ISSN: 0973-7510
E-ISSN: 2581-690X
The World Health Organization identifies antimicrobial-resistance (AMR) among the top ten global health threats, potentially causing 10 million deaths annually by 2050. New global infections have created the need to track disease outbreaks and antibiotic-resistance to develop effective public health solutions. This systematic review aims to update the knowledge on antibiotic-resistant bacterial pathogens through wastewater surveillance carried out at different levels and geographical locations. The study initially screened 4467 articles based on the search criteria set for the current study after eliminating duplicates, review articles, systematic reviews, and articles published in languages other than English. Finally, we identified 156 articles, of which 53 were relevant articles for the systematic review and contained wastewater surveillance along with a comparison with clinical strains. After a careful review of the articles, we found two levels that were very important for antibiotic-resistance, one being the level of wastewater surveillance and the other being the method of screening for antibiotic- resistance, such as culture-based methods or genomic screening approaches. From these studies, we found that 52% were conducted at the single-sewer level, followed by clinical settings and international studies. Most international studies used the genomic screening approach, while, as regional studies, national-level studies used culture-based approaches. Although advanced genomic approaches, such as next-generation sequencing, offer greater advantages in predicting antibiotic-resistance genes and AMR surveillance, they cannot overcome the limitations associated with AMR monitoring, such as contamination from animal sources. Overall, this study indicates that the Enterobacteriaceae family is a highly evolving antibiotic-resistant pathogen, followed by the Enterococcaceae family. The use of research with clinical comparisons in wastewater surveillance avoids false-positive predictions and simplifies the process and interpretation of data.
Wastewater Surveillance, Antimicrobial-resistance, Multidrug-resistance, Sewage Water
Antimicrobial-resistance (AMR) is a broad term that refers to the resistance of all types of microorganisms, such as bacteria, viruses, fungi and parasites. In contrast, antibiotic-resistance is specifically related to bacteria. AMR and antibiotic- resistance are often used interchangeably but are not exactly the same. AMR in bacteria occurs when changes, such as DNA mutations during cell replication or gene transfer, make drugs less effective in treating infections. This has become a serious health issue worldwide in the 21st century.1 The recent overuse of antimicrobials has made AMR a growing problem. As a result, AMR is developing and spreading rapidly, making it a major cause of death worldwide, with the most significant effect observed in nations with few resources.2 Recent estimates suggest that by 2050, AMR could lead to 10 million deaths worldwide each year and cost approximately $1.2 trillion.1,2
Globally, 73.4% of deaths linked to bacterial AMR are caused by six pathogens: Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. Combinations of pathogens and drugs have led to more than 50,000 deaths per year, emphasizing the urgent need to create policies that focus on these particularly deadly pairings. This could be achieved by strengthening infection prevention programs, making it easier for people to receive important second-line antibiotics when needed, and by promoting the development of vaccines and new antibiotics.
Bacteria that are resistant to antibiotics present a health risk as serious as major illnesses such as HIV and malaria. This issue affects every region worldwide, with sub-Saharan Africa experiencing the highest levels of antibiotic resistance. To make smart policy decisions, especially regarding access to important antibiotics, infection prevention programs, and the development of new vaccines and antibiotics, it is crucial to understand the impact of AMR and the key pathogen-drug combinations that drive this problem.1
AMR surveillance is crucial for tracking trends, assessing the effectiveness of interventions, and creating evidence-based treatment guidelines, as outlined in the World Health Organization (WHO) global AMR action plan.3 Large networks, such as the European Antimicrobial-resistance Surveillance Network (EARS-Net) and Global Antimicrobial Resistance and Use Surveillance System (GLASS), have been established to meet this need.4 Keeping track of AMR is essential as it collects information on the locations and timing of resistance occurrences, identifies novel or uncommon resistance characteristics, and monitors developing patterns. This information is crucial for managing AMR infections. This helps in deciding what actions to take, assessing the success of past efforts, guiding treatment choices, reducing adverse effects, and developing new antibiotics. Surveillance data are valuable to healthcare providers, policymakers, and scientists.5 Wastewater surveillance, that is, monitoring municipal sewage, is an approach used to monitor community AMR patterns at the sewer shed, hospital, national, and global population levels.1 Wastewater surveillance can reveal information about bacteria and their resistance traits, helping to predict potential issues for entire communities before they become widespread.6-9
Municipal sewage can be a valuable resource for tracking antibiotic-resistant bacteria (ARB) because it contains a mixture of antibiotics and antibiotic-resistance genes (ARGs). It also includes bacterial matter from all ARB including pathogens found throughout the community. Even before the first symptoms appear, individuals can begin to shed pathogenic biomarkers in municipal sewage systems contributed by bodily excretions, such as nasal mucus, sputum, urine, and faces, even before the first symptoms appear, making early detection possible with wastewater surveillance.10,11 However, clinical surveillance can be quite slow, often taking several days because it involves many steps. These include exposure to pathogens, colonization, display of symptoms, sample collection, analysis, and then reporting of results.12,13 Therefore, by monitoring wastewater, outbreaks can be detected earlier than in clinical reports. This method is also beneficial because it faces fewer legal and ethical issues related to individual privacy, as it does not involve the direct sampling from people.14 Additionally, wastewater surveillance can help track the presence of multidrug-resistant (MDR) pathogens in a community.11
In this review, we present our literature survey on antibiotic resistance in effluents, treatment plants, and hospital sewage treatment plants, as well as a comparison with clinical isolates, to improve our understanding of how environmental pollution affects pathogen evolution, transmission, and development of resistant clinical variants.
For this systematic review, an electronic search strategy was carried out by conducting a comprehensive literature search for all pertinent peer-reviewed published studies using bibliographic databases such as MEDLINE (National Library of Medicine), EMBASE (Excerpta Medica dataBASE), PubMed, Google Scholar, Web of Science, and Scopus. The results were limited to the English language, with no restrictions on the year of publication or duplications. We manually reviewed the references of the selected articles to identify additional studies. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for searching and reporting the literature review.15
The searches were designed to align with our review goals by using keywords related to publication titles. Our systematic review aimed to identify the literature on human clinical epidemiology and the monitoring of AMR in municipal and hospital wastewater. To narrow down the search results, we used several screening strategies. First, we looked at the titles, then read the abstracts carefully, and finally, we thoroughly examined the full articles. Studies that compared AMR in wastewater surveillance with clinical isolates were included. The exclusion criteria were as follows: duplicates, publications without ARB, literature related to AMR only in wastewater surveillance, and no comparison with clinical isolates. We excluded papers written in languages other than English. Studies on wastewater surveillance of AMR that lacked comparison with clinical data, letters/short communications, book chapters, mini-reviews, literature reviews, systematic reviews, and meta-analyses were excluded.
First, we imported all the identified articles into Zotero, a reference management tool. We then used Zotero’s “merge duplicates” feature to combine duplicate articles from different search engines. Next, the titles of the remaining articles were reviewed to identify potential articles for further review. Subsequently, we manually read the abstracts to identify the studies relevant to the focus of our systematic review. This study reviewed the literature on wastewater surveillance for AMR, including findings from clinical research studies and reports. It also covers surveys conducted by government authorities at various levels such as individual or multiple sewer systems, hospitals in different geographical areas (including global, international, national, and regional scales), and data from single sewer systems and hospitals.
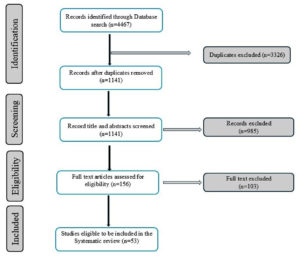
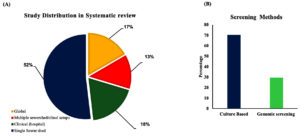
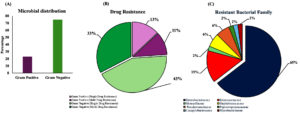
Figure 1 illustrates the literature search process. We found 4,467 articles in the database search. After removing duplicates, 1,141 articles were retained for review. Of these, 985 were irrelevant and were excluded from the study. We then examined the full text of 156 studies to determine whether they met our review criteria, and 103 of them were ultimately excluded. We excluded articles that focused solely on surveillance without comparing the primary results with clinical evidence. Finally, 53 studies were included in the systematic analysis. Of these, 52% were conducted at a single-sewer shed level, 18% at a clinical setting, 17% at an international level (global), and 13% at a national or regional level (covering two or more cities or multiple sewer systems) (Figure 2A). The majority of the studies were conducted in Europe, comprising 35 of the 53 studies. This was followed by Asia (ten studies), North America (four studies), Africa (two studies), and then Oceania and South America (one study each). Culture- and genomic-based approaches are used to detect AMR. Culture-based approaches were found to be the most commonly used for AMR screening (71.6%) (Figure 2B). In all the studies included in this systematic review, we found that 75% of the pathogens were gram-negative, 43% were single-drug resistant, and 33% were MDR strains (Figure 3A, 3B). However, the survey showed that resistance among Gram-positive bacteria was only 13% for single-drug resistance and 12% for multidrug-resistance (Figure 3B). A general surveillance study indicated that Enterobacteriaceae and Enterococcaceae were the dominant families of AMR-associated pathogens (Figure 3C).
Figure 2. (A) Pie chart representing percentage of studies conducted at different levels: global studies (including intercontinental, international, multinational studies), multiple sewer shed/clinical studies (studies conducted at national, multiple hospital setups), studies from hospitals, and studies from single sewer shed. (B) Studies grouped based on the screening methods
Figure 3. Systematic review addressing the drug-resistance survey across studies: (A) studies grouped based on the drug-resistant pathogens. (B) Studies grouped based on the distribution of single drug-resistant pathogen and multidrug-resistant pathogens. (C) Pie chart representing percentage of studies conducted at different levels: global studies (including intercontinental, international, multinational studies), multiple sewer shed/clinical studies (studies conducted at national and multiple hospital setups), studies from hospitals, and studies from single sewer shed
Furthermore, we found that most of the studies included in this survey used culture-based methods: single sewer shed level (n = 25), hospital level (n = 9), national level (n = 6), and international level (n = 1). In studies that applied culture-based methods, 15 targeted Escherichia coli.8,9,11,16-27 Other studies targeted ESBL-producing bacteria,26,28-31 M. tuberculosis,32 Acinetobacter baumannii,33 Staphylococcus aureus,34-36 Pseudomonas spp.,36-38 Salmonella spp.,10,39 cephalosporin-resistant Klebsiella pneumoniae,40 Clostridioides difficile (previously called Clostridium difficile),41 bacteria of the coliform group,42 and Campylobacter spp.43 We grouped the non-culture-based studies as genomic studies, which included studies using polymerase chain reaction (PCR)-based, metagenomic, and next-generation sequencing approaches. We found that two studies that focused on a single-sewer system used PCR-based method,30,32 whereas another study conducted at a hospital also used PCR analysis.44 Furthermore, two studies used metagenomic analysis.45,46 Three of the identified studies used whole genome sequencing (WGS), one of which used a combination of culturing and WGS.31,33,44 Most of these studies compared the results of effluent analyses with clinical isolates obtained from hospitals; however, a small number of these studies directly collected clinical isolates from human patients. Based on the included studies, we found that approximately half of the genome research (42.8%, 6/14 studies) that used metagenomic analysis and next-generation sequencing was carried out at the global level. Genomic analysis has provided more information on ARGs and their prevalence in various geographic locations.7,47,48 Reports and studies published using the World Bank’s Human Development Index (HDI) data47,49 and cases reported in the European Antimicrobial Resistance Surveillance Network (EARS-Net)7,8,49 indicate an association between ARB in hotspots and factors such as increased antibiotic use, inadequate sanitation, and lower socioeconomic status. However, studies carried out at the national level have indicated the prevalence of antibiotic-resistance18,41,50 in wastewater from areas with/without hospitals,9 which is in agreement with government-reported data and secondary literature.26,35,51 Seven studies used metagenomic sequencing and WGS, whereas four studies used culture-based methods to explore the potential connection between ARB in wastewater and clinical isolates. Although there have been various studies at the national and international levels demonstrating antibiotic resistance in wastewater effluents, we chose studies with clinical comparisons of ARB isolates from wastewater.
Antibiotics are highly effective for treating bacterial infections in both humans and animals. However, the extensive use of antibiotics in medical treatment has led to elevated concentrations of these medications52 in the human microbiota and significant amounts end up in urban wastewater. This is because drugs are not fully broken down by the body or are discarded improperly.53 The presence of antibiotics in the environment has led to an increase in ARB and ARGs, which are now commonly found in wastewater.54 Routine surveillance of wastewater for the presence of resistant bacteria and genes is crucial to assess and anticipate the public health risks associated with drug-resistant infections. The rise in ARB and the widespread impact of drug-resistant infections constitute a significant health crisis.3 Furthermore, it has been observed that the death rates from AMR are highest in certain low- and middle-income countries in sub-Saharan Africa and South Asia. Therefore, AMR is a significant global health issue and a serious problem for the world’s poorest nations.1 Unfortunately, according to our literature survey, we found that lower-income countries adopt culture-based methods for AMR surveillance; thus, these studies lacked data on ARGs.
The surveillance of wastewater for AMR focuses mainly on the following areas: (1) to evaluate the level of AMR in people and compare it by analyzing wastewater data, (2) to determine how AMR bacteria avoid treatment in wastewater, and (3) to understand how AMR might develop. Monitoring wastewater can be a valuable and affordable way to spot areas where resistant bacteria are common and track their presence in real-time.55 Currently, there is little global monitoring of ARB using wastewater analysis. Our review shows that, while there are various studies on wastewater surveillance, many do not have clinical evidence to support their results. AMR has been highlighted in the Global Action Plan on AMR, UN Interagency Coordination Group, One Health Global Leaders Group, and several others. Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Streptococcus pneumoniae, and Acinetobacter baumannii are recognized as key pathogens by the WHO and are the main contributors to the burden of AMR. Drug-resistance in these pathogens poses a significant global health risk. To effectively address this issue, increased monitoring, funding, research, and targeted actions from the global health community are needed.1,3
In the current surveillance study, we identified the aforementioned infections and their levels of drug-resistance. This gives us a clear understanding of how common antibiotic -resistance is and how these strains are developing into multidrug-resistant forms in the areas we studied. However, the data and methods required to predict the origin of these infections are currently lacking. The final example is A. baumannii. In this study, we detected an MDR isolate of A. baumanni, although there is no evidence to support the source (whether it is human or animal).
Antibiotics such as carbapenems, cephalosporins, penicillins, and fluoroquinolones, which are typically the first choice for treating serious infections, are responsible for over 70% of deaths linked to antibiotic-resistance.56 We identified the highest number of studies related to resistance against β-lactam antibiotics, particularly within the Enterobacteriaceae family. We believe that this resistance was most likely caused by extensive use of these antibiotics. Our speculation agrees with the findings and conclusions of studies made by Zanotto et al.57
In 2017, the WHO released a list highlighting the need for novel and effective antibiotics. This list aims to guide research and development efforts, focusing on MDR infections that are particularly problematic in hospital environments. There are five key strategies for facing the issue of bacterial resistance. (1) Infection prevention and control of infections are essential to prevent infections and fight AMR. This includes hospital programs to prevent infections and community initiatives focused on enhancing water quality, sanitation, and hygiene practices. These community programs are especially crucial in low- and middle-income countries, where access to clean water and sanitation is limited and AMR is a significant issue. (2) Vaccinations are crucial for stopping infections and reducing reliance on antibiotics. Vaccination programs are a key strategy to prevent diseases such as S. pneumoniae; developing vaccines for pathogens that currently lack one is also essential.58,59 (3) Reducing consumption of antibiotics that are not needed to treat diseases might be the key to reducing risks. (4) Reducing or termination of antibiotic use when not needed, especially for viral infections for which they are not effective. It is important to use antibiotics only when necessary to treat diseases.52 (5) Considering the importance of bacterial resistance to antibiotics worldwide, it is crucial to invest in developing new antibiotics and ensure that second-line antibiotics are available in regions where they are not widely accessible.60 ARB wastewater surveillance is performed primarily using culture-based methods, metagenomics-based approaches, and quantitative real-time PCR (qPCR).61 Based on our literature survey in the present study, 71% of the surveyed studies used culture-based screening methods to identify AMR. Culture-based methods are affordable and effective in identifying specific types of bacteria. Molecular methods, including qPCR and high-throughput qPCR (HT-qPCR), can be used alongside culture-based methods to enhance their effectiveness. These methods mainly focus on monitoring ARGs as evidence of ARB61. A metagenomics-based approach offers a broader coverage of ARGs and is better suited for screening without a specific target. However, this method and other methods have limitations: they cannot set clinical thresholds and cannot distinguish between live and dead cells.62,63 The best approaches for detecting AMR are through phenotypic susceptibility tests and genotypic (q)PCRs. However, these techniques require a significant amount of time and are not flexible in the continuous search for new mutations or genes. WGS is a fast and effective method for analyzing and identifying AMR genes.62 Genomic methods offer more detailed and adaptable results but are associated with a significantly higher cost. Furthermore, the sensitivity of AMR gene detection depends on the depth of sequencing and the accurate association of specific markers of the AMR gene with the strains.4 One of the main challenges in monitoring ARB in wastewater is the presentation of accurate quantitative data because the concentration of the target bacteria can vary widely in different samples. Furthermore, bacteria can develop and grow in their environment, adding another layer of complexity to the analysis. Using wastewater to track ARB can be challenging when attempting to estimate the prevalence of a disease in a population accurately, especially in studies focusing on gene-based antibiotic resistance. The presence of ARB in wastewater does not pinpoint the exact source of contamination. In addition, pathogens in wastewater can originate from both animals and humans.64,65
This review demonstrates that bacterial AMR is a major global health concern. This study sheds light on the identification of AMR at different levels and in different geographic regions. This study also provides information on the predominant bacterial families associated with drug evolution and resistance. It also warrants more multicenter studies across the country to profile AMR in pathogens and develop mitigation strategies to reduce the burden of bacterial AMR.
ACKNOWLEDGMENTS
The authors extend their appreciation to the “Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia” for funding this research work through project number IFP2021-085. In addition, the authors extend their appreciation to Shaqra University for providing the basic research infrastructure and guidance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This research was funded by the “Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia” through project number IFP2021-085.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655.
Crossref - O’Neill J. AMR Review: Review on antimicrobial resistance; Tackling drug-resistant infections globally: final report and recommendations. 2020. Accessed October 17, 2024. https://amr-review.org/
- World Health Organization. Global Action Plan on Antimicrobial Resistance. World Health Organization; 2015. https://iris.who.int/handle/10665/193736. Accessed July 25, 2024.
- Chau KK, Barker L, Budgell EP, et al. Systematic review of wastewater surveillance of antimicrobial resistance in human populations. Environ Int. 2022;162:107171.
Crossref - Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318-327.
Crossref - Khan FA, Hellmark B, Ehricht R, Soderquist B, Jass J. Related carbapenemase-producing Klebsiella isolates detected in both a hospital and associated aquatic environment in Sweden. Eur J Clin Microbiol Infect Dis. 2018;37(12):2241-2251.
Crossref - Parnanen KMM, Narciso-da-Rocha C, Kneis D, et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci Adv. 2019;5(3):eaau9124.
Crossref - Huijbers PMC, Larsson DGJ, Flach CF. Surveillance of antibiotic resistant Escherichia coli in human populations through urban wastewater in ten European countries. Environ Pollut. 2020;261:114200.
Crossref - Blaak H, Kemper MA, de Man H, et al. Nationwide surveillance reveals frequent detection of carbapenemase-producing Enterobacterales in Dutch municipal wastewater. Sci Total Environ. 2021;776:145925.
Crossref - Diemert S, Yan T. Clinically Unreported Salmonellosis Outbreak Detected via Comparative Genomic Analysis of Municipal Wastewater Salmonella Isolates. Appl Environ Microbiol. 2019;85(10):e00139-19.
Crossref - Flach CF, Hutinel M, Razavi M, Ahren C, Larsson DGJ. Monitoring of hospital sewage shows both promise and limitations as an early-warning system for carbapenemase-producing Enterobacterales in a low-prevalence setting. Water Res. 2021;200:117261.
Crossref - Thakali O, Raya S, Malla B, et al. Pilot study on wastewater surveillance of dengue virus RNA: Lessons, challenges, and implications for future research. Environ Chall. 2022;9:100614.
Crossref - Tiwari A, Adhikari S, Kaya D, et al. Monkeypox outbreak: Wastewater and environmental surveillance perspective. Sci Total Environ. 2023;856(part 2):159166.
Crossref - Auguet OT, Niehus R, Gweon HS, et al. Population-level faecal metagenomic profiling as a tool to predict antimicrobial resistance in Enterobacterales isolates causing invasive infections: An exploratory study across Cambodia, Kenya, and the UK. EClinicalMedicine. 2021;36:100910.
Crossref - Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Crossref - Jakobsen L, Sandvang D, Hansen LH, et al. Characterisation, dissemination and persistence of gentamicin resistant Escherichia coli from a Danish university hospital to the waste water environment. Environ Int. 2008;34(1):108-115.
Crossref - Yang CM, Lin MF, Liao PC, et al. Comparison of antimicrobial resistance patterns between clinical and sewage isolates in a regional hospital in Taiwan. Lett Appl Microbiol. 2009;48(5):560-565.
Crossref - Reinthaler FF, Galler H, Feierl G, et al. Resistance patterns of Escherichia coli isolated from sewage sludge in comparison with those isolated from human patients in 2000 and 2009. J Water Health. 2013;11(1):13-20.
Crossref - Zarfel G, Galler H, Feierl G, et al. Comparison of extended-spectrum-β-lactamase (ESBL) carrying Escherichia coli from sewage sludge and human urinary tract infection. Environ Pollut. 2013;173:192-199.
Crossref - Jorgensen SB, Soraas AV, Arnesen LS, Leegaard TM, Sundsfjord A, Jenum PA. A comparison of extended spectrum β-lactamase producing Escherichia coli from clinical, recreational water and wastewater samples associated in time and location. PLoS One. 2017;12(10):e0186576.
Crossref - Ojer-Usoz E, Gonzalez D, Vitas AI. Clonal Diversity of ESBL-Producing Escherichia coli Isolated from Environmental, Human and Food Samples. Int J Environ Res Public Health. 2017;14(7):676.
Crossref - Hutinel M, Huijbers PMC, Fick J, Ahren C, Larsson DGJ, Flach CF. Population-level surveillance of antibiotic resistance in Escherichia coli through sewage analysis. Euro Surveill. 2019;24(37):1800497.
Crossref - Paulshus E, Kuhn I, Mollby R, et al. Diversity and antibiotic resistance among Escherichia coli populations in hospital and community wastewater compared to wastewater at the receiving urban treatment plant. Water Res. 2019;161:232-241.
Crossref - Raven KE, Ludden C, Gouliouris T, et al. Genomic surveillance of Escherichia coli in municipal wastewater treatment plants as an indicator of clinically relevant pathogens and their resistance genes. Microb Genom. 2019;5(5):e000267.
Crossref - Adator EH, Narvaez-Bravo C, Zaheer R, et al. A One Health Comparative Assessment of Antimicrobial Resistance in Generic and Extended-Spectrum Cephalosporin-Resistant Escherichia coli from Beef Production, Sewage and Clinical Settings. Microorganisms. 2020;8(6):885.
Crossref - Taro U, Mitsuhiro O, Hirofumi T. Prevalence of ESBL-producing Escherichia coli and carbapenem-resistant Enterobacteriaceae in treated wastewater: a comparison with nosocomial infection surveillance. J Water Health. 2020;18(6):899-910.
Crossref - Tiwari A, Paakkanen J, Osterblad M, Kirveskari J, Hendriksen RS, Heikinheimo A. Wastewater Surveillance Detected Carbapenemase Enzymes in Clinically Relevant Gram-Negative Bacteria in Helsinki, Finland; 2011-2012. Front Microbiol. 2022;13:887888.
Crossref - Drieux L, Haenn S, Moulin L, Jarlier V. Quantitative evaluation of extended-spectrum β-lactamase-producing Escherichia coli strains in the wastewater of a French teaching hospital and relation to patient strain. Antimicrob Resist Infect Control. 2016;5:9.
Crossref - Rodriguez EA, Ramirez D, Balcazar JL, Jimenez JN. Metagenomic analysis of urban wastewater resistome and mobilome: A support for antimicrobial resistance surveillance in an endemic country. Environ Pollut. 2021;276:116736.
Crossref - Bich VTN, Thanh LV, Thai PD, et al. An exploration of the gut and environmental resistome in a community in northern Vietnam in relation to antibiotic use. Antimicrob Resist Infect Control. 2019;8:194.
Crossref - Davidova-Gerzova L, Lausova J, Sukkar I, et al. Hospital and community wastewater as a source of multidrug-resistant ESBL-producing Escherichia coli. Front Cell Infect Microbiol. 2023;13.
Crossref - Mtetwa HN, Amoah ID, Kumari S, Bux F, Reddy P. Wastewater-Based Surveillance of Antibiotic Resistance Genes Associated with Tuberculosis Treatment Regimen in KwaZulu Natal, South Africa. Antibiotics. 2021;10(11):1362.
Crossref - Odih EE, Sunmonu GT, Okeke IN, Dalsgaard A. NDM-1- and OXA-23-producing Acinetobacter baumannii in wastewater of a Nigerian hospital. Microbiol Spectr. 2024;11(6):e02381-23.
Crossref - Rahimi F, Bouzari M. Biochemical Fingerprinting of Methicillin-Resistant Staphylococcus aureus Isolated From Sewage and Hospital in Iran. Jundishapur J Microbiol. 2015;8(7):e19760.
Crossref - Meir-Gruber L, Manor Y, Gefen-Halevi S, et al. Population Screening Using Sewage Reveals Pan-Resistant Bacteria in Hospital and Community Samples. PLOS ONE. 2016;11(10):e0164873.
Crossref - Kolokotsa A, Leotsinidis M, Kalavrouziotis I, Sazakli E. Effects of tourist flows on antibiotic resistance in wastewater of a Greek island. Journal of Applied Microbiology. 2021;130(2):516-527.
Crossref - Golle A, Janezic S, Rupnik M. Low overlap between carbapenem resistant Pseudomonas aeruginosa genotypes isolated from hospitalized patients and wastewater treatment plants. PLoS One. 2017;12(10):e0186736.
Crossref - Aljanaby AAJ. Antibiotics Susceptibility Pattern and Virulence-associated Genes in Clinical and Environment Strains of Pseudomonas aeruginosa in Iraq. Asian J Sci Res. 2018;11(3):401-408.
Crossref - Pignato S, Coniglio MA, Faro G, Lefevre M, Weill FX, Giammanco G. Molecular epidemiology of ampicillin resistance in Salmonella spp. and Escherichia coli from wastewater and clinical specimens. Foodborne Pathog Dis. 2010;7(8):945-951.
Crossref - Rocha J, Ferreira C, Mil-Homens D, et al. Third generation cephalosporin-resistant Klebsiella pneumoniae thriving in patients and in wastewater: what do they have in common? BMC Genomics. 2022;23:72.
Crossref - Moradigaravand D, Gouliouris T, Ludden C, et al. Genomic survey of Clostridium difficile reservoirs in the East of England implicates environmental contamination of wastewater treatment plants by clinical lineages. Microb Genom. 2018;4(3):e000162.
Crossref - Khan FA, Söderquist B, Jass J. Prevalence and Diversity of Antibiotic Resistance Genes in Swedish Aquatic Environments Impacted by Household and Hospital Wastewater. Front Microbiol. 2019;10:688.
Crossref - Mourkas E, Florez-Cuadrado D, Pascoe B, et al. Gene pool transmission of multidrug resistance among Campylobacter from livestock, sewage and human disease. Environ Microbiol. 2019;21(12):4597-4613.
Crossref - White L, Hopkins KL, Meunier D, et al. Carbapenemase-producing Enterobacteriaceae in hospital wastewater: a reservoir that may be unrelated to clinical isolates. J Hosp Infect. 2016;93(2):145-151.
Crossref - Majeed HJ, Riquelme MV, Davis BC, et al. Evaluation of Metagenomic-Enabled Antibiotic Resistance Surveillance at a Conventional Wastewater Treatment Plant. Front Microbiol. 2021;12:657954.
Crossref - Kelly SA, O’Connell NH, Thompson TP, et al. Large-scale characterization of hospital wastewater system microbiomes and clinical isolates from infected patients: profiling of multi-drug-resistant microbial species. J Hosp Infect. 2023;141:152-166.
Crossref - Hendriksen RS, Munk P, Njage P, et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat Commun. 2019;10:1124.
Crossref - Riquelme MV, Garner E, Gupta S, et al. Wastewater Based Epidemiology Enabled Surveillance of Antibiotic Resistance. medRxiv. 2021:6(1):21258164.
Crossref - Karkman A, Berglund F, Flach CF, Kristiansson E, Larsson DGJ. Predicting clinical resistance prevalence using sewage metagenomic data. Commun Biol. 2020;3(1):711.
Crossref - Gouliouris T, Raven KE, Moradigaravand D, et al. Detection of vancomycin-resistant Enterococcus faecium hospital-adapted lineages in municipal wastewater treatment plants indicates widespread distribution and release into the environment. Genome Res. 2019;29(4):626-634.
Crossref - Su JQ, An XL, Li B, et al. Metagenomics of urban sewage identifies an extensively shared antibiotic resistome in China. Microbiome. 2017;5:84.
Crossref - Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176-187.
Crossref - Triggiano F, Calia C, Diella G, Montagna MT, De Giglio O, Caggiano G. The Role of Urban Wastewater in the Environmental Transmission of Antimicrobial Resistance: The Current Situation in Italy (2010-2019). Microorganisms. 2020;8(10):1567.
Crossref - Bouki C, Venieri D, Diamadopoulos E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol Environ Saf. 2013;91:1-9.
Crossref - Tiwari A, Krolicka A, Tran TT, et al. Antibiotic resistance monitoring in wastewater in the Nordic countries: A systematic review. Environ Res. 2024;246:118052.
Crossref - World Health Organization (WHO). The Selection and Use of Essential Medicines. 2015-TRS 994. Accessed October 23, 2024. https://www.who.int/publications/i/item/9789241209946
- Zanotto C, Bissa M, Illiano E, et al. Identification of antibiotic-resistant Escherichia coli isolated from a municipal wastewater treatment plant. Chemosphere. 2016;164:627-633.
Crossref - Jansen KU, Knirsch C, Anderson AS. The role of vaccines in preventing bacterial antimicrobial resistance. Nat Med. 2018;24(1):10-19.
Crossref - Klugman KP, Black S. Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects. Proc Natl Acad Sci U S A. 2018;115(51):12896-12901.
Crossref - World Health Organization. 2019 antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline. Accessed August 21, 2024. https://www.who.int/publications/i/item/9789240000193
- Karkman A, Do TT, Walsh F, Virta MPJ. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018;26(3):220-228.
Crossref - Berbers B, Saltykova A, Garcia-Graells C, et al. Combining short and long read sequencing to characterize antimicrobial resistance genes on plasmids applied to an unauthorized genetically modified Bacillus. Sci Rep. 2020;10(1):4310.
Crossref - Ferreira C, Otani S, Aarestrup FM, Manaia CM. Quantitative PCR versus metagenomics for monitoring antibiotic resistance genes: balancing high sensitivity and broad coverage. FEMS Microbes. 2023;4:xtad008.
Crossref - Diebold PJ, New FN, Hovan M, Satlin MJ, Brito IL. Linking plasmid-based beta-lactamases to their bacterial hosts using single-cell fusion PCR. Elife. 2021;10:e66834.
Crossref - Hultman J, Tamminen M, Parnanen K, Cairns J, Karkman A, Virta M. Host range of antibiotic resistance genes in wastewater treatment plant influent and effluent. FEMS Microbiol Ecol. 2018;94(4):fiy038.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.