ISSN: 0973-7510
E-ISSN: 2581-690X
Plant extracts are now being used to improve animals’ hematological activity, biochemistry, and immunology. This study aimed to assess the antimicrobial activity of Ziziphus nummularia against E. coli bacteria, which causes bovine mastitis in cattle. In this study, the experimental animals were divided into four groups. The control group (Group 0) was fed a normal diet throughout the experiment. In contrast, Groups 1, 2, and 3 received 100 g, 200 g, and 300 g of Ziziphus nummularia with their normal diet, respectively. At the end of the experiment, hematological parameters showed significant differences in the experimental groups. Biochemical parameters had significant differences in creatinine, ALT, and bilirubin; however, albumin values were moderately significant. The antimicrobial activity of Ziziphus nummularia extract was also assessed at different dilutions (0.6 ml, 0.8 ml, and 1 ml), with the 1 ml dilution showing a larger zone of inhibition compared to the others. Analysis of somatic cell count in milk revealed that Groups 2 and 3 showed a significant decrease compared to the control and Group 1. Therefore, incorporating Ziziphus nummularia into the regular diet of dairy animals is recommended to boost immunity against diseases like bovine mastitis, enabling farmers to manage mastitis effectively and enhance milk production in dairy cattle.
Antimicrobial Activity, Biochemical Parameters, Bovine Mastitis, E. coli, Haematological, Somatic Cell Count, Ziziphus nummularia
The livestock sector in Pakistan is classified as a subsistence sector. It provides a stable source of income and employment for small-scale farmers, landless settlers, and large corporations.1 Even farmers with limited land often have 3 to 4 milk-producing animals, which meet their daily needs and cover other routine expenses. This sector is crucial for both small farmers and large companies, offering a reliable source of income and jobs.2 The population of major milk-producing animals in the country is growing at a steady annual rate of 3.6 percent.3
The primary issue farmers deal with is a variety of bacterial, viral, or fungal infections that can drastically lower their rates of output.4 Mastitis, in particular, is caused by physical or microbial pathogens that inflame the mammary gland. Dairy cattle experience physical injuries and chemical irritations in their milk-secreting tissues when pathogenic (mostly bacterial) invaders enter into their teat canals. This invasions lead to bacteria multiplying in the milk-secreting tissues, producing toxins that cause further injury.5 Numerous bacterial pathogens are implicated in mastitis development, which can be classified as contagious, opportunistic, or environmental based on their epidemiological and patho-physiological characteristics.6 Mastitis is characterized by abnormal milk production (e.g., watery milk or the presence of flakes) and udder inflammation, resulting in swelling, redness, and hardening.7 According to widely accepted standards, normal mammary glands in dairy animals have a somatic cell count (SCC) lower than 1×105 cells/ml and are free from bacterial infection.8 Animals with subclinical mastitis have an SCC greater than
1 × 106 cells/ml of milk and are contaminated with bacteria.9 In some cases, bovine mastitis caused by Escherichia coli can present as a subclinical infection of the mammary gland or as a severe systemic disease. Currently, there is limited evidence supporting the efficacy of antimicrobial treatment for E. coli in mastitis.10
Studies have found that numerous herbs possess varying degrees of antimicrobial activity, indicating that natural medicinal plants may have the potential to be used as antibiotics.11 Several studies have demonstrated the antibacterial activity of natural plant extracts and their compounds, which have proven effective against bacterial isolates obtained from mastitis cases. Many breeders and veterinarians have successfully used phytotherapy to prevent and treat mastitis.12 Medicinal ointments or herbal solutions are applied tropically, while green or dried plants are administered orally for treatment. In the case of cattle mastitis, E. coli is one of the most common pathogenic organisms. The use of phytotherapeutic medications has shown satisfactory results in its treatment.13
Plants contain numerous active compounds, including phenolic acids, alkaloids, flavonoids, steroid hormones, volatile oils, tannins, resins, glycosides, and fixed oils, which can be found in specific parts of the plant such as flowers, leaves, fruits, seeds, roots, and bark. The beneficial medicinal effects of plant materials are often due to the combination of these secondary products. Usually, these substances function by specifically blocking the activity of biological targets.14
Ziziphus nummularia, commonly known as jangli beri in Pakistan, belongs to the family Rhamnaceae and is found in Africa, Australia, America, and predominantly in Asia. This plant is primarily used to treat inflammation, relieve pain, promote healing, and reduce bacterial infections.15 Additionally, it serves as a remedy for fever, a hypoglycemic agent for diabetics, a mild sedative, a treatment for insomnia and dental infections.16 In Arabia, the seeds and leaves are used to treat old wounds in camels.17
The use of antibiotics against disease-causing bacteria eventually leads to the development of resistance. This natural process of adaptation and antimicrobial resistance renders antibiotics less effective for treating diseases. In recent years, antimicrobial resistance has increased, primarily due to the overuse and misuse of antibiotics, especially in treating infections. Consequently, numerous bacterial strains have undergone hypermutation, spreading resistant bacteria throughout the environment. There is an increasing interest in separating, evaluating and using the antibacterial properties of natural compounds made from plants as they are an inexpensive and plentiful source of secondary metabolites for pharmaceutical research. This study examined the antimicrobial activity of Ziziphus nummularia against the pathogenic bacteria E. coli, which causes mastitis.17
Hypothesis
Ziziphus nummularia helps prevent and treat bovine mastitis by improving hematological, biochemical, and immunological parameters, lowering the somatic cell count in milk, and demonstrating antibacterial action against Escherichia coli.
Objectives
- To examine the effect of Ziziphus nummularia on milk’s somatic cell count, especially in relation to cow mastitis caused by Escherichia coli.
- To investigate the possibility of using Ziziphus nummularia as a food supplement to boost immunity and treat mastitis in dairy cattle.
Twelve cattle infected with E. coli-induced bovine mastitis were selected for the experiment from various villages in the district of Khushab, Punjab, Pakistan. After sampling, they were divided into four groups, each consisting of three cattle. Three groups of the cattle received a powdered extract of Ziziphus nummularia (Jangli beri) for 21 days at concentrations of
100 g, 200 g, and 300 g, respectively, along with their normal diet. The fourth group (G0) served as the control and received a normal diet. Additionally, to assess the antimicrobial activity of Ziziphus nummularia, an E. coli strain was obtained from the microbiology laboratory at The University of Lahore, Punjab, Pakistan.
Fresh Ziziphus nummularia plant leaves were gathered, cleaned, and allowed to air dry in a shaded area. The dried material was then ground into a fine powder, extracted using a solvent (ethanol) through maceration, and filtered. The dried powder of Ziziphus nummularia leaves and 200 ml of ethanol were mixed in a flask to form a solution, which was then incubated at 37 °C overnight. After incubation, it was centrifuged at 1000 rpm for three minutes. Finally, the total extract was filtered using Whatman No. 1 filter paper to remove impurities. To evaluate the bioactivity and potential compounds in Ziziphus nummularia, the following components were analyzed experimentally.
Total flavonoids
To analyze flavonoids, 1 ml of the filtered sample mentioned earlier was mixed with 30 ml of distilled water. From this solution, 3 ml was transferred to another test tube, to which 70 µL of sodium nitrite was added and left for 10 minutes. Afterward, 145 µL of aluminum chloride was added and left for another 10 minutes, followed by the addition of 45 µL of sodium hydroxide. The absorbance of the solution was measured using a spectrophotometer at a wavelength of 510 nm.
Total phenols
A prepared 400 ml sample was put into various test tubes. A total of three triplicates of each sample were prepared. 100 µL of 10% Folin-Ciocalteu reagent was added to each test tube and the tubes were left for 8 minutes. After that, 1500 µL of 15% Na2CO3 was added and left it for 40 minutes at 25 °C. A spectrophotometer was used to observe the absorbance at 750 nm.
Alkaloids
In a 300 ml beaker, 5 g of powdered Ziziphus nummularia was mixed with 200 ml of acetic acid and 10% ethanol. The solution was covered with aluminum foil and allowed to stand for 5 hours. Afterward, the solution was filtered by using filter paper to obtain a concentrated extract. Ammonium hydroxide in concentrated form was added dropwise until precipitation was complete. The resulting precipitate was washed with ammonium hydroxide and filtered again. The filtrate, containing alkaloids, was dried and then weighed using a weighing scale.
Blood sampling and parameters
A sterilized syringe was used to collect 5 ml of blood from the tail region of the cattle, which was then stored in EDTA tubes. The blood samples were analyzed for complete blood count (CBC) using a BC-2800 analyzer.
Biochemical analysis
For biochemical analysis, blood plasma was obtained by centrifugation of blood at 4000 rpm for 8 minutes. Aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), and urea levels were determined using the calorimetric technique described by Dachs and Bertoldi. Additionally, creatinine, bilirubin, and albumin levels were measured using methods outlined by Moore and Sharer, HPLC and the bromocresol green (BCG) method, respectively.
Milk sampling and Somatic Cell Count
Milk samples were collected and tested for mastitis using the California Mastitis Test (CMT), which is widely recognized as the most reliable method for determining somatic cell count.
Antimicrobial activity of Ziziphus nummularia against E. coli
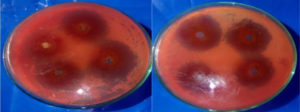
The minimum inhibitory concentration (MIC) and zone of inhibition were determined using the broth micro-dilution method and the Kirby-Bauer test. Nutrient media was prepared, autoclaved, poured into petri dishes, and allowed to cool and solidify. Extracts were diluted to concentrations of 0.6 ml, 0.8 ml, and 1 ml. E. coli bacteria were streaked onto the petri dishes using the streak method and then incubated at 37 °C for 24 hours to promote bacterial growth. After bacterial growth, sterilized filter paper discs were placed on the plates, and the prepared sample dilutions were applied to the discs. The plates were returned to the incubator at 37 °C for another 24 hours. The zone of inhibition for each extract was measured after 24-48 hours. This experiment was repeated for each group of animals as previously described.
Statistical analysis
The data obtained underwent statistical analysis using SPSS software (version 16). Analysis of variance (ANOVA) and calculation of mean standard error were applied to the data. Tukey’s multiple range tests were used to compare the means of all treatments. A significance level of P < 0.05 was adopted.
Antioxidant activity of Ziziphus nummularia
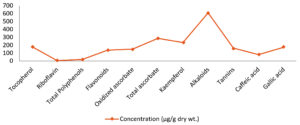
The antioxidant activity of Ziziphus nummularia extract was examined, revealing the presence of antioxidants (Table 1). Quantification of these compounds was achieved by comparing their peak areas to those of standard compounds. Gallic acid, caffeic acid, tannins, quercetin, and kaempferol were detected at retention times in the HPLC analysis of the extract, shown in Figure 1.
Haematological analysis of cattle affected by mastitis
Different hematological parameters are presented in Table 1. The control group showed no differences after the experiment. In contrast, Group 1, exhibited moderate differences in some hematological parameters. Similarly, Group 2, showed more significant differences compared to Group 1 in their hematological parameters. Group 3, exhibited significant results for all hematological parameters, as detailed in Table 1.
Table (1):
Hematological analysis of control and treated groups
| Parameters | Group 0 (control) | Group 1 | Group 2 | Group 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before test | After test | p-value | Before test | After test | p-value | Before test | After test | p-value | Before test | After test | p-value | |
| WBC | 7.7 | 7.8 | 0.9 | 7.2 | 9.5 | 0.005 | 7.0 | 10.1 | 0.01 | 7.30 | 12.1 | 0.001 |
| LYMP | 2.9 | 2.7 | 0.006 | 2.3 | 3.3 | 0.000 | 2.6 | 4.80 | 0.000 | 2.70 | 5.00 | 0.009 |
| MON | 0.86 | 0.79 | 0.14 | 0.93 | 1.23 | 0.005 | 0.76 | 1.69 | 0.03 | 0.85 | 1.95 | 0.01 |
| GRA | 5.74 | 6.20 | 0.02 | 5.73 | 6.83 | 0.001 | 5.71 | 7.50 | 0.004 | 14.72 | 18.5 | 0.004 |
| LYMP% | 31.7 | 32.8 | 0.21 | 30.86 | 32.86 | 0.07 | 31.4 | 34.33 | 0.01 | 32.23 | 37.5 | 0.005 |
| MONO% | 3.94 | 4.0 | 0.05 | 3.54 | 5.55 | 0.04 | 3.84 | 5.90 | 0.03 | 3.984 | 7.98 | 0.01 |
| GRA% | 64.13 | 63.03 | 0.008 | 63.46 | 65.23 | 0.03 | 63.56 | 66.53 | 0.04 | 63.68 | 70.0 | 0.01 |
| RBCs | 5.46 | 4.63 | 0.08 | 5.23 | 6.7 | 0.001 | 5.6 | 6.4 | 0.05 | 5.7 | 7.63 | 0.01 |
| HGB | 13.53 | 11.9 | 0.04 | 13.3 | 13.93 | 0.2 | 13.33 | 14.7 | 0.09 | 13.7 | 16.7 | 0.07 |
| HCT | 42.07 | 39.2 | 0.04 | 39.32 | 41.62 | 0.002 | 40.65 | 43.6 | 0.000 | 38.6 | 46.00 | 0.000 |
| MCV | 93.46 | 91.8 | 0.09 | 94.83 | 95.86 | 0.03 | 93.66 | 97.8 | 0.01 | 94.9 | 98.9 | 0.07 |
| MCH | 13.7 | 11.3 | 0.07 | 13.3 | 14.8 | 0.1 | 13.7 | 14.7 | 0.1 | 13.7 | 17.7 | 0.000 |
| MCHC | 27.3 | 26.3 | 0.03 | 27.8 | 29.0 | 0.1 | 28.4 | 30.1 | 0.005 | 28.6 | 30.3 | 0.06 |
| RDW | 22.1 | 20.1 | 0.05 | 21.3 | 22.5 | 0.1 | 21.8 | 24.7 | 0.01 | 21.7 | 25.8 | 0.008 |
| PLT | 290.7 | 288.3 | 0.02 | 290.4 | 291.3 | 0.1 | 289.0 | 295.6 | 0.01 | 291.3 | 297.6 | 0.01 |
| MPV | 7.6 | 7.5 | 0.1 | 8.2 | 9.7 | 0.004 | 8.1 | 8.8 | 0.008 | 7.2 | 9.7 | 0.003 |
| PCT | 0.2 | 0.2 | 0.3 | 0.4 | 0.9 | 0.000 | 0.4 | 0.5 | 0.06 | 0.1 | 1.1 | 0.008 |
| PDW | 31.9 | 30.0 | 0.02 | 32.3 | 32.9 | 0.1 | 32.6 | 35.8 | 0.002 | 32.9 | 38.5 | 0.003 |
| ESR | 1.8 | 1.6 | 0.01 | 1.9 | 2.0 | 0.2 | 1.9 | 2.9 | 0.002 | 1.6 | 3.5 | 0.003 |
Antimicrobial activity of Ziziphus nummularia against E. coli
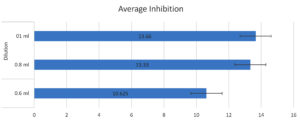
Figures 2 and 3 illustrate the average zone of inhibition for all three dilutions.
Somatic cell count
The detailed results of the somatic cell count (SCC) for all groups are presented in Table 2.
Table (2):
Somatic cell count
| Groups | Animal Cattle | Weight of Cattle in Kg | SCC Before start cells/ml | SCC at Day-0 cells/ml | SCC at Day-1st cells/ml | SCC at Day-2nd cells/ml | SCC at Day-3rd cells/ml | SCC at Day-4th cells/ml | SCC at Day-5th cells/ml | SCC Day-6th cells/ml | SCC at Day-7th cells/ml |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1 | 650 | 250,805 | 250,805 | 250,805 | 255,805 | 255,805 | 259,805 | 257,805 | 257,805 | 257,805 |
| 2 | 600 | 258,004 | 257,004 | 257,004 | 257,004 | 257,002 | 256,004 | 256,224 | 257,004 | 256,004 | |
| 3 | 620 | 259,887 | 259,887 | 259,888 | 259,889 | 259,879 | 259,897 | 259,887 | 259,887 | 259,887 | |
| Average | 623.33 | 256,232 | 255,898.66 | 255,899 | 257,566 | 257,562 | 258,568.66 | 257,972 | 258,232 | 257,898.66 | |
| Treated group 1
|
1 | 670 | 265,000 | 263,000 | 262,000 | 261,000 | 260,000 | 259,090 | 258,000 | 255,000 | 254,000 |
| 2 | 640 | 265,000 | 265,000 | 264,000 | 263,000 | 262,589 | 262,000 | 261,500 | 261,000 | 260,560 | |
| 3 | 630 | 265,000 | 265,000 | 264,000 | 263,000 | 262,589 | 262,000 | 261,500 | 261,000 | 260,560 | |
| Average | 646.66 | 265,000 | 264,333.33 | 263,333.33 | 262,333.33 | 261,726 | 261,030 | 260,333.33 | 259,000 | 258,373.33 | |
| Treated group 2 | 1 | 650 | 265,000 | 262,000 | 261,000 | 260,000 | 250,789 | 250,657 | 250,500 | 250,200 | 250,000 |
| 2 | 630 | 265,589 | 265,200 | 264,578 | 263,578 | 262,585 | 262,000 | 261,589 | 261,000 | 260,879 | |
| 3 | 650 | 265,000 | 264,000 | 263,000 | 262,000 | 261,589 | 260,000 | 250,200 | 251,000 | 250,540 | |
| Average | 643.33 | 265,196.33 | 263,733.33 | 262,859.33 | 261,859.33 | 258,321 | 257,552.33 | 254,096.33 | 254,066.66 | 253,806.33 | |
| Treated group 3 | 1 | 680 | 265,000 | 262,000 | 261,000 | 260,000 | 250,789 | 250,657 | 250,500 | 250,200 | 250,000 |
| 2 | 690 | 265,589 | 265,200 | 264,578 | 263,578 | 262,585 | 262,000 | 261,589 | 261,000 | 260,879 | |
| 3 | 670 | 265,000 | 260,000 | 258,000 | 252,000 | 250,589 | 250,000 | 250,000 | 240,100 | 240,000 | |
| Average | 680 | 265,196.33 | 262,400 | 261,192.66 | 258,526 | 254,654.33 | 254,219 | 254,029.66 | 250,433.33 | 250,293 |
This research aimed to investigate the effects of Ziziphus nummularia, a natural product, on bovine mastitis caused by bacteria commonly found in domestic animals such as cows, cattle, and buffaloes. To reduce bacterial infection rates, Ziziphus nummularia was incorporated into the animals’ fortified feed in varying amounts, serving as an antimicrobial agent against E. coli, a bacterium involved in mastitis. The aim of this research was to determine the antibacterial properties of plant extracts. The herbal remedies not only replace antibiotics but also enhance growth, improve body composition, and strengthen the immune system of animals against viral, bacterial, and fungal infections.18 The results of the present study align with findings by Avancini et al.,19 which investigated the use of various plant extracts, including Foeniculum vulgare, Sida rhombifolia, Aloe arborescens, and Alternanthera brasiliana, for the prevention and control of bovine mastitis in Southern Brazil.
According to studies by Mohammed et al.,20 and Girling et al.,21 the analysis of key biochemical and hematological parameters provides insights into metabolic activities and potential illnesses, whether in a latent or clinical state. They conducted research on bovine mastitis in cows, collecting blood samples from 20 non-mastitic cows and 20 infected individuals. The results indicated that all hematological parameters showed no significant differences (p > 0.05) except for MCH, which exhibited significant variation. Additionally, the biochemical parameters showed that all values were not significantly different except for total protein, AST, ALT, and globulin, which showed significant differences (p < 0.05). The findings of our study align closely with these observations. Any discrepancies noted could be attributed to differences in species or seasonal variations. Mastitis encompasses bacteriological, physical, and chemical changes affecting milk production and pathological alterations in udder glandular tissues, impacting both milk quality and quantity.
In our study, the analysis of somatic cell counts in milk showed a significant decrease was observed in Groups 2 and 3 compared to both the control and Group 1. Moreover, the results of the methanolic extract at a 1 ml dilution were more significant compared to the other dilutions. Sharma et al.22 attributed the antimicrobial activity of Ziziphus nummularia to the presence of alkaloids, flavonoids, glycosides, and saponins in the leaf extracts of the plant, proposing that leaf extracts may be an effective agent for the treatment of several diseases. Antioxidants sourced from plants offer an additional avenue to investigate important plants that could help mitigate the prevalence of this chronic bacterial disease.
Previous studies by Sharma et al.23 reported that acetone extracts of A. nilotica had antibacterial activity with MIC of 6.25 mg/ml and 12.5 mg/ml against S. aureus, for leaves and bark respectively. This study exhibits the methanolic extract of Z. nummularia has antibacterial activity with MIC of 13.66 mg/ml against E. coli.
This study emphasizes Ziziphus nummularia’s potential as a natural supplement to raise cattle productivity and health. The plant’s abundant phytochemical content increased biochemical markers including creatinine, ALT, bilirubin, and albumin, as well as hematological parameters like hemoglobin and red blood cell count. Strong antibacterial activity was shown by the methanolic extract of Ziziphus nummularia, especially against Escherichia coli, the main cause of cow mastitis. The largest zone of inhibition was seen in the 1 ml dilution. Additionally, the extract demonstrated efficacy in reducing inflammation and supporting udder health by drastically lowering somatic cell counts in milk from animals with mastitis. These results imply that Ziziphus nummularia is a natural and sustainable substitute for synthetic antibiotics, offering a workable way to treat mastitis and improve the general well-being and output of dairy cattle.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Ethical Review Committee on Animal Subject, Department of Zoology, The University of Lahore, SGD Campus, Pakistan (Zool-SGD 412).
- Abedullah N, Khalid M, Kouser S. The role of agricultural credit in the growth of livestock sector: A case study of Faisalabad. Pakistan Vet. J. 2009; 29(2): 81-84.
- Khan S, Din A, Ali GM, et al. Screening of lactic acid bacteria for their use as buffalo probiotic. JAPS: Journal of Animal & Plant Sciences. 2020; 30(6).
Crossref - Rana HAA, Iftikhar M, Watto MA, Bilal MQ. Institutional role in coping livestock diseases on farm level in rural areas of Punjab, Pakistan. Pak J Agric Sci. 2021; 58(4).
Crossref - Duguma B, Kechero Y, Janssens GP. Survey of major diseases affecting dairy cattle in Jimma town, Oromia, Ethiopia. Global veterinaria. 2012; 8(1):62-66.
- Khan MJ, Abbas A, Naeem M, Ayaz MM, Akhter S. Current issues and future prospects of dairy sector in Pakistan. Sci. Tech. Dev. 2013; 32(2): 126-139.
- Petit T, Spergser J, Rosengarten R, Aurich JJRIDA. Prevalence of potentially pathogenic bacteria as genital pathogens in dairy cattle. Reproduction in Domestic Animals. 2009; 44(1): 88-91.
Crossref - Manyi-Loh CE, Mamphweli SN, Meyer EL, Okoh AI, Makaka G, Simon M. Inactivation of selected bacterial pathogens in dairy cattle manure by mesophilic anaerobic digestion (balloon type digester). International journal of environmental research and public health. 2014; 11(7): 7184-7194.
Crossref - Hamann J. Diagnosis of mastitis and indicators of milk quality. In: Hogeveen H., editor. Mastitis in Dairy Production: Current Knowledge and Future Solutions. Wageningen Academic Publishers; Wageningen, The Netherlands. 2005;82-91.
Crossref - Krishnamoorthy P, Suresh KP, Saha S, Govindaraj G, Shome BR, Roy P. Meta-analysis of prevalence of subclinical and clinical mastitis, major mastitis pathogens in dairy cattle in India. (2017).
Crossref - Burvenich C, Van Merris V, Mehrzad J, Diez-Fraile A, Duchateau L. Severity of E. coli mastitis is mainly determined by cow factors. Veterinary research. 2003; 34(5): 521-564.
Crossref - Awad E, Awaad A. Role of medicinal plants on growth performance and immune status in fish. Fish & shellfish immunology. 2017; 67: 40-54.
Crossref - Tomanić D, Samardžija M, Kovačević Z. Alternatives to Antimicrobial Treatment in Bovine Mastitis Therapy: A Review. Antibiotics. 2023; 12(4): 683.
Crossref - Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. African journal of traditional, complementary and alternative medicines. 2013; 10(5): 210-229.
Crossref - Mummed B, Abraha A, Feyera T, Nigusse A, Assefa S. In vitro antibacterial activity of selected medicinal plants in the traditional treatment of skin and wound infections in eastern Ethiopia. BioMed research international. 2018.
Crossref - Mesmar J, Abdallah R, Badran A, Maresca M, Shaito A, Baydoun E. Ziziphus nummularia: A Comprehensive Review of Its Phytochemical Constituents and Pharmacological Properties. Molecules. 2022; 27(13): 4240.
Crossref - Kumar MS, Chauhan UK. A study of antimicrobial activity of Zizyphus nummularia leaf extract. Journal of Environmental Biology. 2010; 12(3): 273-277.
- Suroowan S, Mahomoodally MF. Alternative Antimicrobials: Medicinal Plants and their Influences on Animal Infectious Diseases. In Ethnoveterinary Medicine. 2020;23-56.
Crossref - Still J. Use of animal products in traditional Chinese medicine: environmental impact and health hazards. Complementary therapies in medicine. 2003; 11(2): 118-122.
Crossref - Avancini C, Wiest JM, Dall’Agnol R, Haas JS, von POSER GL. Antimicrobial activity of plants used in the prevention and control of bovine mastitis in Southern Brazil. Latin American Journal of Pharmacy. 2008; 27(6): 894-9.
- Mohammed A, Campbell M, Youssef FG. Serum copper and haematological values of sheep of different physiological stages in the dry and wet Seasons of Central Trinidad. Veterinary Medicine International. 2014.
Crossref - Girling SJ, Campbell-Palmer R, Pizzi R, Fraser MA, Cracknell J, Arnemo J, Rosell F. Haematology and serum biochemistry parameters and variations in the eurasian beaver (Castor fiber). PLoS One. 2015; 10(6): e0128775.
Crossref - Sharma C, Aneja KR, Surain P, Dhiman R, Jiloha P, Meashi V, Kaur M. In vitro evaluation of antimicrobial spectrum of Acacia nilotica leaves and bark extracts against pathogens causing otitis infection. Journal of Innovative Biology. 2014;1(1):51-56.
- Sharma S, Singh J, Maherchandani S, Kashyap SK. Antibacterial activity of Ziziphus nummularia and Prosopis cineraria leaves extracts against Staphylococcus aureus and Escherichia coli. Veterinary Practitioner. 2012; 13(1):28-32.
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.