Microbes play a crucial role for us and are present everywhere, such as in human and plant microbiota, and in each ecosystem, they have various medicinal and industrial applications to ease our livelihood. The microbes act as catalyst to enhance useful content of plants such as secondary metabolites and also ameliorate the health, growth and development of the plant. Microbes are eco-friendly in nature, they are effective and low-cost in production of valuable things. Several studies revealed that microbes induced plants are excellent modulator of secondary metabolites of human interest. In this review article, the authors try to compile various microorganisms which are key enhancer of natural antioxidant compounds in plants. This study based on beneficial plant microbes that enhance the plant’s growth and also enhance the content of biologically active compounds in the host plants. Among the microbes, some bacteria, actinobacteria, cyanobacteria and fungi were excellent modulator of secondary metabolites present in the plant. Additionally, they improve plant health and development and also protect from various infections and stresses. This study concluded that among the microbes, bacteria and fungi showed enhancement in flavonoids, phenolics and carotenoid content and how they modulate their metabolite content after microbial inoculation in plants; and also increase their immunity against biotic and abiotic stresses, whereas actinobacteria enhanced growth factors which increase the defence mechanism of plants against various infections.
Microbes, Induced, Plant, Secondary Metabolites, Carotene, Flavonoid
Nowadays, we are frequently using chemical fertilizers and harmful synthetic chemicals for crop productivity that causes deforestation, damage agricultural resources degrading land, salinizing irrigated fields, and leading to over-extraction of groundwater. This study introduces some beneficial plant microbes that enhance growth and productivity of plant and also increase the content of plant secondary metabolites and higher biologically active compounds content to improve the quality of life. Microorganisms are at the top in attention by researchers because of their high caliber to synthesize novel secondary metabolites which are helpful in plant disease management and human beings.1-3
Microbial inoculation is a low-cost commercial, agricultural input and it also helps to decrease environmental pollution.4,5 The major microbes-mediated defense mechanism acquired by plants include the activation of antioxidants of the plant by the catalyst like defense-related enzymes.5 It seems that microbes are frequently used in the form of bio-fertilizers and bio-pesticides to improve the quality and quantity of useful content.6 Inoculation of microbes in soil used as bio-fertilizer to decrease the consumption of chemical fertilization, these bio-fertilizers are more effective and cost-saving.7 The hiring of biological solutions for crop production and protection is necessary due to the rising costs and detrimental effects of chemical fertilizers and pesticides for agricultural production. These pesticides affect our immune system and reduced consumption of unrenewable resources. The PGP microbes inoculation in plants frequently used to improve plant health. This method has been emerged as one of the most alluring methods for creating sustainable agricultural practices.8 Now days to improve the cost efficiency of plants, PGP microbes are used as biological nutrients to enhance nutrients content in plants and also increase the crop productivity, physiological immunity, and defense capacity of plants.6,9
Microbes are present everywhere in the biosphere, and their presence always affects the environment in which they grow. Among the all microbes, the ability of bacteria to recycle the basic components that comprise biosphere such as carbon, oxygen, and nitrogen, is their most significant impact on the planet. Bacteria are small single-celled organisms, and they support many forms of life such as plant and animal, in which they can rapidly grow.10 In the plant species bacteria are present into two types on the basis of niche, one is as an endophyte, which are residing in the inner part of the plant species. Another is rhizobacteria or root-associated bacteria, which are present in the rhizospheric region, and in the adhered soil of the plant. Both types of bacteria are beneficial for plant.11 Besides these microbes, fungi also play a crucial role in the plant health management. Fungi belong to eukaryotes and they are heterotrophic organisms that derive their food from non-living organic sources.12 They are incredibly diverse and have a variety of functions in the environment. They act as decomposers, mutualists, and predators.13 They are also chemoorganotrophs that grow aerobically on simple carbohydrates and amino acids when oxygen is present.14
The roots of plants secrete a variety of exudates in their rhizosphere that are well off in monomers. These exudates affect the actively microbial populations in the plant root which either directly or indirectly aid in the promotion of growth and disease or stress management.15 Among the microbes, cyanobacteria also play a crucial role in the plant health management and secondary metabolite enhancement. Cyanobacteria are present in the water ecosystem and also photosynthetic in nature.16 Usually, water serves as an electron donor during photosynthesis, which causes oxygen to evolve. The cyanobacteria are strong fixer of atmospheric N2, decompose organic wastes, detoxify pesticides and heavy metals, catalyze the nutrients cycling, inhibit the growth of microbial pathogens in aquatic ecosystem, and also synthesize some biological substances such as vitamins, hormones, and enzymes that support plant health and development.17,18
Among the secondary metabolites antioxidants are key components, they serve as immunity boosters for us. An antioxidant is a biological compound that inhibits oxidation.19,20 These antioxidants may play a crucial role in preventing carcinogens and heart disease and enough level of antioxidants enhance immunological processes and increase defensive abilities.21,22
These antioxidants are crucial in scavenging ROS (reactive oxygen species) and preventing the harmful effects of oxidative stress on most sensitive molecules like proteins, lipids, and nucleic acids.23 Recently increased interest of researchers in antioxidants because of their high capacity against various diseases, nutritional properties, and therapeutic effects. Antioxidants are mostly found in medicinal plants and dietary products that may help in less effect of oxidative damage and provide many health benefits to us.22,24
Antioxidants like polyphenolics, ascorbic acid, carotenoids, terpenoids, and tocopherol compounds are found in plants. Among all antioxidants, carotenoids have essential molecules for light harvesting and also give photo-protection and pigmentation to the plants and their products.25 These carotenoids are responsible for the colour of leaves, flowers, and food.26 The conjugated series of C=C bonds is the core structure of carotenoids and also it can interact with singlet oxygen species and it also act as a strong antioxidant.27 Carotenes serve as a precursor molecule for vitamin A synthesis. Vitamin is an antioxidant that is only available in food and is believed to help prevent some diseases such as night blindness.28 Plants, fungi, algae, and bacteria can synthesize carotenoids. Intake of carotenoids decreases the risk of cancer, and cardiovascular or ophthalmological diseases and also protecting cells and tissues from oxidative damage.26 Among the carotenoids, lycopene act as major antioxidants and anti-carcinogenic compound found in the Solanum lycopersicum L. fruits. In a study some endophytic bacterial strains such as Bacillus cereus, B. flexus, Pseudomonas aeruginosa, Methylophillus flavus and Rhizobium pusense inoculated in the Solanum lycopersicum L. plants, the content of lycopene and some secondary metabolites such as phenol, flavonoid increased many folds after inoculation with tomato seed and seedling when compared with control.29 In another study inoculation of two bacterial gene viz. CrtB gene (phytoene synthase) and CrtI gene (carotene desaturase) enhanced the vitamin A content in wheat (Triticum aestivum L.).30
In plants, the phenolic compound also acts as an antioxidant by donating electrons to highly reactive oxygen species, i.e. guaiacol peroxidase for detoxification of peroxide produced under stress phase and also influences host plant growth. Phenolic compounds have caliber to inhibit several enzymes which are associated with the development of human diseases, including hypertension, and neurodegenerative diseases. It also has anti-inflammatory properties to treat various skin diseases.31,32 Materials like spices, herbs, cereals, seeds, fruit or vegetable products are rich in phenolic compounds.22
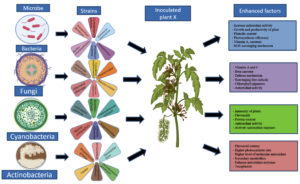
Besides this, selenium is an antioxidant; it has capability to breakdown peroxides which can damage tissue and DNA. It is a strong element in terms of antioxidant enhancement. In a study influencing plant metabolism, particularly antioxidant activity, simultaneous usage of Selenium or micronutrients can reduce the detrimental effects of biotic stress on corn output.33 In another study, Brassica juncea L. plants were inoculated with rhizospheric bacteria, had five times higher Selenium content in roots when compared with control, it seems that this selenium content might play’s crucial role as an antioxidant34 (Figure 1).
Figure 1. Graphical representation of the microbial role in plants to enhance their metabolite content, which can act as an antioxidant
Role of various microbes to enhance the content of plant’s antioxidants
Antioxidant enhancement in plants via bacteria
Bacteria are excellent modulator of bioactive compounds in the plants. Some beneficial microbes can enhance crop productivity and also protect the host plant from various stresses via a variety of mechanisms. Microbial inoculation in the plants modulates the biologically active compounds synthesis such as antioxidants with high medicinal properties, several microorganisms enhanced the content of bioactive compounds after microbial inoculation, which are discussed in Table.54,55 In a study results revealed that the effect of two bacterial strains (Pseudomonas fluorescens WCS417r and Bacillus amyloliquefaciens GB03) inoculated in peppermint plant enhanced the bioactivity of antioxidative enzymes like peroxidase (PX) and superoxide dismutase (SOD) and total phenolic content (TPC).36 In another study the impact of two bacterial strains Bacillus amylolequefaciens BChi1 and Paraburkholderia fungorum BRRh-4 when inoculated with strawberries, results revealed that bacterized plant have increased fruit quality and yield, and also enhance the antioxidant capacity in comparison with control.32 In many plants species such as rice, inoculation with few bacterial strains such as Pseudomonas, Rhizobium, Bacillus, Serratia, and Azospirillum sp. enhanced the crop productivity of plants and enhanced the enzymes which play key role to reduce salinity stress.40
Table:
Some microbe-induced plants and the enhancement of antioxidants after inoculation
No. |
Microbe |
Strain |
Species |
Enhanced compounds |
Ref. |
|---|---|---|---|---|---|
1. |
Bacteria |
Actinomycetes |
Date palm (Phoenix dactylifera L.) |
Antioxidant activity |
[35] |
2. |
Bacteria |
Paraburkholderia fungorum BRRh-4, Bacillus amylolequefaciens BChi1 |
strawberry fruit (Fragaria x ananassa) |
Antioxidants |
[32] |
3. |
Bacteria |
Rhizobacteria, Pseudomonas fluorescens sp., and Bacillus amyloliquefaciens sp., |
Peppermint (Mentha piperita) |
Antioxidant enzymes i.e. peroxidase (PX) and superoxide dismutase (SOD) |
[36] |
4. |
Bacteria |
Pseudomonas sp.,Rhizobium sp., Bacillus sp. |
Rice (Oryza sativa L.) |
Activating antioxidant defense system |
[37] |
5. |
Bacteria |
Bacillus subtilis BCRC |
Black soybeans (Glycine max) |
antioxidant activity, total phenolic and flavonoid |
[21] |
6. |
Bacteria |
Pseudomonas aeruginosa |
Devil’s Horsewhip(Achyranthes aspera L.) |
Antioxidant Activity |
[38] |
7. |
Bacteria |
Pseudomonas sp., Azotobacter sp. and Arbuscular Mycorrhiza Fungi |
Tomato (Solanum lycopersicum) |
Carotenoids constituents (lycopene) |
[39] |
8. |
Bacteria |
Azospirillum lipoferum, Azotobacter chroococcum |
Maize (Zea mays) |
Antioxidative enzyme activities, carotenoids, peroxidase (POD) and catalase (CAT). |
[40] |
9. |
Bacteria |
Pseudomonas aeruginosa and Bacillus megaterium |
Maize (Zea mays) |
Secondary metabolites |
[3] |
10. |
Bacteria |
Actinomycetes |
Leguminous seeds (Pea, chickpea soybean, kidney bean, lentil) |
Boosting Nitrogen, metabolism, primary and secondary metabolites |
[35] |
11. |
Cyanobacteria |
Desmonostoc muscorum, Anabaena oryzae, and Arthrospira platensis |
Pepper (Piper nigrum) |
Phenolic compounds |
[16] |
12. |
Cynobacteria |
Microalgae polysaccharides (Arthrospira platensis, Dunaliella salina, Porphyridium sp) |
Tomato (Solanum lycopersicum L.) |
Carotenoid |
[41] |
13. |
Arehaebacteria |
Haloarchaeal species strain |
Maize (Zea mays) |
Phenolics and tocopherols |
[42] |
14. |
Fungi |
Arbuscular mycorrhizal fungi
|
Egyptian Pea (Sesbania sesban L.) |
Catalase, Superoxide dismutase (SOD), ascorbate peroxidase (APX), |
[23] |
15. |
Fungi |
Arbuscular mycorrhizal fungi |
Sunflower (Helianthus Annuus L.) |
Inhibition of toxic ROS ( Reactive oxygen species) |
[43] |
16. |
Fungi |
Aspergillus flavus, Aspergillus niger, Mucor circinelloides |
Tomato (Solanum lycopersicum L.) |
Carotenoid, phenolic content |
[44] |
17. |
Fungi |
Arbuscular mycorrhizal fungi |
Green and red leaf lettuces (Lactuca sativa) |
Carotenoids, chlorophylls and tocopherol |
[45] |
18. |
Fungi |
Arbuscular mycorrhizal Fungi |
Lettuces (Lactuca sativa) |
Carotenoids, vitamin E and C |
[46] |
19. |
Fungi |
Arbuscular mycorrhizal fungi |
Marigold (Calendula officinalis) |
Phenols, flavonoids, carotenoids and β-carotene |
[47] |
20. |
Fungi |
Sclerotinia sclerotiorum |
Pea (Pisum sativum) |
Phenols |
[9] |
21. |
Fungi |
Arbuscular mycorrhizal fungi (AMF) |
Grape (Vitis vinifera L.) |
Carotenoid |
[48] |
22. |
Fungi |
Filamentous fungi(Aspergillus oryzae) |
Soybean koji |
Antioxidant capacity |
[19] |
23. |
Fungi |
Yeast glucan (as fungal elicitors |
Mungbean (VignaRadiata) |
Potential phenolic |
[49] |
24. |
Fungi |
Fungal elicitor |
Tipton Weed (Hypericum perforatum L.) |
Phenylpropanoid and naphtodianthrone |
[50] |
25. |
Fungi |
Trichoderma longibrachiatum T6 |
Wheat (Triticum astivam) |
Antioxidative Defense System |
[51] |
26. |
Algae |
Cylindrospermum sp. |
Great plantain (Plantago major L.) |
Total Flavonoid and Phenolic |
[52] |
27. |
Algae |
Chlorella zofingiensis |
Green Alagae (Chlamydomonas reinhardtii) |
Carotenoids, lutein |
[53] |
In a study, to obtain healthier and antioxidant-rich Achyranthes aspera L. plants were inoculated with Pseudomonas aeruginosa AL2-14B strain, resulted showed that the content of antioxidant increased also showed antifungal properties against Rhizoctonia solani pathogen.38
In a study paddy plants were inoculated with Curtobacterium albidum SRV4 strain, results showed that the content of antioxidantive enzymes viz. peroxidase, ascorbate peroxidase and catalase were enhanced significantly.56 Beneficial bacterial strains Azospirillum lipoferum and Azotobacter chroococcum increased the plant physiological activity and also antioxidant activity in maize by enhancing the ROS scavenging mechanism and increasing the selectivity of sodium and potassium ions absorption by the plant, authors were able to reduced stress brought on by the saline circumstances and boost K+ uptake while restricting Na+ absorption.40 Pseudomonas fluorescence is a rhizobacterial strain inoculated in rice (Oryza sativa L.), it activated an antioxidant defence system and the majority of antioxidant enzymes such as ascorbate peroxidase, superoxide dismutase and catalase, content enhanced after inoculation.37
Inoculation of fungi in plants for the enhancement of phenolic and carotenoid content
Some fungi are single-celled microorganisms which are beneficial for the crops and vegetables, due to their unique medicinal and economical features. Some PGP fungi such as Fusarium have several mechanisms to control plant mycopathogens via antioxidant compounds synthesis that recover the oxidative burst to ameliorate plant defence, combat the mycotoxin synthesis. In a study, Inonotus obliquus (Chaga), the medicinal fungus produces a phenolic compound that is hispidin and melanin analogues. This hispidin analogues shows the defence response of plants and boost the stress tolerance capacity.57 Inoculation of some fungal strains such as (Alternaria alternata) in certain plants increases the productivity of useful secondary metabolites in plant cells, and also enhance the content of phenolic compounds and their mechanism of action.44 Carotenoid compounds are key components of our human body; they provide essential vitamins to the body to catalyze the proper functions of cells. In a study arbuscular mycorrhizal fungi (AMF) was inoculated in tomato plants the phenolic content was increased many folds.45 In another study Arbuscular mycorrhizal fungi induced in lettuce (Lactuca sativa) to activate their antioxidant compounds like carotenoids, vitamins E, and C to enhance (ROS) reactive oxygen species and immunity of the plant.46 Arbuscular mycorrhizal fungus was inoculated with Calendula officinalis L. (Marigold) after inoculation it increases its antioxidant activity, and it exhibits the highest vitamin A value (β-carotene). Because of the linear link between antioxidant activity and phenolic content. Phenolic compounds may have a significant role in the production of antioxidant activity.47 Symbiotic relationships between plants and AM fungus can encourage the production of significant secondary metabolites in herbs and increase their potential for antioxidants.46 Besides the fungus, yeasts are single-celled microorganisms and play significant role in the antioxidant accumulation. In a study mung bean (Vigna radiata) plants were treated with yeast glucan (as fungal elicitors), xanthan gum, and yeast extract, and microbial polysaccharide inoculation observed that the enhancement of phenolic content and antioxidant activity.49 In a pot experiment of marigold flowers, AM fungus encouraged the production of significant secondary metabolites (phenols, flavonoids, and carotenoids), which improved the flower’s antioxidant potential. The primary specified elements of the mycorrhizal plant’ carotenoid profiles differed significantly from one another (lutein, lycopene, β-carotene). The marigold inoculated with Funneliformis mosseae contained the highest levels of β-carotene (vitamin A) and lycopene.47 In a study the enhancement of carotenoid in grapes obtained due to inoculation of Arbuscular mycorrhizal fungi, which is responsible for photosynthesis, and also they contributed as antioxidants by scavenging the free radicals in plant systems and protecting them from being damaged by oxidative stress and diseases.48 Carotenoids are pigments that serve as light-harvesting pigments and aid in the process of photosynthesis. By scavenging free radicals in plant systems and defending them from oxidative stress and diseases, they act as antioxidants. When mycorrhizal fungal strains were inoculated into grape (Vitis vinifera L.) plantlets, the carotenoid content increased many folds.48
Inoculation of arbuscular mycorrhizal fungi (AMF) in sunflower (Helianthus annuus L.) plants showed an increase in their antioxidant activity which helps in the quick scavenging of toxic ROS therefore avoiding the oxidative stress and enhances the productivity of plants.43 Treatment with fungal elicitors Botrytis cinerea, Phoma exigua, and Fusarium oxysporum enhanced the enzymatic and non-enzymatic antioxidants which help in scavenging of ROS and maintain physiological redox status of plant cells and provide protection of plant.50
Arbuscular mycorrhizal fungus also showed significant modification with salinity treatment in terms of antioxidant enzymes, as well as non-enzymatic antioxidants (ascorbic acid and glutathione). Solanum lycopersicum L. has previously reported that inoculating with AMF caused an increase in the production of chlorophyll pigments. AMF vaccination boosted the antioxidant’s activity. Increased antioxidant activity aids plants in removing harmful ROS quickly to maintain steady metabolism and increased antioxidant enzyme activity in AMF inoculated plants.44 Zhang et al., stated that when Trichoderma longibrachiatum T6 inoculated in wheat it showed improvement of the antioxidant defence system and enzymes as well as content i.e peroxidase (POD), and catalase (CAT), superoxide dismutase (SOD) were increased in wheat seedlings.51
Inoculation of microalgae and cyanobacteria for antioxidants enhancement in plant
Microalgae and cyanobacteria are considered as natural sources of biologically and pharmacologically active compounds.58 Cyanobacteria liberate many kinds of biologically active substances like vitamins which help in the growth of plants and stimulate antioxidant activity which enhances their immunity against biotic and abiotic stress.59 Spirulina is nutrient rich cyanobacteria. Spirulina platensis showed enhancement or induction of biologically active compounds.58 Bell pepper plant treated with cyanobacteria Roholtiella sp. enhanced the antioxidant activity (CAT).60
Additionally, MEF5% (microalgae-cyanobacteria extract) extracts improved SOD, POD, and CAT ROS scavenging enzyme activities. Plants activate their ROS-scavenging systems to lessen the oxidative damage outcome of by excessive ROS buildup.61 The capacity of these antioxidant mechanisms to maintain ROS equilibrium boosts plant’s resistance to salt stress. Previous research has shown that the detoxification of ROS by antioxidant enzymes including CAT, SOD, and POD reduces oxidative damage.62 In the hybrids of maize show enhanced peroxidase activity and yield by inoculation of consortia such as A4 (Anabaena-Azotobacter biofilm) and A1 (Anabaena sp.-Providencia sp., CW1 + PW5).63
Luteins are carotenoid compounds which protect plants from photo induced free radicals damages. In a study, Chlamydomonas reinhardtii isolated from green microalgae Chlorella zofingiensis (CzPSY) increases the content of carotenoids, violaxanthin and lutein after inoculation.64,53 The commercial value of carotenoids is very high due to their antioxidant property.65 Tomato plants (Solanum lycopersicum L.) treated with extract from Arthrospira platensis, Dunaliella salina, and Porphyridium sp. showed enhanced carotenoid content in tomato. Another cyanobacterial strain viz. Anabaena spp. inoculated in tomato plant, after inoculation it showed better plant growth and increased its antioxidant (β-carotene) content.66,41
The phenolic compounds and flavonoids are effective antioxidant sources. The use of cyanobacterial extract on way bread (Plantago major) medicinal plant showed significant results such as enhanced content of secondary metabolites, and bioactive compounds and also increased the plant growth.52 Cyanobacteria (Desmonostoc muscorum, Anabaena oryzae, and Arthrospira platensis) induced in pepper plants showed an active immune response against Fusarium wilt.16 In a study wheat was treated with microalgae Spirulina maxima and Chlorella ellipsoida results showed that increase in antioxidant and protein content.67 Inoculation of two cyanobacterial species (Nostoc entophytum and Oscillatoria angustissima) in pea plants increased photosynthetic pigments such as carotene which resulted in increased yield and quality of products.7
Archaea and actinobacteria inoculation in plants to enhance its antioxidant potential
Among the microbes actinobacteria are the strong PGPR, which enhance the plant’s growth, crop productivity, and soil fertility and also decrease pathogens interaction.68 Streptomyces sp. belong to the genus Actinomycetes and it is most occurring actinobacteria genus in soil, it is also used as biopesticides and biofertilizers for sustainable agriculture.20,35 Actinomycetes’s ability to support plant growth and defend against illnesses has been discovered; it to be correlated with their antioxidant capacities as well as their concentration of physiologically active chemicals such as phenols and flavonoids. Secondary metabolites aid in defending plants from microbial infections. Streptomycetes that are associated with mycorrhizae have been found to create bioactive compounds, including the antibiotics, which may have inhibitory effects on various bacterial and fungal plant diseases. The increase in soil fertility and better soil health were a result of the antioxidant capacity.35 In a study plant growth-promoting actinobacteria enhance the secondary metabolites production, increase flavonoid content, photosynthetic rate, and create a higher level of molecular antioxidant (total ascorbate, glutathione, tocopherols, phenolic acids, and flavonoids) in maize (Zea mays L.).69
In addition, the volatile oils like steroids, tannins and pyrocatechols present in plant extracts and essential oils of Summer savory (Satureja hortensis) used to treat various severe diseases such as carcinoma, heart diseases, diabetes mellitus. In this prospects, enhancing the growth and yield of secondary metabolites present in Satureja hortensis would improve its medicinal and nutritional values. To increase the secondary metabolite content of S. hortensis, researchers used actinobacteria Ac9 inoculation on summer savory (Satureja hortensis) plant to enhance the antioxidant activity, results revealed that antioxidant increased under water deficit condition.70 Streptomyces sp. playing key roles in the enhancement of secondary metabolites in several crops. Secondary metabolites, such as total ascorbate, glutathione, and tocophenols increased due to inoculation of Streptomyces sp. in maize plants. Whereas Streptomyces griseoluteus inceased the carotenoid content in bean plants (Phaseolus vulgaris L.).71,72
In a study bacterization was done with Pseudomonas putida, Azotobacter chroococcumin tomato plants which increased the antioxidant content, and also increased the yield and regulated defence mechanism.39 In another study through the stimulation of several antioxidative enzymes and phenolics in chickpeas, the Streptomyces sp. was also able to generate host-plant resistance.73 Endophytic bacteria strain Streptomyces sp. DBT34 isolated from 4 O’ clock (Mirabilis jalapa) plant inoculated in chickpea seedlings, it enhanced the content of antioxidant enzymes like superoxide dismutase (SOD) which have property to the detoxification of Reactive oxygen species, which provides a defense mechanism in plants.74 Enhancement in antioxidant enzymes like superoxide dismutase, ascorbate peroxidase, phenylalanine ammonia-lyase, guaiacol peroxidase, glutathione reductase, and polyphenol oxidase, and some phenolic compounds, present in leaves of chick peas which was treated with endophytic actinobacteria Streptomyces sp., it also act as host-plant resistance inducers against Botrytis cinerea in chickpea.73
In a study, the plant Date palm (Phoenix dactylifera L.) was treated with Actinobacteria, after inoculation of actinobacteria the biological activity like antioxidant which showed improvement in growth of fruits. Higher antioxidant levels limit the build-up of free radicals that could cause cell harm.75 In watermelon (Citrullus lanatus) plant authors induced salicylic acid which activates the antioxidant capacity and enhanced the cold tolerance capacity also.20 Two endophytic actinobacterial strains viz. Streptomyces zaomyceticus Oc-5 and Streptomyces pseudogriseolus isolated from medicinal plant Yellow Wood Sorrel. It shows anti-microbial potential against strains – Aspergillus niger, Pythium ultimum, Alternaria alternate, and Fusarium oxysporum and also showed strong free radical and scavenging activity.76
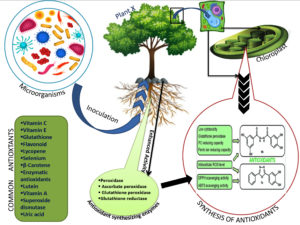
A Haloarchaeal species strain, belonging to the Haloferax genus inoculated in maize, its leaves showed an increased production of antioxidant metabolism (phenolics and tocopherols) and ROS scavenging enzyme capacity42 (Figure 2).
Figure 2. Schematic representation of mechanism of antioxidant synthesis in plants after microbial inoculation
Major medicinal plants that enhanced their antioxidant activity via microbes
The world’s most traditional kind of medicine has been secondary metabolites since ancient times. Medicinal plants have been employed since ancient times in many different traditional medical systems. The secondary metabolites are natural compounds, and a key source of natural drugs. Many modern therapeutic techniques that are developed from conventional medicinal plants are presently used in modern pharmacotherapy. Several medicinal plants bioactive compounds are currently using to cure serious diseases such as cancer. Microbes are key enhancer of these metabolites in medicinal plants. In a study Lemongrass (Cymbopogon citratus) treated with Pseudomonas and Azotobacter sp., plant growth promoting rhizobacterial strains. After bacterization the antioxidaive enzymes enhanced and their catalase (CAT), superoxide dismutase (SOD) activity, total flavonoid content (TFC) production were also enhanced.77 Using arbuscular mycorrhiza fungal species (Rhizoglomus irregular and Funneliformis mosseae) combined with Trichoderma atroviride biostimulants inoculated in tomato plant which increases its carotenoid compounds- b-carotene, z-carotene, total phenolic content, and total antioxidant activity.78 In another study some microbial strains Bacillus lentus, Azospirillum brasilens, Pseudomonas sp., and a consortia of these three bacterial strains used as PGPR, these were inoculated in basil (Ocimum basilicum L.) plant, after bacterization the plant health, antioxidant activity and chlorophyll content were increased several folds.79
Role of flavonoid in plants and their enhancement via microbial mechanism
A diverse family of plant polyphenolic compounds known as flavonoids are secondary metabolites and there are currently more than 8000 flavonoid compounds known and isolated from plants. Flavonoids have drawn a lot of attention recently in plant metabolite research because of their significant bioactivity linked to anti-cancer, antioxidant, anti-inflammatory, and anti-microbial activities.80,81 To maintain plant development and health, flavonoids play diverse functions for plant homeostasis and these are also involved in coping with biotic and abiotic challenges. More specifically, flavonoids play a role in stress-induced morphogenic responses in plants under several abiotic circumstances, such as salinity, drought, heat, cold, metal toxicity, the presence of xenobiotics, and UV radiation.82 Flavonoids influencing plant growth and development as well as defense mechanisms against biotic and abiotic stimuli, by controlling auxin polar transportation and male fertility in plants like rice (Oryza sativa), petunias (Petunia hybrid), tomatoes (Solanum lycopersicum L.), and maize (Zea mays).83 Flavonoids can be adsorbed to cation binding sites in soil, their solubility, structure, microbial availability, and binding sites may all affect how long they persist and move around in the rhizosphere. Flavonoid glycosides are the core form of flavonoids present in the plant. They are sparsely aqueous soluble and are anticipated to be less adsorbed to binding sites, improving mobility and availability. Additionally, environmental stresses, such as the availability of nutrients (nitrogen and phosphorus) in the soil, may affect the secretion of flavonoids.84 Flavonoids are released into the rhizosphere or the area around the plant’s roots. The release via root exudation is also linked to the intracellular transport and delivery of flavonoids. The exudation of flavonoids in the rhizosphere would either dynamically absorb onto soil organic matter or be quickly broken down by soil-dwelling microbes. Many of these interactions are effectively mediated by flavonoids. Perhaps best known for its role in the rhizobium nodulation process in plants of the Fabaceae family. Flavonoids have been found to either stimulate rhizobial nod gene expression, cause rhizobia to chemo attract to the root, inhibit phytopathogens, stimulate mycorrhizal hyphal branching and spore germination, mediate alelopathic interactions between plants, influence quorum sensing, and chelate soil nutrients, depending on the rhizosphere’s structure.83,85
Essential chemicals called flavonoids influence how plants grow and develop as well as how they defend themselves from biotic and abiotic stresses. By influencing pollen germination and pollen tube growth, they influence male fertility in plants.83 Cylindrospermum michailovskoense and Anabaena vaginicola induced in Plantago major L. showed the highest content of flavonoid.52 Flavonoids are important in the interactions between plants and microbes, and they can help bacteria more easily to colonize the roots.86 In the several studies it is found that the content of flavonoids increased many folds after microbial inoculation such as, Anabaena oryzae inoculated in rice plants to enhance its flavonoid content and PGP traits. Plantago major L. treated with cyanobacteria enhanced its flavonoid content in plants. Endophytic fungus isolated from Scutellaria baicalensis, identified as Fusarium and Alternaria, among these Alternaria sp. showed enhancement in flavonoid content in Scutellaria baicalensis. Inoculation of arbuscular mycorrhizal fungi in Trifoliate orange enhanced the flavonoid levels and also increased its stress tolerance in various adverse conditions of plant. In another study, Pseudarthrobacter sp. NIBRBAC000502770 showed positive impact of flavonoid content in Geum aleppicum.52,86-88
In the past decade, several severe microbial diseases, such as SARS-CoV-2, have affected the whole world and have majorly affected the human population. Due to the low immunity of individuals, it was severe in people from various countries. This review study shows the use of various microbes that increase the quality and crop productivity of beneficial crops and medicinal plants, and also enhance the accumulation of antioxidants such as carotene, phenols, flavonoids, etc. Due to their unique medicinal properties, they are being explored more and more by researchers. Therefore, it is the reason for the rising interest in this area. Among all microbes, bacteria, fungi, actinobacteria and some cyanobacteria play a crucial role in the enhancement of secondary metabolites in plants via inoculation. This review might encourage individuals to use biofertilizers, which are eco-friendly and cost-effective. Due to this strategy, we can save lives and our incredible planet, too.
ACKNOWLEDGMENTS
The authors would like to acknowledge the School of Applied and Life Sciences for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MS conceptualized and supervised the stduy. NAS, RS, MD, BK and VR, designed the figures and table. MS, PK and VR wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Kumar A, Singh AK, Kaushik MS, Mishra SK, Raj P, Singh PK, Pandey KD. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: a review. 3 Biotech.2017;7:357.
Crossref - Gupta A, Verma H, Singh PP, Singh P, Singh M, Mishra V, Kumar A. Rhizome endophytes: roles and applications in sustainable agriculture. In: Seed Endophytes; Verma, S., White, Jr, J. (eds) Springer, Cham. 2019:405-421.
Crossref - Jha Y. Enhanced cell viability with induction of pathogenesis related proteins against Aspergillus niger in maize by endo-rhizospheric bacteria. Jordan J. Biol. Sci. 2021;15:1995-6673.
Crossref - Pace L, Pellegrini M, Palmieri S, Rocchi R, Lippa L, Del Gallo M. Plant growth-promoting rhizobacteria for in vitro and ex vitro performance enhancement of Apennines’ Genepi (Artemisia umbelliformis sub sp. eriantha), an endangered phytotherapeutic plant. In Vitro Cell. Dev. Biol. 2020;56: 134-142.
Crossref - Souza RD, AmbrosiniA,Passaglia LMP. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015; 38: 401-419.
Crossref - Mishra S, Singh A, Keswani C, Saxena A, Sarma BK, Singh HB. Harnessing plant-microbe interactions for enhanced protection against phytopathogens. In: Plant Microbes Symbiosis: Applied Facets; Arora, N. (eds). Springer, New Delhi. 2015: 111-125.
Crossref - Osman MEH, Sheekh MME, Naggar AHE, Gheda SF. Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol. Fertil. Soils. 2010; 46: 861-875.
Crossref - Sathya A, Vijayabharathi R, Gopalakrishnan S. Plant growth-promoting actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotech. 2017;7: 102.
Crossref - Jain A, Singh A, Singh S, Singh HB. Phenols enhancement effect of microbial consortium in pea plants restrains Sclerotiniasclerotiorum. Biol. Control. 2015;89: 23-32.
Crossref - Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica(Cairo). 2012: 963401.
Crossref - Ren XM, Guo SJ, Tia W, Chen Y, Han H, Chen E, Chen ZJ. Effects of plant growth-promoting bacteria (PGPB) inoculation on the growth, antioxidant activity, Cu uptake, and bacterial community structure of rape (Brassica napusL.) grown in Cu-contaminated agricultural soil. Front. Microbiol. 2019;10: 1455.
Crossref - Gupta A, Gupta R, Singh RL. Principles and applications of environmental biotechnology for a sustainable future. Microbes Environ. 2017;43-84.
Crossref - Manoharachary C, Sridar K, Singh R, Adholeya A, Suryanarayanan TS, Rawat S, Johri BN. Fungal biodiversity: Distribution, conservation and prospecting of fungi from India. Curr. Sci. 2005;89:58-77.
- Kumar RS, Singh D, Bose SK, Trivedi PK. Biodegradation of environmental pollutant through pathways engineering and genetically modified organism’s approaches. In Microorganisms for Sustainable Environment and Health; Chowdhary P, Raj A, Akhter Y (eds.), 2020; 137-165.
Crossref - Singh M, Singh D, Gupta A, Pandey KD, Singh PK, Kumar A. Plant growth promoting rhizobacteria: application in biofertilizers and biocontrol of phytopathogens. In PGPR amelioration in Sustainable Agriculture; Singh AK, Kumar A, Singh PK (eds.), Elsevier. 2018: 41-66.
Crossref - Abdelaziz AM, Attia MS, Salem MS, Refaay DA, Alhoqail WA, Senousy HH. Cyanobacteria-mediated immune responses in pepper plants against fusariumwilt. Plants. 2022;11:2049.
Crossref - Whitton BA, Potts M. Introduction to the cyanobacteria. In Ecology of cyanobacteria II: their diversity in space and time,pp. 1-13, 2012. Springer.
Crossref - Higa T. Effective microorganisms: A biotechnology for mankind. In Proceedings of the first international conference on Kyusei nature farming. US Department of Agriculture, Washington, DC, USA; 1991; pp. 8-14.
- Lin CH, Wei YT, Chou CC. Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol. 2000;23: 628-633.
Crossref - Hua YJ, Yuan G, Yan-Man L, Xiao-Hua Q, Zhang MF. Salicylic acid-induced enhancement of cold tolerance through activation of antioxidative capacity in watermelon. Sci. Hortic. 2008;118:200-205.
Crossref - Juan MY, Chou CC. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010;27:586-591.
Crossref - Dziki D, Rozyło R, GawlikDU,Swieca M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci Technol. 2014;40: 48-61.
Crossref - Allah EFA, Hashem A, Alqarawi AA, Bahkali AH, Alwhibi MS. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbaniasesban(L.) Merr using arbuscularmycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2015;22:274-283.
Crossref - Sharififar F, Nudeh GD, Mirtajaldini M. Major flavonoids with antioxidant activity from Teucriumpolium L. Food chem. 2009;112: 885-888.
Crossref - Grabmann J. Terpenoids as plant antioxidants. Vitam. Horm. 2005;72:505-535.
Crossref - Stahl W, Sies H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003;24:345-351.
Crossref - Young AJ, Lowe GL. Carotenoids-antioxidant properties. Antioxidants. 2018;7:28.
Crossref - Singh DP, Beloy J, Mcinerney JK, Day L. Impact of boron, calcium and genetic factors on vitamin C, carotenoids, phenolic acids, anthocyanins and antioxidant capacity of carrots (Daucus carota). Food Chem. 2012;132:1161-1170.
Crossref - Singh M, Pandey KD. Endophytic bacterial strains modulated synthesis of lycopene and bioactive compounds in Solanumlycopersicum L. fruits. Biocatal. Agric. Biotechnol. 2021;35:102088.
Crossref - Wang ZQ,Guillot DO, Lopez-Pujol J. Crassulaovata, a new alien plant for mainland China. Collect. Bot. 2015; 34: e009.
Crossref - Suryanti V, Marliyana SD, Putri HE. Effect of germination on antioxidant activity, total phenolics, β-carotene, ascorbic acid and α-tocopherol contents of lead tree sprouts (Leucaena leucocephala (lmk.) de Wit). Int. Food Res. J. 2016;23:167-172.
- Rahman M, Sabir AA, Mukta JA, Khan MMA,Mohi MUD, Miah MG, Rahman M, Islam MT. Plant probiotic bacteria Bacillus and Paraburkholderiaimprove growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018;8:2504.
Crossref - Sajedi NA, Ardakani MR, Madani H, Naderi A, Miransari M. The effects of selenium and other micronutrients on the antioxidant activities and yield of corn (Zea mays L.) under drought stress. Physiol. Mol. Biol. Plants. 2011;17:215-222.
Crossref - Souza MPD, Chu D, Zhao M, Zayed AM, Ruzin SE, Schichnes TN. Rhizosphere bacteria enhance selenium accumulation and volatilization by Indian Mustard. Plant Physiol. 1999;119:565-574.
Crossref - Abd Elgawad H, Abuelsoud W, Madany MM, Selim S, Zinta G, Mousa AS, Hozzein WN. Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules. 2020;10:1675.
Crossref - Chiappero J, del Rosario Cappellari L, Alderete LGS, Palermo TB, Banchio E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops. Prod. 2019;139:111553.
Crossref - Gusain YS, Singh US, Sharma AK. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryzasativa L.). Afr. J. Biotechnol. 2015;14:764-773.
Crossref - Devi KA, Pandey G, Rawat AKS, Sharma GD, Pandey P. The endophytic symbiont-Pseudomonas aeruginosa stimulates the antioxidant activity and growth of AchyranthesasperaL. Front. Microbiol.,2017;8:1897.
Crossref - Ordookhani K, Zare M. Effect of Pseudomonas, Azotobacter and arbuscularmycorrhiza fungi on lycopene, antioxidant activity and total soluble solid in tomato (Lycopersicon esculentum F1 Hybrid, Delba). Adv. Environ. Biol. 2011;5:1290-129
- Latef AAHA, AlhmadMFA,Kordrostami M, Baker ABA, Zakir A. Inoculation with Azospirillum lipoferumor Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 2020;39:1293-1306.
Crossref - Rachidi F, Benhima R, Sbabou L, El AH. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020;25:e00426.
Crossref - Selim S, Akhtar N, Hagagy N, Alanazi A, Warrad M, El Azab E, Elamir MYM, Al-Sanea MM, Jaouni SKA, Mawgoud MA Abdelgawad H. Selection of newly identified growth-promoting archaea haloferaxspecies with a potential action on cobalt resistance in maize plants. Front. Plant Sci. 2022;13.
Crossref - Allah EFABD, Abeer H, Alqarawi AA, Hend AA. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscularmycorrhizal fungi. Pak. J. Bot. 2015;47:785-795.
- Attia MS, Abdelaziz AM, Askar AAA, Arishi AA, Abdelhakim AM, Hashem AH. Plant growth-promoting fungi as biocontrol tool against fusarium wilt disease of tomato plant. J. Fungi. 2022;8:775.
Crossref - Baslam M, Esteban R, Garcia PJI, Goicoechea N. Effectiveness of arbuscularmycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl. Microbiol. Biotechnol. 2013;97:3119-3128.
Crossref - Baslam M, Garmendia I, Goicoechea N. Enhanced accumulation of vitamins, neutraceuticals and minerals in lettuces associated with arbuscularmycorrhizal fungi (AMF): a question of interest for both vegetables and humans. Agriculture. 2013;3:188-209.
Crossref - Hristozkova M, Geneva M, Stancheva I, BoychinovaM,Djonova E. Contribution of arbuscularmycorrhizal fungi in attenuation of heavy metal impact on Calendula officinalis development. Appl. Soil Ecol. 2016;101:57-63.
Crossref - Krishna H, Singh SK, Sharma RR, Khawale RN, Grover M, Patel VB. Biochemical changes in micropropagated grape (VitisviniferaL.) plantlets due to arbuscularmycorrhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci. Hortic. 2005;106: 554-567.
Crossref - McCue P, Shetty K. A biochemical analysis of mung bean (Vignaradiata) response to microbial polysaccharides and potential phenolic-enhancing effects for nutraceutical applications. Food biotechnol. 2002;16:57-79.
Crossref - Simic SG, Tusevski O, Maury S, Hano C, Delaunay A, Chabbert B, Lamblin F, Laine E, Joseph C,Hagege D. Fungal elicitor-mediated enhancement in phenylpropanoid and naphtodianthrone contents of HypericumperforatumL. Plant Cell. Tissue. Organ. Cult. 2015;122:213-226.
Crossref - Zhang S, Gan Y, Bingliang Xu. Application of plant-growth-promoting fungi Trichodermalongibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidativedefense system and gene expression. Front. Plant Sci. 2016;7.
Crossref - Chookalaii H, Riahi H, Shariatmadari Z, Mazarei Z, Seyed HM. Enhancement of total flavonoid and phenolic contents in Plantago major L. with plant growth promoting cyanobacteria. J. Agric. Sci. Technol. 2020;22:505-518.
- Cordero BF,Couso I, Leon R, Rodriguez H, Vargas MA. Enhancement of carotenoids biosynthesis in Chlamydomonas reinhardtii by nuclear transformation using a phytoene synthase gene isolated from Chlorella zofingiensis. Appl. Microbiol. Biotechnol. 2011;91: 341-351.
Crossref - Singh M, Kumar A, Singh R, Pandey KD. Endophytic bacteria: a new source of bioactive compounds. 3 Biotech. 2017;7:315.
Crossref - Backer R, Rokem JS, Ilangumaran G, Lamont J,Praslickova D, Ricci E, Subramanian S, Smith DL. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018;9:1473.
Crossref - Vimal SR, Patel VK, Singh JK. Plant growth promoting Curto bacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2018;105:553-562.
Crossref - Zheng W, Miao K, Zhang Y, Pan S, Zhang M, Jiang H. Nitric oxide mediates the fungal-elicitor-enhanced biosynthesis of antioxidant polyphenols in submerged cultures of Inonotus obliquus. Microbiol. 2009;155: 3440-3448.
Crossref - Shalaby EA, Shanab SM, Singh V. Salt stress enhancement of antioxidant and antiviral efficiency of Spirulina platensis. J Med Plants Res. 2010;4:2622-2632.
Crossref - Singh S. A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014;17:1221-44.
Crossref - Bello AS, Hamadou RB, Hamdi H, Saadaoui I, Ahmed T. Application of cyanobacteria (Roholtiella sp.) liquid extract for the alleviation of salt stress in bell pepper (Capsicum annuum L.) plants grown in a soilless system. Plants. 2022;11:104.
Crossref - Hanin M, Ebel C, Mariama N, Laplaze L, Masmoudi K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016; 7:1787.
Crossref - Joan CM, Rachidi F, Mohamed HA, Mernissi, NE,Aasfar A, Barakate M, Arroussi HE. Microalgae-cyanobacteria-based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J. Appl. Phycol. 2021;33: 3779-3795.
Crossref - Prasanna R, BidyaraniN,Babu S, Hossain F, Shivay YS, Nain L. Cyanobacterial inoculation elicits plant defense response and enhanced Zn mobilization in maize hybrids. Cogent food agric. 2015;1:998507.
Crossref - Ahn YJ, Kim H. Lutein as a modulator of oxidative stress-mediated inflammatory diseases. Antioxidants. 2021;10:1448.
Crossref - Saini DK, Pabbi S, Shukla P. Cyanobacterial pigments: Perspectives and biotechnological approaches. Food Chem. Toxicol. 2018;120:616-624.
Crossref - AbuyeF,Achamo B. Potential use of cyanobacterial bio-fertilizer on growth of tomato yield components and nutritional quality on grown soils contrasting pH. Glob. J. Biol. Agric. Health Sci. 2016;6:2224-3208.
- Baky HHAE, Baz FKE, Baroty GSE. Enhancing antioxidant availability in wheat grains from plants grown under seawater stress in response to microalgae extract treatments. J. Sci. Food Agric. 2010;90:299-303.
Crossref - Mitra D, Mondal R, Khoshru B, Senapati A, Radha TK, Mahakur B, UniyalN,Myo EM, Boutaj H, Sierra BEG, Panneerselvam P, Ganeshamurthy, AN, Elkovic SA, Vasic T, Rani A, Dutta S, Mohapatra PKD. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere. 2022;32: 149-170.
Crossref - Selim S, Hassan YM, Saleh AM, Habeeb TH, AbdElgawad H. Actinobacterium isolated from a semi-arid environment improves the drought tolerance in maize (Zea mays L.). Plant Physiol. Biochem. 2019; 142:15-21.
Crossref - Ahmed MM, Hagagy N, AbdElgawad H. Establishment of actinobacteria-Satureja hortensis interactions under future climate CO2-enhanced crop productivity in drought environments of Saudi Arabia. Environ. Sci. Pollut. Res. 2021;28:62853-62867.
Crossref - Nassar AM, Tarabily KAE, Sivasithamparam K. Growth promotion of bean (Phaseolus vulgaris L.) by a polyamine-producing isolate of Streptomyces griseoluteus. J. Plant Growth Regul. 2003;40: 97-106.
Crossref - Warrad M, Hassan YM, Mohamed MSM, Hagagy N, Maghrabi OAA, Selim S, Saleh AM,Elgawad H. A bioactive fraction from Streptomyces sp. enhances maize tolerance against drought stress. J. Microbiol. Biotechnol. 2020; 30:1156-1168.
Crossref - Vijayabharathi R, Gopalakrishnan S, Sathya A, Kumar MV, Srinivas V, Mamta S. Streptomyces sp. as plant growth-promoters and host-plant resistance inducers against Botrytis cinerea in chickpea. Biocontrol. Sci. Technol. 2018: 1360-0478.
Crossref - Passari AK, Leo VV, Singh G,Samanta L, Ram H, Siddaiah CN, Hashem A, Arjani ABFA, Alqarawi AA, Allah EFA, Singh BP. In vivo studies of inoculated plants and in vitro studies utilizing methanolic extracts of endophytic Streptomyces sp. strain DBT34 obtained from Mirabilis jalapa L. exhibit ROS-scavenging and other bioactive properties. Int. J. Mol. Sci. 2020;21: 7364.
Crossref - AbdElgawad H, Saleh, AM, Jaouni SAI, Selim S, Hassan MO, Wadaan MA., Shuikan AM, Mohamed HS and Hozzein WN. Utilization of actinobacteria to enhance the production and quality of date palm (Phoenix dactylifera L.) fruits in a semi-arid environment. Sci. Total Environ., 2019; 665:690-697.
Crossref - Hassan SED, Fouda A, Radwan AA, Salem SS, Barghoth MG, Awad MA, Abdo AM, Gamal MSE. EndophyticactinomycetesStreptomycessp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 2019;24:377-393.
Crossref - Mirzaei M, Moghadam AL, Hakimi L, Danaee E. Plant growth promoting rhizobacteria (PGPR) improve plant growth, antioxidant capacity, and essential oil properties of lemongrass (Cymbopogoncitratus) under water stress. Iran. J. Plant Physiol. 2020;10: 3155-3166.
- Ganugi P, Fiorini A, Tabaglio V, Capra F, Zengin G, Bonini P, Caffi T, Puglisi E, Trevisan M, Lucini L. The functional profile and antioxidant capacity of tomato fruits are modulated by the interaction between microbial biostimulants, soil properties, and soil nitrogen status. Antioxidants. 2023;12:520.
Crossref - HeidariM,Golpayegani A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (OcimumbasilicumL.). J. Saudi Soc. Agric. Sci. 2012, 11, 57-61.
Crossref - Tariq H, Asif S,Andleeb A, Hano C, Abbasi BH. Flavonoid production: current trends in plant metabolic engineering and de novo microbial production. Metabolites. 2023;13:124.
Crossref - Bag S, Mondal A, Majumder A, Mondal SK, Banik A. Flavonoid mediated selective cross-talk between plants and beneficial soil microbiome. Phytochem. 2022;21:1739-1760.
Crossref - Ghitti E, Rolli E, Crotti E, Borin S. Flavonoids are intra- and inter- kingdom molulator signals. Microorganism. 2022;10:2479.
Crossref - Wang L, Chen M, Lam PY,Andreote FD, Dai L, Wei Z. Multifaceted roles of flavonoids mediating plant-microbe interactions. Microbiome. 2022;10:233.
Crossref - Shah A, Smith DL. Flavonoids in agriculture: chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy. 2020;10:1209.
Crossref - Hassan S, Mathesius U. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012;63: 3429-3444.
Crossref - Cui XX, Wang L, Fang HY, Zheng YG, Su CY. The cultivable endophytic fungal community of Scutellaria baicalensis: diversity and relevance to flavonoid production by the host. Plant Signal Behav. 2022;17: 2068834.
Crossref - Ham SH, Yoon AR, Oh HE, Park YG. Plant growth-promoting microorganism Pseudarthrobacter sp. NIBRBAC000502770 enhances the growth and flavonoid content of Geumaleppicum. Microorganisms. 2022;10:1241.
Crossref - Liu XQ, Cheng S, Aroca R, Zou YN, Wu QS. Arbuscular mycorrhizal fungi induce flavonoid synthesis for mitigating oxidative damage of trifoliate orange under water stress. Environ. Exp. Bot. 2022;204:105089.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.