Diabetes mellitus is known to be a long-term metabolic disorder identified by high blood glucose levels, affects millions of individuals worldwide, and considerably amplifies the risk of infections, including those caused by fungi. Fungal infection is considered a silent killer and the number of cases is increasing globally. This review explores the complex relationship between diabetes and fungal infections, emphasizing the elevated risk and severity of these infections in diabetic patients. Elevated blood glucose levels and impaired immune function in diabetic individuals create an environment favorable to fungal growth, leading to fosters conditions favorable to infections, from minor to severe systemic cases. Commonly occurring fungal pathogens such as Candida, Aspergillus, and dermatophytes are discussed, along with their clinical implications and treatment challenges. The article also includes case studies from diverse geographic regions that underscore the prevalence and severity of fungal infections in diabetic populations. It has been observed that the pharmacokinetics of antifungal agents are greatly impacted by diabetes and cause alteration in drug absorption, distribution, and metabolism. Prevention and management strategies, including strict glycemic control, regular screening, hygiene practices, and patient education, are emphasized to mitigate the risk of fungal infections. This review calls for ongoing research and the development of new antifungal treatments tailored to diabetic patients to improve outcomes and enhance patient care.
Fungal Infection, Diabetes, Candida, Aspergillus, Dermatophytes
Diabetes mellitus is known to be a long-term metabolic disorder identified by high blood glucose levels and affects millions of individuals worldwide.1 According to WHO data in 2024, around 422 million people globally have diabetes disorder, with approximately 1.5 million fatalities reported annually.2 According to the assumption given by the International Diabetes Federation, diabetes cases will increase to 700 million by 2045.3
The major cause of diabetes is due to the arises in insulin secretion, insulin action, or a combination of both, leading to various complications that significantly impact the quality of life.4 Due to these complications the susceptibility to the infection is increased, which leads to severe health outcomes. Diabetes patients face a greater risk of mortality from infections compared to non-diabetic adults. In various studies, it has been observed that diabetes mellitus acts as a silent killer by affecting various organs and acting as a primary cause of bacterial or fungal infection.5
While much attention has been given to the relationship between diabetes and fungal infections, the impact of fungal infections on diabetic patients is a growing area of interest. Fungal disease can cause serious illnesses and death.6 According to the Centers for Disease Control and Prevention, there are a total of 75,000 hospitalizations and nearly 9 million outpatients visit each year for fungal disease.7
According to various studies, compromised immune systems and elevated blood sugar levels play a major role in fungal infection as well as in increasing the colonization of fungus. Diabetic patients are notably more severe and can cause superficial infections, such as skin and mucosal infections, to systemic and life-threatening conditions. The most frequent fungal pathogens affecting diabetic individuals include Candida species, Aspergillus species, and Dermatophytes. These pathogens can cause various forms of infections, each presenting unique clinical challenges and necessitating specific treatment approaches.8,9
According to the data of Medical News Today, a 2018 report stated that around 300,000 people show a higher risk of infection which includes yeast infection in type 1 and 2 diabetic patients.10 Managing fungal infections in diabetic patients requires a multifaceted approach, including strict glycemic control, prompt diagnosis, and appropriate therapy. The early identification of fungal pathogens and the initiation of targeted treatments are crucial to prevent complications and improve patient outcomes. Additionally, understanding the underlying pathophysiological mechanisms that predispose diabetic patients to fungal infections can aid in developing preventive strategies and therapeutic interventions.11
In this article, we delve into the complex interplay between fungal microorganisms and diabetes, exploring the implications of fungal infections for patient care and management.
Hyperglycemia and immune dysfunction: key factors in fungal infection risk for diabetic patients
Hyperglycemia
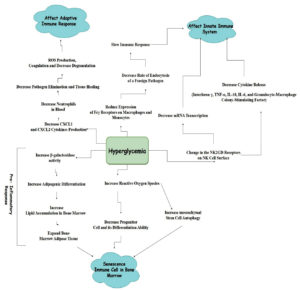
Various types of bacteria and fungi grow on the surface of the skin and beneath it. In healthy individuals, there is a balance between fungi or microorganisms and the immune system, which can fight against infections. However, certain conditions can cause bacteria and fungi to grow uncontrollably, leading to infections. Diabetes is one such condition that leads to weakening of the immune system and causes thrush.12
Thrush, widely known as Candidiasis or a yeast infection, occurs due to the presence of Candida fungus. It is more likely to develop in diabetic patients because high blood sugar levels promote the growth of Candida. High blood sugar means there is more sugar in sweat, saliva, and urine, providing an ideal environment for yeast to thrive, particularly in the mouth and genital areas.13
Mandal et al. conducted a study showing that glucose not only promotes the growth of Candida spp. but also causes resistance to antifungal drugs, they concluded that increasing the glucose concentration by 2% decreases the activity of the antifungal drug voriconazole by 4%. They mentioned in the article that simple sugars like glucose, sucrose, maltose, and lactose are likely to cause rapid growth and increase fungal colonization.14
Impaired immune function
Another major factor contributing to fungal infections is impaired immune function. Various studies have reported that patients with diabetes have impaired immune responses compared to healthy individuals. This leads to the increased concentration of interleukin-6 and interleukin-8 in diabetic individuals, as observed in in vivo studies, indicative of a chronic inflammation state. This has been observed in several ex vivo studies where pathogenic stimulation of whole blood, mononuclear cells, or monocytes was analyzed from both diabetic and nondiabetic individuals. However, these studies were conducted on small sample sizes or focused on specific infections, such as fungal infections by C. albicans or bacterial infection by S. aureus.15-17
It is therefore considered that a diabetic individual has an impaired immune system, leading to a higher risk of fungal infections. The factors triggered by diabetes and hypoglycemia that impair various immune responses and promote the growth of fungal infections are detailed in Figure 1. Due to the increased risk of infections in diabetes, especially the innate and adaptive immune responses in type 1 diabetes, it is crucial to address the health issue. 18,19
Insights into fungal infections in diabetic patients: Case studies and clinical findings across different settings
Fungal infections represent a growing challenge in the management of diabetes, giving the susceptibility of diabetic patients to these infections due to their impaired immune system and increased blood glucose levels.20 The given collection of case studies and research highlights various aspects of fungal infections in diabetic patients, ranging from local studies in Nepal to individual case reports and broader retrospective analysis in Southern China. Each study provides a valuable understanding of the prevalence, clinical presentation, and treatment outcomes of fungal infections among diabetic individuals, underscoring the critical need for heightened awareness, early diagnosis, and effective management strategies to address these infections.
In Bhaktapur, Nepal, a case study was conducted to examine the occurrences of fungal infections in diabetic and non-diabetic individuals. The study was conducted from July 2019 to January 2020. In total, 670 samples were gathered from 134 participants, with a larger percentage of females than males in both diabetic and non-diabetic participants. A random total of five samples was collected. The samples include oral wash, toe swabs, mid-stream urine, hair fiber, and nail scraping. The result showed that out of the 670 samples, 130 exhibited fungal growth, with 114 from diabetic participants and 16 from non-diabetic participants. In both groups, the most frequently isolated fungus was Candida species, then the list included Aspergillus species, Trichophyton species, Mucor species, and Rhizopus species. Among diabetic individuals, the highest number of fungal growths was observed from oral wash samples, then by the toe swabs, mid-stream urine, and hair fiber, and the least fungal growth was observed in nail samples. The study confirms that diabetic patients are more prone to fungal growth as compared to non-diabetic individuals.21
Another case study was reported which highlights the increasing cases of fungal infections, particularly Aspergillosis, in patients with uncontrolled diabetes. It shows the rare case of aspergillosis in the oral cavity of a 50-year-old woman with a history of uncontrolled diabetes and also includes symptoms like severe pain and swelling in the upper left back tooth region. Investigations included RT-PCR for COVID-19, blood sugar levels, Orthopantomogram (OPG), and Enhanced Computed Tomography (CECT) of the facial part. Surgical debridement and curettage of necrotic bone were performed, followed by a 10% potassium hydroxide (KOH) test and histopathological examination. The KOH test and histopathological examination confirmed the presence of Aspergillosis. The patient was treated with injectable amphotericin-B oral voriconazole, and also antidiabetic drugs. Follow-up revealed uneventful healing and the patient remained asymptomatic.22
Another case study was reported in Southern China. The case includes invasive fungal disease (IFD) in patients with type 2 diabetes were investigated. The study found a 0.4% prevalence of IFD among individuals suffering from type 2 diabetes, with candidiasis and cryptococcosis being the most common types of yeast infections. Treatment was administered to 90% of the patients, with the majority showing improvement. However, the study noted that poor prognosis was associated with conditions such as diabetic nephropathy, mixed fungal infections, disseminated IFD, and co-infections.23
Pharmacokinetic alternation of antifungal agents in diabetic patients
Diabetes can significantly impact the pharmacokinetics and pharmacodynamics of antifungal drugs. Several factors contribute to these alterations in diabetic individuals, including delayed gastric emptying, changes in drug distribution, and liver disease. These factors play a major role in modifying the effectiveness and metabolism of antifungal agents.
Delayed gastric emptying, or gastroparesis, is a common issue in diabetic patients, affecting drug absorption. Studies indicate that between 28% and 65% of diabetic individuals experience this condition, which can lead to delayed absorption of antifungal medications like tolazamide.24,25
Drug distribution is another factor influencing the pharmacokinetics of antifungal drugs. In diabetes, non-enzymatic glycation of albumin alters the protein structure, reducing drug-protein interactions. This results in higher free drug concentration and lower albumin levels in the blood. Antifungal drugs with significant protein binding, such as amphotericin B, itraconazole, ketoconazole, miconazole, caspofungin, anidulafungin, and micafungin, are particularly affected by these changes.26,27
Impaired drug distribution into tissue has also been observed in diabetic individuals, due to changes in the microvasculature or reduced vascular permeability. This is especially observed in patients with diabetic foot infections, where effective tissue penetration of antimicrobial agents is crucial. Although the data on antifungal drugs for the treatment of diabetic foot is limited, it has been observed that drugs like vancomycin, Fosfomycin, and macrolides (excluding telithromycin) exhibit reduced tissue distribution in diabetic patients.28,29
Additionally, diabetes is also associated with liver diseases such as non-alcoholic fatty liver disease (NAFLD) and diabetic hepatosclerosis, which can impair hepatic function and affect drug metabolism. In animal models, the impact of diabetes on drug biotransformation has been observed. Antifungals such as itraconazole, voriconazole, and micafungin, which undergo hepatic metabolism, are particularly affected by liver impairment. For instance, micafungin has shown reduced exposure in patients with modern hepatic impairment compared to healthy individuals.30-32
Common Fungal Infections in Diabetic Patients and Their Impact
Diabetes patients are more prone to various fungal infections due to several factors, including elevated blood sugar levels, which create an environment conducive to fungal infections. High glucose levels can lead to increased glycation. Additionally, reduced blood circulation, especially in the extremities, can contribute to the development and persistence of infections.33 Here are some of the most common fungal infections observed in diabetic patients:
Candidiasis
Candida species, particularly Candida albicans, are known for causing opportunistic infections in diabetic individuals. These infections can have various onsite effects on the body and lead to significant morbidity if not managed properly.34
Normally, candida is present in the gut microflora, with a colony-forming unit count of 10. However, in diabetic individuals, this can rise to up to 40%, disrupting the ecological balance of intestinal flora. Studies have shown that interleukin-12 serum levels can help inhibit yeast colonization in the gastrointestinal tract.34,35 Various factors increase the pathogenicity of candida species, including:
- Enzymatic activity: increased hydrolytic enzyme activity36
- Biofilm formation: formation of a film of microorganisms in the extracellular matrix of the cell which resists antifungal therapy.37,38
- Hydrophobicity: adhesion mediated by agglutinin-like proteins, which enhances virulence.39,40
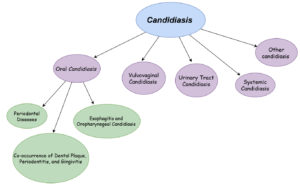
Candidiasis can occur in different body parts in diabetic patients, including the oral cavity, vulvovaginal area, urinary tract, and systemically. The physiology and treatment strategies vary depending on the location of the infection, as illustrated in Figure 2.
- Oral candidiasis: linked to uncontrolled hyperglycemia, high glucose levels in saliva, lower the salivary pH, the demised response of the tissue to injury, poor oral hygiene, and the presence of prostheses or medications like xerostomia.41
- Vulvovaginities: caused by uncontrolled hyperglycemia, high glucose levels, pregnancy, diabetes type, and aging.42,43
- Urinary tract infections: result from uncontrolled hyperglycemia, high sugar levels in urinary tract mucosa or systemic circulation, influenced by factors like gender and medications such as SGLT2 inhibitors.44,45
- Nail infections: Associated with high glucose levels in vaginal mucosa, duration of diabetes, and factors such as gender and age.46
Aspergillosis
Aspergillus is a filamentous, saprophytic fungus found in soil, hospital dust and construction sites. Out of around 900 species, 12 are known to cause disease in humans.47 Aspergillus infections cover a broad clinical spectrum including:
- Allergic Bronchopulmonary Aspergillosis (ABPA): occurs in individuals with cystic fibrosis or asthma, triggered by sensitization to A. fumigatus.
- Chronic Pulmonary Aspergillosis (CPA): affects immunocompetent individuals with preexisting lung conditions, presenting as simple aspergilloma, Aspergillosis nodules, chronic cavitary pulmonary aspergillosis (CCPA), and fibrosing pulmonary aspergillosis.
- Invasive Pulmonary Aspergillosis (IPA): this form, including invasive aspergillosis (IA), poses significant challenges due to high morbidity and mortality rates. While primarily affecting immunocompromised individuals, an increasing number of immunocompetent patients are also susceptible.48,49
Diagnostic approaches include high-resolution chest CT scans, CT pulmonary angiograms, and biomarkers, with varying sensitivity and specificity based on the patient’s immune system and sample type. The primary treatment involves antifungal medications, with voriconazole being the drug of choice. Alternatives like isavuconazole and echinocandins are used for intolerant cases. For severe cases, itraconazole and posaconazole serve as second-line treatment. Surgical intervention may be considered for patients with low surgical risk or those with aspergillomas. Recent advances in early detection and treatment have reduced mortality rates from around 90% to 40-50%. Ongoing follow-up is crucial for assessing treatment efficacy and patient recovery.50
Mucormycosis
Mucormycosis, also known as phycomycosis or zygomycosis. Mucormycosis is caused by fungi from the Mucorales order and the Mucoraceae family. It represents the third most common angio-invasive fungal infection after candidiasis and aspergillosis. The infection typically begins in the nose and paranasal sinuses following the inhalation of fungal spores. Genera involved include Absidia, Mucor, Rhizomucor, and Rhizopus. Despite the rich blood supply of maxillofacial areas, mucormycosis can affect the maxilla, particularly in immunocompromised individuals such as those with diabetes. Early diagnosis and prompt treatment are crucial to reduce mortality rates.51,52
Dermatophyte infections
Dermatophyte infections are widespread cutaneous infections involving the skin, hair, and nails. Dermatophytes are fungi that utilize keratin to cause infections. A study conducted by Dinesh et al. examined 80 individuals diagnosed with dermatophytosis. The male-to-female ratio was 51% to 48%. Skin infections were the most common, followed by nail infections, with hair infections being the least frequent. The predominant isolates were Trichophyton spp., followed by Microsporum spp. and Epidermophyton spp. Among these, Trichophyton rubrum was the most commonly found and isolated from skin scrapings, and T. mentagrophyte was second on the list. M. gypseum was isolated from both diabetic and non-diabetic patients, while E. floccosum was the least frequently isolated dermatophyte.53
Cryptococcosis
Cryptococcosis, particularly cryptococcal meningitis, is considered a significant cause of morbidity and mortality globally, with an estimated 181,100 deaths each year. Its role in diabetes remains unclear. A study by Archuleta et al. investigated patients with cryptococcal infections. Out of 96 cases, cryptococcal meningitis (49.0%) and pneumonia (36.5%) were the most commonly found fungal infection. The study found that pulmonary cryptococcosis with uncontrolled diabetes had a significantly higher mortality rate at 10 weeks compared to cases with controlled or no diabetes. Uncontrolled diabetes was associated with an increased rate of death. Multivariable analysis revealed increased odds of mortality for uncontrolled diabetes, with an 11% increase in mortality risk per 1% increase in HbA1c levels. The study concludes that uncontrolled diabetes can increase mortality from pulmonary cryptococcosis by up to 4 to 6 times at 10 weeks and 1 year, respectively.54
Common fungal infections in diabetic patients
The fungal infection occurs in diabetic individuals and affects various parts of the body. This led to significant mobility if not treated properly. The Table provides an overview of the most common fungal infections in diabetic individuals, including the specific body parts affected, recommended treatments, and references for further reading. By understanding the relationship between diabetes and these infections, healthcare professionals can better diagnose, treat, and prevent these conditions, thereby improving patient outcomes and quality of life.55
Table:
List of fungal infections that usually occur in diabetic patients
No. |
Fungal infection |
Body part |
Treatment |
Ref. |
|---|---|---|---|---|
1 |
Onychomycosis |
Usually occurs in nails, which over time results in dystrophy and disfigurement. |
Itraconazole, ketoconazole, and clotrimazole. Shows high resistance to fluconazole |
56 |
2 |
Candida parapsilosis |
Urinary tract infection |
fluconazole and amphotericin |
57 |
3 |
Aspergillus flavus |
Spinal infection |
Terbinafine hydrochloride |
58 |
4 |
Trichophyton rubrum |
Foot infection |
– |
59 |
5 |
Candida albicans, Candida tropicalis, Candida parapsilosis |
Foot infection |
Antifungal drugs |
60 |
6 |
Mucormycosis |
Nose and paranasal sinuses |
amphotericin B for 3 months |
51 |
7 |
Rhizopus oryzae |
Oral |
antifungal therapy with antitumor activity |
61 |
8 |
Cryptococcus neoformans |
CNS |
– |
62 |
Prevention and management strategies for fungal infections in diabetic patients
Effective prevention and management of fungal infections in diabetic patients involves a multifaceted approach that includes glycemic control, regular screening, hygiene practices, prompt treatment, and patient education. Below are detailed descriptions of these strategies:
Glycemic control
Maintaining optimal blood sugar levels is critical for reducing the risk of fungal infections in diabetic patients. High blood glucose levels can impair the immune system and create an environment that promotes fungal growth. Glycemic control helps to improve immune function and reduce the incidence of infections. A study was performed by Ooi et al., in which they took 9 studies involving 1459 patients and observed that insulin therapy helps to reduce the infection and also better neurological outcomes. It has also been observed that severe hypoglycemia shows no correlation with infection control.33,63
Regular Screening
Regular screening for fungal infections is essential for early detection and treatment, especially in high-risk areas such as the feet, nails, and mucous membranes. Diabetic patients should undergo periodic examinations by healthcare professionals to identify any signs of infection. Early detection allows for prompt intervention, which can prevent the progression of the infection and reduce complications.64
Hygiene Practices
Proper hygiene practices are vital for preventing fungal infections in diabetic patients. These practices include keeping the skin clean and dry, regularly washing and drying feet, and avoiding walking barefoot in public places. Additionally, wearing breathable footwear and changing socks daily can help prevent moisture buildup, which is a common breeding ground for fungi.65
Prompt treatment
Prompt treatment of fungal infections is crucial to prevent their spread and minimize complications. At the first sign of infection, diabetic patients should seek medical advice and begin appropriate antifungal therapy. Delayed treatment can lead to more severe infections that are harder to manage and can result in significant morbidity.66 Depending upon the type of fungal and on the site of fungal infection the type of drug used also differs for vaginal yeast infection caused due to non-albicans species being treated by fluconazole more efficiently.67
Patient education
Educating patients about the risks of fungal infections and the importance of preventive measures is essential. Patients should also be informed about proper fungal infections most likely to occur in diabetic patients. The importance of maintaining good hygiene, and the need for regular check-ups. They should also be taught how to recognize early signs of infection and the importance of adhering to prescribed treatments to ensure effective management.68
Diabetes mellitus increases the susceptibility to fungal infections, posing serious health challenges for affected individuals. The interplay between elevated blood glucose levels and compromised immune function creates an ideal environment for fungal pathogens to thrive. The review highlights the risk and the types and sites where fungal infections in diabetic patients were most likely to occur, emphasizing the importance of understanding the pathophysiological mechanisms involved. Common fungal pathogens like Candida, Aspergillus, and dermatophytes are often observed in diabetic individuals. They require specific treatment approaches and close monitoring. The case studies have also been discussed from the different geographical regions and the prevalence and severity of the infection among diabetic individuals gives the critical need for awareness and early diagnosis. By addressing the challenges caused by fungal infections in diabetic patients, it helps to improve the diagnostic and treatment approach.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Jitender Joshi, Chancellor, and Prof. (Dr.) Dharam Buddhi, Vice Chancellor of Uttaranchal University, Dehradun, for encouraging the publication of this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This work was funded under the seed money grant by Uttaranchal University, UU/DRI/SM/2024-25/017.
DATA AVAILABILITY
All datasets generated or analysed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Duklan S, Saha S, Jakhmola V, et al. Molecular Docking, Synthesis, In vitro Alpha Amylase and Antibacterial Activities of Newer Generation Pyrimidine Derivatives. Adv J Chem A. 2024;7(4):459-476.
Crossref - World Health Organisation. WHO report on diabetic cases https://www.who.int/europe/news-room/fact-sheets/item/diabetes Assessed 23 July, 2024
- Kumar A, Gangwar R, Abrar AZ, Kumar R, Sharma A. Prevalence of diabetes in India: A review of IDF diabetes atlas 10th edition. Current Diabetes Reviews. 2024;20(1):105-14.
Crossref - Arabi II, Hossain MA, Kawsar SMA, et al. Glucopyranoside Derivatives as Antibacterial and Antifungal Agents: QSAR, Molecular Docking and ADMET Analyses. Adv J Chem A .2024;6(4):334-411
Crossref - Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the US. Diabetes Care. 2001;24(6):1044-1049.
Crossref - Negm EM, Mohamed MS, Rabie RA, et al. Fungal infection profile in critically ill COVID-19 patients: a prospective study at a large teaching hospital in a middle-income country. BMC Infect Dis. 2023;23(1):246.
Crossref - Centers for Disease Control and Prevention data for fungal infection. https://www.cdc.gov/fungal/data-research/facts-stats/index.html Assessed 22 July 2024
- Chen X, Chen C, Wu M, et al. Causal relationship between type 1 diabetes mellitus and mycoses: a Mendelian randomization study. Front Med. 2024;11:1408297.
Crossref - Ledoux MP, Herbrecht R. Invasive pulmonary aspergillosis. J Fungi. 2023;9(2):131.
Crossref - Medical News Today report for yeast infection in diabetic patient https://www.medicalnewstoday.com/articles/317824. Assessed 20 July, 2024.
- Khanam A, Hithamani G, Naveen J, Pradeep SR, Barman S, Srinivasan K. Management of invasive infections in diabetes mellitus: A comprehensive review. Biologics. 2023;3(1):40-71.
Crossref - Padder SA, Padder RA, Ramzan A, et al. Glucose metabolic reprogramming and modulation in glycerol biosynthesis regulates drug resistance in clinical isolates of Candida. J Appl Microbiol. 2023;134(5):lxad091.
Crossref - Qadir MI, Asif H. An overview to candidiasis-a Candida infection. Int J Adv Res Microbiol Immunol. 2020;2(1):1-3.
- Mandal SM, Mahata D, Migliolo L, et al. Glucose directly promotes antifungal resistance in the fungal pathogen, Candida spp. J Biol Chem. 2014;289(37):25469-25473.
Crossref - Janssen AWM, Stienstra R, Jaeger M, et al. Understanding the increased risk of infections in diabetes: innate and adaptive immune responses in type 1 diabetes. Metabolism. 2021;121:154795.
Crossref - Zozulinska D, Majchrzak A, Sobieska M, Wiktorowicz K, Wierusz-Wysocka B. Serum interleukin-8 level is increased in diabetic patients. Diabetologia. 1999;42(1):117-118.
Crossref - Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interluekin-6 with metabolic syndrome X. Diabetologia. 1997;40(11):1286-92.
Crossref - Larkin JG, Frier BM, Ireland JT. Diabetes mellitus and infection. Postgrad Med J. 1985;61(713):233-237.
Crossref - Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl1):S27-36.
Crossref - Chavez-Reyes J, Escarcega-Gonzalez CE, Chavira-Suarez E, et al. Susceptibility for some infectious diseases in patients with diabetes: the key role of glycemia. Front Public Health. 2021;9:559595.
Crossref - Saud B, Bajgain P, Paudel G, et al. Fungal infection among diabetic and nondiabetic individuals in Nepal. Interdiscip Perspect Infect Dis. 2020;2020(1):7949868.
Crossref - Singhal I, Arora M, Dave A, Saluja P. Diabetes and fungal infection-a didactic relationship. J Clin Diagn Res. 2023;17(4):ZD01-ZD04.
Crossref - Lao M, Li C, Li J, Chen D, Ding M, Gong Y. Opportunistic invasive fungal disease in patients with type 2 diabetes mellitus from Southern China: clinical features and associated factors. J Diabetes Investig. 2020;11(3):731-44.
Crossref - Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13(9 Suppl 5):S16-22.
- Mohd Sazlly Lim S, Sinnollareddy M, Sime FB. Challenges in antifungal therapy in diabetes mellitus. J Clin Med. 2020;9(9):2878.
Crossref - Szkudlarek A, Sulkowska A, Maciazek-Jurczyk M, Chudzik M, Rownicka-Zubik J. Effects of non-enzymatic glycation in human serum albumin. Spectroscopic analysis. Spectrochim Acta A Mol Biomol Spectrosc. 2016;152:645-53.
Crossref - Arredondo G, Suarez E, Calvo R, Vazquez JA, Garcia-Sanchez J, Martinez-Jorda R. Serum protein binding of itraconazole and fluconazole in patients with diabetes mellitus. J Antimicrob Chemother. 1999;43(2):305-307.
Crossref - Muller M, dela Pena A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob Agents Chemother. 2004;48(5):1441-1453.
Crossref - Ray A, Malin D, Nicolau DP, Wiskirchen DE. Antibiotic tissue penetration in diabetic foot infections: a review of the microdialysis literature and needs for future research. J Am Podiatr Med Assoc. 2015;105(6):520-531.
Crossref - Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Non alcoholic fatty liver disease in diabetics-prevalence and predictive factors in a multiracial hospital clinic population in Malaysia. J Gastroenterol Hepatol. 2013;28(8):1375-1383.
Crossref - Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45(6):737-79.
Crossref - Lim SMS, Sinnollareddy M, Sime FB. Challenges in antifungal therapy in diabetes mellitus. J Clin Med. 2020;9(9):2878.
Crossref - Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16(5):442-449.
Crossref - Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. infections in patients with diabetes mellitus. J Clin Med. 2019;8(1):76.
Crossref - Bramono K, Yamazaki M, Tsuboi R, Ogawa H. Comparison of proteinase, lipase and alpha-glucosidase activities from the clinical isolates of Candida species. Jpn J Infect Dis. 2006;59(2):73-76.
- Ingham CJ, Boonstra S, Levels S, de Lange M, Meis JF, Schneeberger PM. Rapid susceptibility testing and microcolony analysis of Candida spp. cultured and imaged on porous aluminum oxide. PloS one. 2012;7(3):e33818.
Crossref - Chandra J, Mukherjee PK. Candida biofilms: development, architecture, and resistance. Microbiol Spectr. 2015;3(4):115-34.
Crossref - Rodrigues CF, Silva S, Henriques M. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis. 2014;33:673-88.
Crossref - Richardson JP, Ho J, Naglik JR. Candida-epithelial interactions. J Fungi. 2018;4(1):22.
Crossref - Hoyer LL, Cota E. Candida albicans agglutinin-like sequence (Als) family vignettes: a review of Als protein structure and function. Front Microbiol. 2016;7:280.
Crossref - Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78(922):455-459.
Crossref - Atabek ME, Akyurek N, Eklioglu BS. Frequency of vagynal Candida colonization and relationship between metabolic parameters in children with type 1 diabetes mellitus. J Pediatr Adolesc Gynecol. 2013;26(5):257-60.
Crossref - Deorukhkar SC, Saini S, Mathew S. Non albicans Candida infection: an emerging threat. Interdiscip Perspect Infect Dis. 2014;2014(1):615958.
Crossref - Yismaw G, Asrat D, Woldeamanuel Y, Unakal C. Prevalence of candiduria in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia. Iran J Kidney Dis. 2013;7(2):102.
- Esmailzadeh A, Zarrinfar H, Fata A, Sen T. High prevalence of candiduria due to non-albicans Candida species among diabetic patients: A matter of concern? J Clin Lab Anal. 2018;32(4):e22343.
Crossref - Michalopoulos A, Kriaras J, Geroulanos S. Systemic candidiasis in cardiac surgery patients. Eur J Cardiothorac Surg. 1997;11(4):728-31.
Crossref - Fernandez-Trujillo L, Eraso I, Morales EI, Sua LF. Invasive aspergillosis in a patient with diabetes mellitus as the only risk factor: case report and literature review. J Investig Med High Impact Case Rep. 2023;11:23247096231175443.
Crossref - Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med. 2018;141:121-31.
Crossref - Tudesq JJ, Peyrony O, Lemiale V, Azoulay E. Invasive pulmonary aspergillosis in nonimmunocompromised hosts. Semin Respir Crit Care Med. 2019;40(4):540-547.
Crossref - Bassetti M, Peghin M, Vena A. Challenges and solution of invasive aspergillosis in non-neutropenic patients: a review. Infect Dis Ther. 2018;7(1):17-27.
Crossref - Afroze SN, Korlepara R, Rao GV, Madala J. Mucormycosis in a diabetic patient: a case report with an insight into its pathophysiology. Contemp Clin Dent. 2017;8(4):662-6.
Crossref - Mallis A, Mastronikolis SN, Naxakis SS, Papadas AT. Rhinocerebral mucormycosis: an update. Eur Rev Med Pharmacol Sci. 2010;14(11).
- Dinesh K, Saikumar C. A study on the dermatophytic pattern in diabetic and non-diabetic patient. J Res Med Dent Sci. 2021;9(6):101-106.
- Archuleta S, Gharamti AA, Sillau S, et al. Increased mortality associated with uncontrolled diabetes mellitus in patients with pulmonary cryptococcosis: a single US cohort study. Ther Adv Infect Dis. 2021;8:20499361211004367.
Crossref - Labib A, Rosen J, Yosipovitch G. Skin manifestations of diabetes mellitus. Endotext. 2022.
- Idris AM, Hafiz TR, Getso MI, Umar MB, Kabuga AI. Onychomycosis: Prevalence, Fungal Pathogens, Risk Factors, and Antifungal Susceptibility Profile among People Living with Diabetes Mellitus in Kano, Northwestern Nigeria. Nigerian Journal of Basic and Clinical Sciences. 2024;21(3)196-200.
Crossref - Mathiasen ASF, Andersen MFB, Antsupova V, Fode M. ‘Case of the Month’from Herlev and Gentofte Hospital, Denmark: candidaemia and fungus ball in a patient with diabetes on sodium glucose co transporter 2 inhibitor. BJU Int. 2024.
Crossref - Li H, Pan H, Lei Y, Wang H, Li S, Xiao C. Spinal infection caused by Aspergillus flavus in a diabetic: a case report and literature review. Front Med. 2024;11:1348203.
Crossref - Eckhard M, Lengler A, Liersch J, Bretzel RG, Mayser P. Fungal foot infections in patients with diabetes mellitus-results of two independent investigations. Mycoses. 2007;50(Suppl 2):14-19.
Crossref - Kandregula S, Behura A, Behera CR, et al. A clinical significance of fungal infections in diabetic foot ulcers. Cureus. 2022;14(7):14-19.
Crossref - Beiglboeck FM, Theofilou NE, Fuchs MD, et al. Managing mucormycosis in diabetic patients: A case report with critical review of the literature. Oral Diseases. 2022;28(3):568-576.
Crossref - Nsenga L, Kajjimu J, Olum R, et al. Cryptococcosis complicating diabetes mellitus: a scoping review. Ther Adv Infect Dis. 2021;8:20499361211014769.
Crossref - Ooi YC, Dagi TF, Maltenfort M, et al. Tight glycemic control reduces infection and improves neurological outcome in critically ill neurosurgical and neurological patients. Neurosurgery. 2012;71(3):692-702.
Crossref - Zhang J, Zhang Z, Zhang K, Ge X, Sun R, Zhai X. Early detection of type 2 diabetes risk: limitations of current diagnostic criteria. Front Endocrinol. 2023;14:1260623.
Crossref - Bloomfield SF, Aiello AE, Cookson B, O’Boyle C, Larson EL. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am J Infect Control. 2007;35(10):S27-64.
Crossref - Scorzoni L, de Paula e Silva AC, Marcos CM, et al. Antifungal therapy: new advances in the understanding and treatment of mycosis. Front Microbiol. 2017;8:36.
Crossref - MONISTAT® 7 meets the CDC recommendation for treatment of yeast infections in people with diabetes. MONISTAT. https://hcp.monistat.com/diabetes#:~:text=MONISTAT%C2%AE%207%20meets%20the,butoconazole%2C%20miconazole%2C%20or%20terconazole. Assessed 24 July, 2024.
- Ferreira PL, Morais C, Pimenta R, et al. Knowledge about type 2 diabetes: its impact for future management. Front Public Health. 2024;12:1328001.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.