Thus, a mature biofilm consists of microcolonies surrounded by water channels for the transport of nutrients and signaling molecules.
- Dispersion: To disperse the microorganisms and start a new cycle of biofilm, the mature biofilm ruptures by active or passive mode.
Treatment hurdles amid biofilm
Biofilms produced by bacteria interfere with the antimicrobial action against organisms. It has the potential to reduce susceptibility patterns by up to 1000-fold.11 Biofilm-associated infections are difficult to treat due to various factors such as slow onset of disease, foreign material used in the diagnosis or treatment, antibiotic ineffectiveness and failure of early detection.12
At the beginning of the infection, biofilm-producing organisms remain dormant and slowly colonize causing acute infection in the host. They also remain unexposed to the host immune system and form a slimy matrix. Within this, they become adapted to the oxygen – and nutrient-limited host environment by lowering their metabolic rate and causing persistent infection.4
The presence of foreign material in the body significantly contributes to the process of biofilm production and enhancement by providing a free surface for bacterial colonization. The infection rate of biofilm-forming organisms is significantly higher in the presence of such foreign bodies than when organisms are present alone, without being associated with any foreign objects. This phenomenon is explained by the fact that in the presence of foreign bodies, the action of neutrophils is reduced or injured; hence, there is downregulated phagocytosis and neutrophil action12,13 and the treatment of such cases becomes difficult. For example, a 64-year-old woman who underwent arthroplasty developed persistent MRSA infection in her hip and knee despite receiving prompt treatment with intravenous vancomycin and oral linezolid. She was treated with 2-stage revision surgery and insertion of an antibiotic-loaded PROSTALAC hip spacer to cure Prosthetic Joint Infections. Despite undergoing the DAIR (debridement, antibiotic, and implant retention) procedures, infection was unavoidable. Deep-seated recalcitrant MRSA infection is the primary reason why conventional antibiotics cannot eliminate the pathogen. Over 4 years, the patient was subjected to a wide range of treatments, but they proved ineffective. Even escalating antibiotics did not help in the case of deep-seated infections.14
All the adaptations made by bacteria to fit into the stressful host environment alter the antimicrobial targets in the organism and reduce the cell division rate. It aids the bacteria in becoming resilient to antibiotic agents, and the host immune responses exaggerate collateral tissue damage, which adds more burden to the treatment.15 The matrix does not participate in the inhibition of antibiotic penetration into the cell; however, the changes induced during biofilm formation, such as changes in gene expression or protein production within the biofilm, mediate this antibiotic recalcitrance.16,17 To combat the adverse effects of biofilms, the foremost option is to remove the infected medical devices or replace them with sterile ones.18 However, the changing time of the medical devices also plays a major role. If replacement or removal of foreign devices is not possible, sensitive & aggressive antibiotic treatments are considered.12
Antibiofilm strategies
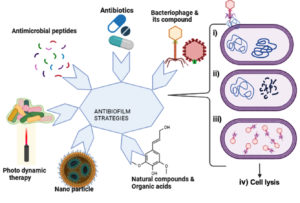
Biofilm-associated infections are very difficult to treat as they either do not respond or show a poor response to classic antibiotic therapy. The barrier formed by the biofilm must diffuse to reverse the resistance mechanisms. Disruption of the biofilm and restoration of the organism to its original free-living planktonic state and inhibiting it solve the quest of the biofilm hurdle. There are various strategies to inhibit biofilm formation, such as exposing the biofilm-forming bacteria to antimicrobial agents and antimicrobial peptides with a broad spectrum of antimicrobial activity19 and photodynamic therapy is potentially active against biofilm-related resistance. Photodynamic therapy uses visible light of a specific wavelength to form cytotoxic reactive oxygen species (ROS),20 organic acids,21 and extracellular enzymes. Extracellular enzymes, such as glycoside hydrolases, proteases, and deoxyribonucleases, potentially target the extracellular polymeric substances of biofilms and revert the cells into a planktonic state.22 Targeting biofilms with enzymatic degradation demonstrates the highest efficacy on both developing and existing biofilms.19 Various strategies that are potentially effective against biofilm-related resistance are summarized in Figure 2.
Figure 2. Graphical representation of various commonly available anti-biofilm strategies, including antibiotics, antimicrobial peptides, photodynamic therapy, nanoparticles, natural compounds, and organic acids. The figure also depicts the mechanism of action of lytic bacteriophages by (i) enzymatic activity and penetration of phage genetic material into the host cell, (ii) synthesis of viral genome and protein, (iii) assembly of virions, and (iv) lysis of host cells and release of progeny virions (Source: created by Biorender.com)
Surface topography is one of the newer innovative techniques in which the surface of the implant device is coated without altering its original characteristics. Various compounds such as antimicrobial peptides, quorum-sensing inhibitor enzymes, and antibiotics can be stably coated onto these devices.23 To avoid low penetration of drugs or antibacterial compounds, nanoparticles are used as an efficient drug delivery system for disease treatment and as a bacterial detection system for microbial diagnostics. Most of the nanoparticles are also potential antimicrobial agents, along with an effective delivery vehicle.24 Nanoparticles have applications in the creation of antibacterial coatings for implantable devices and medical materials to prevent infections. Despite these advantages, some nanoparticles also serve as promoters of drug-resistance. Previous studies have shown that aluminum nanoparticles can enhance the conjugative transfer of plasmids such as RP4, PK2, and pCF10, leading to the spread of multidrug-resistance not only within the same bacterial species but also across different genera.25
Bacteriophages
In the early 1900s, Twort and D’Herelle isolated a category of viruses called bacteriophages from the feces of convalescing patients with dysentery. However, the isolated virus is not pathogenic to humans and is hosted by bacteria.26 Bacteriophages are viruses that infect and replicate only in the bacterial cells. Phage therapy is a blooming hope in preventive and therapeutic medicine. In the early 1940s, the therapeutic application of bacteriophages was first tested for the treatment of bacterial infections.
Until recently, antibiotics overshadowed the effect of bacteriophages; however, in the new era, where multidrug-resistant organisms are evolving with each mutation, the bacteriophage again flashes in the limelight. Since bacteriophages are non-antibiotic tools used to inhibit bacterial growth and prevent infection, they have attracted the interest of researchers as a favorable therapy in the context of an antimicrobial crisis.27
Antibiotics are known to target either Gram-positive or Gram-negative bacteria, including beneficial flora, which is now seen as undesirable because of its negative impact on the overall microbiota and potential to promote antibiotic resistance. Phage therapy offers a solution to these challenges because of its exceptional specificity and efficacy against drug-resistant strains. In addition, the degradation of phages through antibodies and other mechanisms does not result in the generation or buildup of toxic by-products.28
The efficient use of target-specific bacteriophages at an effective dose, route, and frequency on an appropriate diseased condition will sufficiently inhibit bacterial growth and result in the improvement of patient health.29 This is explained by successful case reports through the administration of either a single phage or a phage cocktail. The success rate was measured based on the microbiological or clinical outcomes. The utilization of distinct bacteriophages tailored to target individual bacterial strains is a key factor that contributes to this success. Nevertheless, existing manufacturing constraints, pharmacoeconomic models, and marketing demands tend to support predetermined phage cocktails that have already been employed in phage therapy clinical trials. Also, it is noted that the phage cocktails exhibit more immunogenicity compared to monovalent phage preparation, potentially leading to adverse effects on their efficacy when used.30
Recent data suggests that the strategy of combined treatment with phages and antibiotics may not be suitable for all phages and antibiotics, as aminoglycoside antibiotics have been found to exhibit Mycobacteriophage DNA replication, potentially interfering with pathogen elimination by phages.31
Antibacterial properties of bacteriophage
The antibacterial or antibiofilm action of phages could be explained by the mechanism involving 2 key enzymes of phages: depolymerase and lysins.32 Depolymerase is the tail spike protein of bacteriophage. It acts as an adjuvant, favoring the elimination of bacteria.33 Lysins are phage enzymes with the ability to hydrolyze the cell wall and help release phage progeny during bacterial attack.34 Phages are capable of penetrating biofilms, dissolving the extracellular polymeric matrix, and reaching target organisms. The antibiofilm properties of bacteriophages can be explained by the direct dispersion of the biofilm matrix, intra-to-extracellular degradation, or extra-to-intracellular degradation of the bacterial structure.32
The injection of the phage genome into the host is essential for the phage to initiate bacterial infection. Therefore, self-replicating phages can cause cell lysis. This mechanism occurs when the receptor protein present on the tail fiber tip initiates an interaction with specific bacterial surface receptor molecules.35 Thus, the discovery of antibacterial mechanisms of bacteriophages has shown their remarkable action in the treatment and prevention of infectious diseases. Unfortunately, the antibiotic revolution has pulled phage therapy behind this screen. However, the modern era is again turning towards the use of phages and their derivatives in the healthcare progress.
Protocol
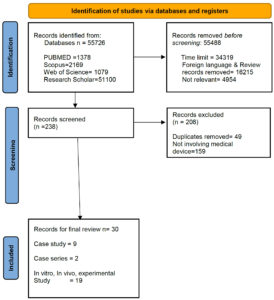
This systematic review was prepared and reported according to ‘The Preferred Reporting Items for Systematic Review and Meta-Analysis.36 The PRISMA flow diagram in Figure 3 depicts this review’s detailed data-screening method. The present systematic review included studies published between January 2014 and October 2023.
Search strategy & eligibility criteria
Multiple sources of electronic databases such as PubMed, Scopus, Web of Science, and Google Scholar were included to identify and extract data on the use of bacteriophages to inhibit biofilms formed on medical devices. A combination of search strategies involving Medical Subject Headings (MeSH terms) and Boolean characters were employed. The search was limited to the use of bacteriophages to prevent or inhibit biofilm formation in medical devices. These included devices experimenting on humans, animals, and in vitro studies. MeSH terms used for the search were biofilm, bacteriophage, antibiofilm, medical device, implant, and prosthetic.
Inclusion criteria
We screened full-text articles published in English between January 2014 and October 2023. Original articles, including in vitro and in vivo studies, case reports, clinical studies, trials, and controlled clinical trials, were included. Using the MeSH terms like ‘Bacteriophage’, and ‘Biofilm’ connected with the Boolean character ‘AND’ the full-text articles were filtered, and later, individual searches for different medical devices were used. For example, ‘Catheter’, ‘Endotracheal tube’, ‘Prosthetic’, etc., for the final list of articles from the 4 databases. Research involving the synergistic action between bacteriophages and antibiotics was included in this systematic study. The PRISMA plot flow diagram (Figure 3) depicts the data identified, screened, and retrieved for systematic review.
Figure 3. Systematic review flow diagram. PRISMA flowchart for the present review detailing the process of literature screening and inclusion of studies
The main objectives of these studies were to describe the potential of isolated or library-chosen bacteriophages to inhibit or prevent bacterial biofilm infections associated with indwelling medical devices, and at the end of the study, a conclusion of either positive or negative effects was described.
Exclusion criteria
Non-medical device-related studies on bacterial infection and phage therapy, phage therapy involving phage-derived products such as phage proteins, enzymes, engineered or tagged phages, nano-formulated phages, and older research articles published before 2014 were excluded. To ensure uniformity and consistency in the analysis, the studies included in this systematic review were strictly limited to those investigating the therapeutic application of whole bacteriophages to mitigate bacterial infection.
Data extraction
The first author independently collected the data and eligible articles were screened for the final review, which the co-author then verified. Various factors, such as language, type of article, year of publication, and MeSH terms were considered for data filtering.
The initial data search yielded 55,726 results, distributed as follows: 1378 in PubMed, 2169 in Scopus, 1079 in Web of Science, and 51,100 in Google Scholar. After screening for the year of publication, articles in English, and removing the irrelevant articles, 238 articles were retrieved. Finally, 30 articles were included in the systematic review, after applying the inclusion and exclusion criteria, as detailed above. The details of the included studies are summarized in Table.
Table:
Summary of the studies used in this systematic review
Bacteria involved |
Bacteriophage used |
Route of phage administration |
Multiplicity of Infection/ Dose |
Treatment effect |
Country (Year) Reference |
|---|---|---|---|---|---|
Proteus mirabilis |
Isf-Pm1, Isf-Pm2 |
Phage suspension is added to the bacteria colonized on the catheter |
0.001 to 10 (106 to 109 PFU/ml |
Significant reduction in levels of encrustation & significant decrease in 24 hr biofilm mass compared to that of the untreated control |
Iran (2024)65 |
Pseudomonas aeruginosa |
vB_PaeM_USP_2 and vB_PaeM_USP_18 |
Phage cocktail is coated onto the surface of the endotracheal tube |
4 × 107 (PFU/mL) |
Significant reduction in microbial load |
Brazil (2021)64 |
Pseudomonas aeruginosa |
ΦJHS-PA1139 and ΦSMK-PA1139 |
Phage lysates were coated on the catheter and the endotracheal tube (pre and post treatment) |
Pre-treatment 106 PFU/mL, Post treatment 102, 104 and 106 PFU/mL |
The most efficient log10 reductions were achieved when phages were applied at titers of 106 PFU/mL |
Ethiopia (2022)39 |
Staphylococcus aureus |
S. aureus Phage K |
Intraluminal inoculation into the catheter in the animal model |
108 PFU/ml |
Antimicrobial-lock technique significantly reduced bacterial colonization and biofilm presence |
North Carolina (2014)47 |
Pseudomonas aeruginosa, MSSA, Polymicrobial also |
Details Not given |
3- Intraoperative, 1-IV, 1- Local |
Details not given |
Microbiological eradication was achieved in 3 patients, however, in 1 case relapse with emergence of phage-resistant Pseudomonas aeruginosa |
Germany (2022)41 |
Pseudomonas aeruginosa and Proteus mirabilis |
Paer4, Paer14, M4, 109, E2005-A, and E2005-C & Pmir1, Pmir32, Pmir34, and Pmir37 |
Phage suspension exposed to the catheter |
1 X 109 PFU/ml anti-pseudo phage and 3 X 108 PFU/ml antiproteus phage |
Combination of bacterial interference with both anti-P. aeruginosa and anti-P. mirabilis phage cocktails conceivably offer broad protection against uropathogen colonization |
Georgia (2015)66 |
Pseudomonas aeruginosa |
Details not given |
Local application |
108 PFU/ml |
10 months after reimplantation, the patient reported no pain in the right knee; the soft tissue at the surgical site was unremarkable and the mobility satisfactory. The serum C-reactive protein was normal. |
Germany (2020)53 |
Enterococcus faecalis |
EPA, EPB, EPC, EPD, EPE, and EPF |
In vitro coating onto the catheter |
107 phage lysates |
Phages reduced the formed and preformed biofilms to a range of 38.02–45.7% and 71.0–80.0%, respectively, as compared to the control |
Egypt (2022)40 |
Proteus mirabilis |
vB_PmiP_5460 and vB_PmiM_5461 |
In vitro coating onto the catheter |
109 PFU/mL−1 |
A significant reduction of P. mirabilis biofilm formation up to 168 hr of catheterization |
Portugal (2016)67 |
Escherichia coli |
HP3, ES12, ES17, ES19, ES21, and ES26 |
Phage cocktail is added to the Biofilm formed on the catheter |
107 PFU/mL and 109 PFU/mL |
Phage cocktail comprising 6 phages lyses 82% of the strains in the E. coli library & increasing the dose of phage cocktails and incubation time resulted in a higher reduction (∼4-log) in bacterial burdens |
USA (2022)63 |
Pseudomonas aeruginosa |
Details not given |
The catheter was dipped into the mixture of phage-bacteria |
Details not given |
The imipenem-resistant strains were 100% sensitive and decreased the production of biofilm & 80% of the strains produced less amount of biofilm |
India (2021)62 |
Staphylococcus aureus |
Staphage |
Phage is coated onto the biofilm developed implant |
2 × 106 PFU |
Reduction of >98% biofilm in 8-hour cultures on exposure to phage cocktail, whereas no significant reduction on exposure to Cefazolin (100 times the MIC) |
Australia (2018)68 |
Pseudomonas aeruginosa |
vB_PaeM_USP1, vB_PaeM_USP_2, vB_PaeM_USP_3, vB_PaeM_USP_18, vB_PaeM_USP_25 |
Phage suspension added to a preformed Biofilm on an Endotracheal tube |
108 PFU/ml |
The viability rate, the metabolic activity of the organisms, reduced with disrupted biofilms |
Brazil (2020)69 |
Methicillin-resistant Staphylococcus aureus |
MR-5 |
Phage mixed with HPMC gel coated on the orthopaedic grade Kirschner-wires |
109 PFU/ml |
Phage as well as linezolid coated wires showed maximum reduction in bacterial adherence, associated inflammation, and faster resumption of locomotion and motor function |
India (2016)70 |
Staphylococcus aureus |
S. aureus bacteriophage 191219 |
Phage suspension injected into the abdomen of larvae |
109 PFU/larva (50 µL) |
Singular bacteriophage application was not effective against S. aureus infection. Simultaneous treatment with bacteriophages and antibiotics slightly enhances the effect of the antibiotic. |
Germany (2022)71 |
Staphylococcus aureus |
Staph phage K, WTP113011 and WTP092811 |
Phage lysate is added to the biofilm formed on the PEEK membrane and disc |
109 PFU/mL |
Phage-antibiotic combined treatment synergistically decreased bacterial concentration in both the static and dynamic biofilm conditions |
USA (2023)72 |
Staphylococcus aureus |
PP1493, PP1815, and PP1957 |
Personalized cocktail of phages was injected in the joint after closure |
1 × 109 PFU/ml |
During the follow-up, 2 patients had no signs of infection, negative CRP & pain and were able to walk. 3rd patient showed mild intermittent synovial fluid discharge with pain-free walk |
France (2020)42 |
Klebsiella pneumoniae |
KpJH46Φ2 |
Intravenous |
Daily infusions of 6.3 × 1010 phages in 50 ml normal saline (40 doses) |
During 34 weeks of follow-up, Resolution of local symptoms, signs of infection, and recovery of function |
USA (2020)73 |
MRSA |
Sb-1 |
Injecting the phage lysate into the proleg of larvae |
107 PFU/mL phage pretreatment & 108 PFU/mL phage post infection |
Sb-1 had a lytic activity similar to that exhibited by the antibiotic, and the combination of phage and daptomycin showed more reduction |
Italy (2022)74 |
Escherichia coli |
Coliphage |
Phage suspension was added to the biofilm formed on the catheter piece |
Details not given |
Significant reduction in the biofilm formation on using the crude bacteriophage on all three types of catheters |
India (2023)75 |
Enterococcus faecalis |
EF phage 1 |
direct injection into the knee followed by Intravenous bacteriophage therapy |
1 × 1010 PFU/mL |
In 24 months follow up, patient is without clinical signs of left knee PJI recurrence but developed right leg MRSA bacteremia requiring below the knee amputation. |
USA (2023)43 |
MRSA |
SaGR51ϕ1 |
IA & IV |
5.4 × 109 PFU IA and 2.7 × 109 PFU IV |
Two months of treatment, Intraoperative cultures negative, severe chronic infection was eradicated. |
USA (2020)44 |
Pseudomonas aeruginosa |
PT07 & PNM |
IV & local application |
107 PFU/mL |
No signs of recurrence 21 months after treatment |
Riga, Latvia (2023)76 |
Staphylococcus epidermidis |
PM448 |
Intra articular |
1 × 1010 PFU/mL |
Patient has full range of motion of knee and no clinical signs of PJI recurrence |
USA (2021)45 |
MRSA |
SaWIQ0488ø1 |
Intra-articular and intravenous |
1.2 × 108 PFU/mL |
No evidence of recurrence |
USA (2022)14 |
Providencia stuartii |
2 lytic phages |
Phage suspension added to a preformed biofilm on the catheter |
Details not given |
1.9- and 2-fold reduction in 3-day old P. stuartii biofilms built on latex or silicone catheters, respectively |
Israel (2021)77 |
methicillin-susceptible Staphylococcus aureus |
PYO bacteriophage & Staph phage Sb-1 |
Local application |
106 PFU/ml & 107 PFU/ml |
During follow-up 9 months later, the surgical site showed no local signs of infection |
Germany (2020)[46] |
Staphylococcus aureus. |
SniPha 360, Sanubiom GmbH, Fritzens, Austria |
Local application within the wound closure |
1 × 107 CFU/mL |
After 6 months, signs of local infection of the driveline exit site without systemic infection |
Germany (2022)78 |
Klebsiella pneumoniae and Klebsiella oxytoca |
vB_KppSSamwise, vB_KppS-Jiji, vB_KppS-Strom, vB_KppS-Pokey, vB_KppS-Anoxic, vB_KoM_Flushed |
Exposure of the phage suspension to the bacterial biofilm on the catheter |
1 × 102 PFU/mL |
The meropenem treatment in combination with the phage cocktail significantly reduced the viability of the biofilm in three out of five clinical strains; Trimethoprim and phage treatment showed statistically significant reduction in 4 out of 5 strains |
UK (2020)79 |
Proteus mirabilis |
ΦRS1-PmA, ΦRS1-PmB, and ΦRS3-PmA |
Phage cocktail is added to the bacteria colonized on the surface of the catheter |
3 × 1010 PFU |
The phage cocktail completely prevented catheter blockage and eradicated infection, with models draining freely for >8 days compared to the controls which blocked after 2 days |
UK (2016)80 |
Study characteristics
Of the 30 articles included, 9 were case reports, 2 were case series, and the remaining 19 were in vitro/in vivo/experimental studies. These 30 included studies: Seven were from the USA, five from Germany, three from India, two each from Brazil and the United Kingdom, and a single study from Israel, Italy, France, Iran, Ethiopia, Latvia, Australia, Portugal, North Carolina, Georgia and Egypt. All listed articles were published between January 2014 and October 2023.
The list of selected articles employed phage therapy for a variety of biofilm-related infections, mainly focusing on catheter-associated urinary tract infections, orthopedic implant-related periprosthetic joint infections of the knee and hip, cardiovascular implant infections, and models mimicking ventilator-associated infections.
A total of 33 bacterial species were reported in the selected studies, including Staphylococcus aureus (36.4%, n = 12/33), Pseudomonas aeruginosa (24.2%, n = 8/33), Proteus mirabilis (12.1%, n = 4/33), Enterococcus faecalis, Escherichia coli, and Klebsiella pneumoniae in 2 isolates each (18.2%, n = 6/33), and the remaining Klebsiella oxytoca, Staphylococcus epidermidis, and Providentia stuartii in 1 isolate each (9%, n = 3/33).
Biofilm growth on medical devices
Fifteen articles reported on the in vitro biofilm formation method. This included biofilm formation experiments on various medical devices, such as urinary catheters, endotracheal tubes, and Kirschner wires. Biofilm age plays a pivotal role in determining the action of an antimicrobial agent.37 Considering all the chosen articles, the age of biofilm formation or bacterial colonization before treatment with a particular phage ranged between 30 minutes and 20 days. Bacteriophages have dual actions on biofilms. It can eradicate the preformed biofilm and prevent the formation of biofilms on any surface.38 Two in vitro studies explored both the biofilm inhibitory and preventive actions of bacteriophages on medical devices.39,40
Phage characteristics in controlling biofilm
Among the 30 articles included, 14 (46.6%) reported the use of single phages and 15 (50%) included a cocktail of phages for the experiment or treatment. Only one study (3.3%) did not specify the number of phages used in the experiment. Out of 30 studies, in 13 (43.3%) studies, the phages were isolated by themselves, and the remaining 17 (56.6%) studies were conducted by obtaining the phage from other sources, such as commercial pharma companies or phage libraries. In about four (13.3%) and eight (26.6%) articles, the details of genome size and family to which the phage belongs, respectively, were mentioned. The genome size of the phages varied from 44,573 bp to 1,67,727 bp. Twenty-six (86.6%) articles failed to mention the details of the phages such as genome size and other characteristics. Approximately nine (30%) studies mentioned the family name to which the study phages belonged.
In the 30 studies included in this review, various routes of administration were used for the delivery of selected bacteriophages to the target site. Seventeen (56.6%) studies coated the phage lysate into either naked implants or preformed biofilm implants. In two (6.6%) studies involving the use of animal models, the phage suspension was directly injected into organs such as the abdomen or the proleg of an animal model. In the case reports, intra-articular administration was used to treat five patients, while local application of phages was performed for five other patients. The intraoperative mode was selected for three of the patients. The highest number of patients (n = 6) were treated with an intravenous injection of bacteriophages.
Study models
This systematic review includes the studies conducted using animal models, medical device models, and human case reports. Of the studies involving the animal models, one study was carried out using New Zealand white rabbits, one using BAL B/C female mice, and one study conducted with Galleria mellonella.
Eleven case reports dealing with phage therapy were included. Based on these case reports, 16 patients were treated with phage therapy. The ages of the patients ranged from 41 to 84 years. Years with a mean age of 66.7 years. Of the 16 patients who opted for phage treatment, only 4 were female and the remaining 12 were male candidates.
Efficacy of phage therapy
The efficacy of phage therapy was evaluated mainly through microbiological and clinical improvements. In vitro and in vivo studies have been conducted using animal models, microtiter plates or implant devices. In all 19 studies performed on either animals or inanimate objects, there was a significant reduction in microbial load. Phage therapy for the direct treatment of humans with different implant-related infections showed that 15 (n = 15/16, 93.75%) patients had complete microbiological recovery from the target infection until the follow-up period mentioned in the article. Of the 16 patients, one (6.2%) had a relapse of Pseudomonas aeruginosa infection related to the LVAD driveline. According to the authors, the emergence of phage-resistant bacteria and the complications of phage delivery to the infected site could explain the recurrence of infection.41 In another case series from France, three patients who underwent knee arthroplasty recovered completely with only non-specific synovitis symptoms.42 In the case of a left knee prosthetic joint infection with Enterococcus faecalis, the patient recovered. However, the patient developed MRSA right-ankle hardware infection and bacteremia, which resulted in below-the-knee amputation.43
Safety of phage therapy
Only case studies and case series including 16 patients were analyzed to determine the safety of phage therapy. Here 6 (37.5%) patients mentioned that the phages used were safe, without any remarkable adverse effects. Six (37.5%) patients failed to report any significant changes or adverse events during or after the phage therapy. In contrast, four (25%) patients exhibited temporary mild adverse effects such as an increase in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) for 3-4 days,14,44,45 and mild nausea.46 There was a case in which a patient died after treatment, but there was no relationship with phage therapy. The observed reactions could not be confirmed or associated with phage therapy consequences due to data limitations.
This systematic review critically analyzed 30 studies related to phage therapy published between January 2014 and October 2023, and the PRISMA guidelines were followed to emphasize the reproducibility, comprehensiveness, and transparency of the review. All the included articles (in vitro, in vivo, and case reports) dealt with the application of bacteriophages to treat medical indwelling device-related infections caused by different groups of bacteria and offered a comprehensive insight into phage therapy regarding its extent of usage, types of bacterial disease, type of medical device, phage isolation, and antibacterial characteristics.
Indwelling medical devices serve as a niche for harmful opportunistic pathogens. Complete inhibition of bacteria is necessary to prevent this harmful effect. Although the use of antibiotics initially removes a small fraction of pathogens, there is a chance of developing resistance against the antibiotic later. Removal of these implant devices would be ideal for eliminating infection. However, removing and eventually replacing the device presents significant practical challenges for patients requiring parenteral nutrition, chemotherapy, hemodialysis, and other treatments. For instance, to prevent removal, an antibiotic-locked catheter lumen was used to treat catheter-related bacteremia. However, it also shows a reduced success rate along with the chance of developing resistance among pathogens.47
Bacteriophages are self-replicating, natural predators of bacteria. They exhibit significant diversity in terms of size, morphology, and genomic structure. Nevertheless, they share a common feature, each comprising a nucleic acid genome surrounded by phage-encoded capsid proteins, serving to safeguard genetic material and facilitate its transfer into the subsequent host cell. Considering the studies in which bacteriophage details were mentioned, they commonly belonged to Myoviridae, Podoviridae, and Siphoviridae. These three families of bacteriophages belong to the Caudovirale order of phages, which are virulent phages that cause lysis of the host cell to release their progeny. These findings align with observations in phage therapeutic observations, where there is a prevailing preference for the use of virulent-tailed phages belonging to the Caudovirale order.48
Western countries like the USA and Germany utilize the highest number of phage technology in treating infectious diseases compared to other countries. The majority of the organisms encountered in the studies are Staphylococcus aureus, Pseudomonas aeruginosa, Proteus mirabilis, etc. These organisms are associated with severe hospital-acquired infections, often marked by elevated levels of drug resistance. In the case of polymicrobial infections, a cocktail of bacteriophages was used. Phages are species-specific and exhibit a narrow spectrum of activity. They act against a particular target pathogen and are ineffective against various strains of the same species. The efficacy of monophages in combating multi-bacterial infections is challenging unless a phage cocktail comprising active phages against each isolated organism is used. Creating phage banks or conducting in vitro evolution to enhance phage activity and reduce bacterial resistance can be effective strategies for addressing limited host specificity in targeted phage therapy.49
Under physiological conditions, the freely dispersed bacteriophages are prone to inactivation. Immune clearance by phages can also lead to a decrease in phage infectivity. Hence, sustained release and escape from the immune system or harsh physiological environment are necessary in some cases of phage therapy. For example, in medical device-related infections, the bacteriophage used in urinary catheters may lose infectivity owing to the highly acidic condition of the urinary tract. Thus, the use of phage delivery agents plays a pivotal role in enhancing the phage action.50
Microencapsulated, pH-responsive polymers were used in 2017 in the UK for the efficient oral delivery of Clostridium difficile bacteriophage to treat colonic infection. Encapsulated phages demonstrated substantial protection during extended exposure to an acidic environment without the inclusion of an antacid in the formulation.51 In case of wound infections, bacteriophage-loaded functional and biocompatible nanofibers were employed. These polymer fibers retained their antimicrobial effectiveness for almost four weeks at ambient temperature. However, its activity was higher when stored at -20 °C.52 Thus, efficient delivery methods offer significant promise as fundamental technologies that facilitate the clinical implementation of bioactive bacteriophages in phage therapy. Bacteriophages are excellent biocontrol agents. It demonstrates its potential disinfectant action on various surfaces such as glass, hospitals, medical devices, etc.
There was a record of 93.75% microbiological recovery from medical device-associated infection in the included clinical studies. Bacteriophages, as self-replicating microorganisms, theoretically require administration of a single dose to combat bacterial infections. Nevertheless, numerous other studies have suggested that multiple doses may yield superior therapeutic outcomes compared to a single-dose regimen.53
In the included studies, the efficacy of phage therapy correlated with the administered dosage. In 2022, at the German Heart Center Berlin, among the four phage treatments, the patient who experienced a relapse received the lowest dosage compared to the others. This was further compounded by the challenge of delivering bacteriophages locally to the LVAD driveline infection sites during sterile dressing.41 The route of administration is also crucial for determining therapeutic efficacy. Findings from these studies indicate that the local application of phages alone is inadequate to eradicate infection. Administering a targeted bacteriophage at an optimal concentration via intraoperative or intravascular routes can significantly enhance treatment effectiveness. During the data analysis, no association was observed between the bacterial species and the effectiveness of phage treatment. Additionally, the combination of bacteriophages and antibiotics proved to be more effective in conditions such as prosthetic joint infection and ventricular assist device (VAD) driveline infection compared to phage therapy alone.
It is also clear from the study that the included human cases with various implant infections were DAIR (Debridement, Antibiotics, and Implant Retention) failed cases in which the infection recurred. DAIR is considered an appealing treatment option, particularly for cases of acute prosthetic joint infection (PJI), and shows the most promising outcomes.54 However, in all the reviewed case studies, DAIR played the role of only a complementary path to provide a clear target site for phage treatment.
Bacteriophages present novel benefits, such as their heightened specificity toward the host cell, mitigating harm to the patient’s normal microbiome, and diminishing colonization by other pathogens in the absence of in vivo drug interactions. Additionally, they exhibit bactericidal activity, minimal variability in pharmacokinetics and pharmacodynamics, unbiased bacterial targeting regardless of bacterial antibacterial susceptibility profiles, minimal environmental impact, and the potential to induce susceptible bacterial profiles.55
The dynamic evolution of phage resistance poses a challenge in phage therapy. The emergence of bacteriophage resistance through defense mechanisms and other strategies may impede the development of effective phage-based therapies. Phage resistance can be explained by various mechanisms. Bacteria can undergo evolutionary adaptations to modify or lose their phage receptors, thereby hindering phage attachment. These adaptations may involve structural alterations in the receptor protein or the complete elimination of the receptor itself, such as random genetic mutations or phenotypic variations in bacteria that lead to a reduced affinity for phage adsorption. Bacteria can produce restriction enzymes that recognize and cleave foreign DNA-like phage DNA. However, phages can evolve to evade restriction enzymes by modifying their DNA and protecting the bacteria by preventing the phage genome from integrating/replicating inside the host cell.56 Some bacteria produce proteins that directly inhibit phage adsorption, preventing the virus from attaching to the bacterial surface. The CRISPR-Cas system is a bacterial immune system that stores fragments of viral DNA in the bacterial genome and uses this information to recognize and defend against subsequent phage infections.57 Despite these challenges, ongoing research endeavors have sought to overcome phage resistance.
There are various other strategies for overcoming bacterial phage resistance. Bacteriophage (phage) cocktails have become a promising approach for addressing phage-resistant bacterial infections. The selection of phages that can identify distinct surface molecules is crucial for the effectiveness of phage cocktail therapy. Incorporating multiple phages that target different bacterial receptors could minimize the chances of bacteria acquiring resistance.58
Phage engineering techniques, such as gene editing using recombinant technology, are promising avenues for developing targeted therapies against multidrug-resistant bacterial infections. In this method, a DNA template sequence with homologous regions is introduced into host cells, facilitating the modification of bacteriophage DNA during subsequent infection of the bacterial host. The altered bacteriophage DNA, enclosed within the protein capsid, gives rise to engineered bacteriophage progeny.59 CRISPR-Cas technology has been employed to strengthen phage therapy by minimizing bacterial resistance and enhancing phage adaptability. Modified phages equipped with the CRISPR Cas system can specifically attack and deactivate bacterial genes involved in defence mechanisms, rendering bacteria more vulnerable to phage infection. Additionally, CRISPR-Cas can precisely cleave antibiotic-resistance genes within bacterial genomes, reinstating their sensitivity to antibiotic treatments.60
In a few studies, the combined action of antibiotics and phages against bacterial colonization has also been determined. Antibiotics alone can inhibit bacterial growth, however; the development of drug resistance is unavoidable. If a combination of bacteriophages and antibiotics is exposed, the immunomodulatory action of these agents will aid in inhibiting bacterial growth. In addition, it is hypothesized that the sequential exposure of bacterial cells to two selective pressures, bacteriophages, and antibiotics, will reduce the chances of the development of drug resistance.61 It is clear from a previous study that phages are capable of re-sensitizing bacterial cells to previously resistant antibiotics. Bacteriophages also have the potential to minimize biofilm production compared to a sub-inhibitory concentration of a particular antibiotic.62
Clinical and safety trials have consistently shown that utilizing naturally occurring phages for therapy through various administration routes is safe. It has been demonstrated from the reviewed articles that bacteriophage treatment successfully decreased bacterial levels, broke down biofilms, facilitated wound healing, and enhanced results.
Challenges
First, the challenge when opting for the phage treatment is phage selection and isolation. An accurate species-specific selection of bacteriophages alone can combat bacterial infections.61 Even when using a bacteriophage from a phage library, it must show sensitivity to the test bacterial strain. Although phage therapy can inhibit bacterial infection, timely identification and preparation of phage suspensions must be achieved with no delay. Personalized phage preparation is considered superior because it provides strain specificity.
The route of phage administration becomes more challenging when associated with implant devices. In a case series reported from the Berlin Heart Center, phage therapy did not work for one out of 4 patients, as there was a complication in delivering the phages to the LVAD driveline-infected area and the development of a phage-resistant strain of bacteria.41 An inherent challenge in phage therapy revolves around the potential for strains to evolve and develop resistance to phages used for treatment.
Determining the phage dosage to be used in therapy is a task. It is the multiplicity of phage infection, which is defined as the ratio of phages to bacteria, in which only those phages that have attached to, the infected bacteria are considered. Hence, the adsorption of phages, the susceptibility of target bacteria to phages, and the density of target bacteria are pivotal factors in the practical application of phage therapy. However, it must be noted that the FDA-recommended endotoxin limitation for the intravenous route is 5EU/kg of body weight/h. Hence, the determination of endotoxin level is also a challenge.
Limitations
To prevent bacterial colonization and biofilm formation on indwelling devices, efforts must be made to coat or impregnate these medical devices with antimicrobial agents. Thus, phage-coated devices can be used to prevent the initial adherence of bacteria. The delivery or coating of substance must allow the slow and sustainable release of bacteriophages at the target site. There is not much data available in the literature on the coating techniques. However, there are a few other studies that describe the antibiofilm activity of bacteriophages impregnated on the devices.40,64,65 In summary, owing to a lack of enough published data and clinical trials available on phage therapy, this review article aims to draw the attention of scientists worldwide to pursue further research centered on phage therapy.
Phage therapy offers an alternate non-antibiotic method, employing bacteriophages effectively coated on medical devices to inhibit biofilm formation and mitigate antibiotic resistance in a lasting manner. Due to their specificity towards host cells, extensive libraries of phages are necessary to personalize treatments. In addition to the considerable variation in the methods employed to evaluate phage-biofilm interactions, the biological properties of phages and the physical properties of medical devices have emerged as key factors influencing the efficacy of biofilm control through phage interventions. Given the rising crisis of antimicrobial resistance, phage therapy is expected to provide a valuable adjunct or alternative therapeutic option, particularly in clinical cases of medical indwelling device infections in which biofilm-based antibiotic insensitivity is present. In this systematic review, we analyzed reports on phage characteristics, efficacy, and safety of phage therapy for medical device-associated infections. Efforts must be made to develop standardized and reproducible methods for coating indwelling devices with bacteriophages to ensure their long-lasting and effective action. In addition, larger, well-designed clinical trials are required to determine the clinical effectiveness and safety of phage therapy.
ACKNOWLEDGMENTS
The authors would like to thank K.S. Hegde Medical Academy, Nitte (Deemed to be University), Mangalore, India, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
NKR and VS conceptualized the study, performed data curation and formal analysis. VS performed project administration, collected resources and supervised the study. NKR wrote the original draft. PSR wrote and revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Scott VEJ, Younger JG. Implantable device-related infection. Shock. 2016;46(6):597-608.
Crossref - Di Martino P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018;4(2):274-288.
Crossref - Rather MA, Gupta K, Mandal M. Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz J Microbiol. 2021;52(4):1701-1718.
Crossref - Vestby LK, Gronseth T, Simm R, Nesse LL. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics. 2020;9(2):9020059.
Crossref - Schulze A, Mitterer F, Pombo JP, Schild S. Biofilms by bacterial human pathogens: Clinical relevance – Development, composition and regulation – Therapeutical strategies. Microbial Cell. 2021;8(2):28-56.
Crossref - Jamal M, Ahmad W, Andleeb S, et al. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81(1):7-11.
Crossref - Mendelson M, Sharland M, Mpundu M. Antibiotic resistance: Calling time on the “silent pandemic.” JAC Antimicrob Resist. 2022;4(2):dlac016.
Crossref - Torjesen Ingrid. Antimicrobial resistance will lead to an additional 10 million deaths each year worldwide by 2050. Pharm J. 2014.
- Jeske A, Arce-Rodriguez A, Thoming JG, Tomasch J, Haussler S. Evolution of biofilm-adapted gene expression profiles in lasR-deficient clinical Pseudomonas aeruginosa isolates. NPJ Biofilms Microbiomes. 2022;8(1):6.
Crossref - Sauer K, Stoodley P, Goeres DM, et al. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat Rev Microbiol. 2022;20(10):608-620.
Crossref - Prinzi A, Rohde R. The role of Bacterial Biofilms in Antimicrobial Resistance. American Society for Microbiology, 2023.
- Wu H, Moser C, Wang HZ, Hoiby N, Song ZJ. Strategies for combating bacterial biofilm infections. Int J Oral Sci. 2015;7(1):1-7.
Crossref - Arciola CR, Campoccia D, Montanaro L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16(7):397-409.
Crossref - Schoeffel J, Wang EW, Gill D, et al. Successful Use of Salvage Bacteriophage Therapy for a Recalcitrant MRSA Knee and Hip Prosthetic Joint Infection. Pharmaceuticals. 2022;15(2):177.
Crossref - Uruen C, Chopo-Escuin G, Tommassen J, Mainar-Jaime RC, Arenas J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics. 2020;10(1):3.
Crossref - Dincer S, Uslu FM, Delik A. Antibiotic Resistance in Biofilm. In: IntechOpen eBook 2020.
Crossref - Ciarolla AA, Lapin N, Williams D, Chopra R, Greenberg DE. Physical Approaches to Prevent and Treat Bacterial Biofilm. Antibiotics. 2023;12(1):12010054.
Crossref - Rodriguez-Merchan EC, Davidson DJ, Liddle AD. Recent strategies to combat infections from biofilm-forming bacteria on orthopaedic implants. Int J Mol Sci. 2021;22(19):221910243.
Crossref - Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. 2020;19(5):311-332.
Crossref - Hu X, Huang YY, Wang Y, Wang X, Hamblin MR. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front Microbiol. 2018;9:1299.
Crossref - Ji QY, Wang W, Yan H, et al. The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli. Foods. 2023;12(16):3011.
Crossref - Wang S, Zhao Y, Breslawec AP, et al. Strategy to combat biofilms: a focus on biofilm dispersal enzymes. NPJ Biofilms Microbiomes. 2023;9(1).
Crossref - Khalid S, Gao A, Wang G, Chu PK, Wang H. Tuning surface topographies on biomaterials to control bacterial infection. Biomater Sci. 2020;8(24):6840-6857.
Crossref - Al-Wrafy FA, Al-Gheethi AA, Ponnusamy SK, Noman EA, Fattah SA. Nanoparticles approach to eradicate bacterial biofilm-related infections: A critical review. Chemosphere. 2022;288(Pt 2):132603.
Crossref - Qiu Z, Yu Y, Chen Z, et al. Nanoalumina promotes the horizontal transfer of multiresistance genes mediated by plasmids across genera. Proc Natl Acad Sci U S A. 2012;109(13):4944-4949.
Crossref - Brives C, Pourraz J. Phage therapy as a potential solution in the fight against AMR: obstacles and possible futures. Palgrave Commun. 2020;6(1):100.
Crossref - Keen EC. A century of phage research: Bacteriophages and the shaping of modern biology. BioEssays. 2015;37(1):6-9.
Crossref - Romero-Calle D, Benevides RG, Goes-Neto A, Billington C. Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics. 2019;8(3):138.
Crossref - Al-Ishaq RK, Skariah S, Busselberg D. Bacteriophage treatment: Critical evaluation of its application on world health organization priority pathogens. Viruses. 2021;13(1):v13010051.
Crossref - Gorski A, Miedzybrodzki R, Lobocka M, et al. Phage therapy: What have we learned? Viruses. 2018;10(6):288
Crossref - Jiang Z, Wei J, Liang Y, Peng N, Li Y. Aminoglycoside antibiotics inhibit mycobacteriophage infection. Antibiotics. 2020;9(10):1-6.
Crossref - Chang C, Yu X, Guo W, et al. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front Microbiol. 2022;13:825828.
Crossref - Knecht LE, Veljkovic M, Fieseler L. Diversity and Function of Phage Encoded Depolymerases. Front Microbiol. 2020;10:2949.
Crossref - Abdelrahman F, Easwaran M, Daramola OI, et al. Phage-encoded endolysins. Antibiotics. 2021;10(2):124.
Crossref - Islam MZ, Fokine A, Mahalingam M, et al. Molecular Anatomy of the Receptor Binding Module of a Bacteriophage Long Tail Fiber. PLoS Pathog. 2019;15(12):1008193.
Crossref - Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ. 2021;372:n71.
Crossref - Chen X, Thomsen TR, Winkler H, Xu Y. Influence of biofilm growth age, media, antibiotic concentration and exposure time on Staphylococcus aureus and Pseudomonas aeruginosa biofilm removal in vitro. BMC Microbiol. 2020;20(1):264.
Crossref - Pires DP, Melo LDR, Vilas Boas D, Sillankorva S, Azeredo J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr Opin Microbiol. 2017;39:48-56.
Crossref - Amankwah S, Adisu M, Gorems K, Abdella K, Kassa T. Assessment of Phage-Mediated Inhibition and Removal of Multidrug-Resistant Pseudomonas aeruginosa Biofilm on Medical Implants. Infect Drug Resist. 2022;15:2797-2811.
Crossref - El-Atrees DM, El-Kased RF, Abbas AM, Yassien MA. Characterization and anti-biofilm activity of bacteriophages against urinary tract Enterococcus faecalis isolates. Sci Rep. 2022;12(1):1-11.
Crossref - Tkhilaishvili T, Potapov E, Starck C, et al. Bacteriophage therapy as a treatment option for complex cardiovascular implant infection: The German Heart Center Berlin experience. J Heart Lung Transplant. 2022;41(5):551-555.
Crossref - Ferry T, Kolenda C, Batailler C, et al. Phage Therapy as Adjuvant to Conservative Surgery and Antibiotics to Salvage Patients With Relapsing S. aureus Prosthetic Knee Infection. Front Med (Lausanne). 2020;7:1-9.
Crossref - Doub JB, Chan B, Johnson AJ. Salphage: Salvage bacteriophage therapy for a chronic Enterococcus faecalis prosthetic joint infection. IDCases. 2023;33:e01854.
Crossref - Doub JB, Ng VY, Johnson AJ, et al. Salvage bacteriophage therapy for a chronic MRSA prosthetic joint infection. Antibiotics. 2020;9(5):241.
Crossref - Doub JB, Ng VY, Wilson E, Corsini L, Chan BK. Successful treatment of a recalcitrant Staphylococcus epidermidis prosthetic knee infection with intraoperative bacteriophage therapy. Pharmaceuticals. 2021;14(3):14030231.
Crossref - Mulzer J, Trampuz A, Potapov EV, Potapov EV. Treatment of chronic left ventricular assist device infection with local application of bacteriophages. Eur J Cardiothorac Surg. 2020;57(5):1003-1004.
Crossref - Lungren MP, Donlan RM, Kankotia R, et al. Bacteriophage K antimicrobial-lock technique for treatment of Staphylococcus aureus central venous catheter-related infection: A leporine model efficacy analysis. J Vasc Interv Radiol. 2014;25(10):1627-1632.
Crossref - Gulyaeva A, Garmaeva S, Kurilshikov A, et al. Diversity and Ecology of Caudoviricetes Phages with Genome Terminal Repeats in Fecal Metagenomes from Four Dutch Cohorts. Viruses. 2022;14(10):14102305.
Crossref - Friman VP, Soanes-Brown D, Sierocinski P, et al. Pre-adapting parasitic phages to a pathogen leads to increased pathogen clearance and lowered resistance evolution with Pseudomonas aeruginosa cystic fibrosis bacterial isolates. J Evol Biol. 2016;29(1):188-198.
Crossref - Rotman SG, Sumrall E, Ziadlou R, et al. Local Bacteriophage Delivery for Treatment and Prevention of Bacterial Infections. Front Microbiol. 2020;11:538060.
Crossref - Vinner GK, Vladisavljeviז GT, Clokie MRJ, Malik DJ. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLoS One. 2017;12(10):0186239.
Crossref - Kielholz T, Rohde F, Jung N, Windbergs M. Bacteriophage-loaded functional nanofibers for treatment of P. aeruginosa and S. aureus wound infections. Sci Rep. 2023;13(1):8330.
Crossref - Tkhilaishvili T, Winkler T, Muller M, Perka C, Trampuz A. Bacteriophages as Adjuvant to Antibiotics for the Treatment of Periprosthetic Joint Infection Caused by Multidrug-Resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2020;64(1):1-5.
Crossref - Barros LH, Barbosa TA, Esteves J, Abreu M, Soares D, Sousa R. Early Debridement, antibiotics and implant retention (DAIR) in patients with suspected acute infection after hip or knee arthroplasty – safe, effective and without negative functional impact. J Bone Jt Infect. 2019;4(6):300-305.
Crossref - C Duplessis, B Biswas, B Hanisch, et al. Refractory Pseudomonas Bacteremia in a 2-Year-Old Sterilized by Bacteriophage Therapy. J Pediatric Infect Dis Soc. 2018;7(3):253-256.
Crossref - Vasu K, Nagamalleswari E, Nagaraja V. Promiscuous restriction is a cellular defense strategy that confers fitness advantage to bacteria. Proc Natl Acad Sci U S A. 2012;109(20):1119226109.
Crossref - Nami Y, Rostampour M, Panahi B. CRISPR-Cas systems and diversity of targeting phages in Lactobacillus johnsonii strains; insights from genome mining approach. Infect Genet Evol. 2023;114:105500.
Crossref - Yoo S, Lee KM, Kim N, Vu TN, Abadie R, Yong D. Designing phage cocktails to combat the emergence of bacteriophage-resistant mutants in multidrug-resistant Klebsiella pneumoniae. Microbiol Spectr. 2024;12(1):e0125823.
Crossref - Jia HJ, Jia PP, Yin S, Bu LK, Yang G, Pei DS. Engineering bacteriophages for enhanced host range and efficacy: insights from bacteriophage-bacteria interactions. Front Microbiol. 2023;14:1172635.
Crossref - Gencay YE, Jasinskyte D, Robert C, et al. Engineered phage with antibacterial CRISPR-Cas selectively reduce E. coli burden in mice. Nat Biotechnol. 2024;42(2):265-274.
Crossref - Torres-Barcelo C, Hochberg ME. Evolutionary Rationale for Phages as Complements of Antibiotics. Trends Microbiol. 2016;24(4):249-256.
Crossref - Kenjar A, Udayalaxmi J, Suman E, Kotian SM, Samson HP. Effect of bacteriophage and sub-inhibitory concentration of imipenem on biofilm production by Pseudomonas aeruginosa on endotracheal tubing – An in-vitro model system. European Journal of Molecular and Clinical Medicine. 2021;8(2):1998-2008.
- Sanchez BC, Heckmann ER, Green SI, et al. Development of Phage Cocktails to Treat E. coli Catheter-Associated Urinary Tract Infection and Associated Biofilms. Front Microbiol. 2022;13:1-15.
Crossref - Oliveira VC, Macedo AP, Melo LDR, et al. Bacteriophage cocktail-mediated inhibition of Pseudomonas aeruginosa biofilm on endotracheal tube surface. Antibiotics. 2021;10(1):78.
Crossref - Mirzaei A, Wagemans J, Nasr Esfahani B, Lavigne R, Moghim S. A Phage Cocktail To Control Surface Colonization by Proteus mirabilis in Catheter-Associated Urinary Tract Infections. Microbiol Spectr. 2022;10(5):1-11.
Crossref - Lehman SM, Donlan RM. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob Agents Chemother. 2015;59(2):1127-1137.
Crossref - Melo LDR, Veiga P, Cerca N, et al. Development of a phage cocktail to control Proteus mirabilis catheter-associated urinary tract infections. Front Microbiol. 2016;7:1024.
Crossref - Morris J, Kelly N, Elliott L, et al. Evaluation of bacteriophage anti-biofilm activity for potential control of orthopedic implant-related infections caused by Staphylococcus aureus. Surg Infect. 2019;20(1):16-24.
Crossref - Oliveira VC, Bim FL, Monteiro RM, et al. Identification and Characterization of New Bacteriophages to Control Multidrug-Resistant Pseudomonas aeruginosa Biofilm on Endotracheal Tubes. Front Microbiol. 2020;11:1-12.
Crossref - Kaur S, Harjai K, Chhibber S. In Vivo Assessment of Phage and Linezolid Based Implant Coatings for Treatment of Methicillin Resistant S. aureus (MRSA) mediated orthopaedic device related infections. PLoS One. 2016;11(6):e0157626.
Crossref - Mannala GK, Rupp M, Walter N, et al. Microbiological and ultrastructural evaluation of bacteriophage 191219 against planktonic, intracellular and biofilm infection with Staphylococcus aureus. Eur Cell Mater. 2022;43:66-78.
Crossref - Joo H, Wu SM, Soni I, et al. Phage and Antibiotic Combinations Reduce Staphylococcus aureus in Static and Dynamic Biofilms Grown on an Implant Material. Viruses. 2023;15(2).
Crossref - Cano EJ, Caflisch KM, Bollyky PL, et al. Phage Therapy for Limb-threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In vitro Characterization of Anti-biofilm Activity. Clin Infect Dis. 2021;73(1):E144-E151.
Crossref - Materazzi A, Bottai D, Campobasso C, et al. Phage-Based Control of Methicillin Resistant Staphylococcus aureus in a Galleria mellonella Model of Implant-Associated Infection. Int J Mol Sci. 2022;23(23):14514.
Crossref - Renukaprasad AK, Suman E, Jeppu U, Paul SH. Promising catheters to reduce hospital-acquired urinary tract infections. Biomedicine. 2023;43(3):992-995.
Crossref - Racenis K, Lacis J, Rezevska D, et al. Successful Bacteriophage-Antibiotic Combination Therapy against Multidrug-Resistant Pseudomonas aeruginosa Left Ventricular Assist Device Driveline Infection. Viruses. 2023;15(5):1210.
Crossref - Rakov C, Poratc SB, Alkalay-Oren S, et al. Targeting biofilm of MDR Providencia stuartii by phages using a catheter model. Antibiotics. 2021;10(4):375.
Crossref - Rojas SV, Junghans S, Fox H, et al. Bacteriophage-Enriched Galenic for Intrapericardial Ventricular Assist Device Infection. Antibiotics. 2022;11(5):602.
Crossref - Townsend EM, Moat J, Jameson E. CAUTI’s next top model – Model dependent Klebsiella biofilm inhibition by bacteriophages and antimicrobials. Biofilm. 2020;2:100038.
Crossref - Nzakizwanayo J, Hanin A, Alves DR, et al. Bacteriophage can prevent encrustation and blockage of urinary catheters by Proteus mirabilis. Antimicrob Agents Chemother. 2016;60(3):1530-1536.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.