ISSN: 0973-7510
E-ISSN: 2581-690X
Bloodstream infections (BSI) belong to the most detrimental healthcare associated infections (HAI) that have an impact on the treatment result of patients hospitalised to intensive care units. Recently incidence of nosocomial fungal BSI is drastically increased in ICU patients. Although Candida BSI are well studied, there is an acute lack of data relevant to other fungi from underdeveloped countries. The present study aimed to evaluate the fungal bloodstream infection (BSI) in patients admitted to an ICU at a tertiary care teaching hospital. A conventional mycological methodology was used to identify the fungal infections isolated from BSI to the species level, and their antifungal susceptibility profile was examined. Risk factors pertaining to fungal BSI were analyzed. The rate of nosocomial BSI was 1.2%. The rate of central line associated blood stream infection (CLABSI)/1000 central line days was 3.9. The rate of fungal BSI was 5.6%. Contributing factors are gender (Male), advancing age, increased hospital stays, and central venous catheterization were significantly associated with the development of nosocomial BSI. Candida spp. was the predominant pathogen. Fluconazole resistance was observed. resistant to fluconazole was found in 61.5% of Candida isolates. Fungal pathogens have emerged as important cause of nosocomial BSI. From this study, it can be concluded that Hitherto, fungal isolates, once rarely encountered like Non-albicans Candida spp., Trichosporon spp. are now common in invasive mycosis. These pathogens often demonstrate less susceptibility to antifungal drugs, hence are associated with poor/no response to therapy and therefore may present as mayhem to patients. Finally, this emerging mayhem necessitates the importance of being vigilant about predisposing factors, strict implementation of infection prevention and control and initiation of antifungal stewardship program.
Blood Stream Infection, Candida spp., Fungal Pathogens, Healthcare Associated Infections
In recent years, healthcare-associated infections (HAI) have become a significant concern to the safety of individuals admitted to hospitals, particularly in critical care units. HAIs are infections that happen when a patient is in the hospital but are not present or incubating at the time of admission, according to the definition provided by the Centres for Disease Control and Prevention (CDC) National Healthcare Safety Network. Usually, these infections start 48 to 72 hours after admission and manifest within 10 days following discharge from the hospital.1
As per literature, hundreds of millions of patients worldwide are affected by HAI per annum. HAI affects 5-15% of hospitalised patients and can result in problems for 25-50% of patients treated to intensive care units (ICUs) in developed countries.2 In addition to high mortality and morbidity rates, HAI have several implications including increased hospitalization and incremental economic burden.3
Blood stream infections (BSI) is among the most deleterious HAI has affects the therapeutic outcome of the patients admitted to ICUs. The risk factors for nosocomial BSI includes, advanced age, underlying disorders, a history of trauma, associated healthcare, antibiotic therapy and prolonged immobility.
Fungal HAI is less anticipated by clinicians when compared to bacterial HAI. Though fungi are emerging causes of HAI. On recent time, the incidence of nosocomial fungal BSI is drastically increased in ICU patients and widespread use of immunosuppressive medicines, glucocorticoids, invasive surgeries, and broad-spectrum antibiotics is to blame for this.4,5
Studies from developed countries have documented Candida spp., Aspergillus spp., Fusarium, Mucorales and Saccharomyces cerevisiae as important fungal isolates from nosocomial BSI in ICU setup.6-8 Data regarding other fungus from developing nations are extremely scarce, despite the fact that Candida BSI are well researched. In this tertiary care teaching hospital, the goal of the study was to investigate fungal bloodstream infections (BSI) in patients receiving ICU care.
The current descriptive cross-sectional research was carried out across three years (2019-2022) at the Microbiology Department, Government Medical College and Hospital, Kota, Rajasthan. The protocol for the research was approved by the institutional ethics committee. Inclusion criteria include all hospitalized patients suspected of having fungal and bacterial nosocomial infections. Exclusion criteria include those who declined to provide consent, those in the Outpatient Department, and those admitted for less than 48 hours. The study comprised patients who had been hospitalized to intensive care units (MICU or SICU) for more than 48 hours. Using structured format, clinical and demographic data were gathered.
The National Nosocomial Infections monitoring (NNIS) system standards established by the CDC were used for catheter-related blood stream infections (CR-BSI) monitoring.9 A CR-BSI was suspected when a patient with a central venous catheter (CVC) in place for more than two consecutive calendar days developed fever or other unclear sepsis-related symptoms.9
From these patients, paired blood samples were aseptically collected for culture. The catheter supplied the first blood sample, which was followed by the second from the opposite arm. The time of CVC removal was used to collect blood culture specimens. The critical care physician removed the CVC while following all aseptic protocols. Using sterile scissors, the distal 2 cm segment of the CVC was collected in a sterile container.
The inoculated blood culture bottles and CVC tip arrived at the microbiology lab right away. Blood culture and CVC tip samples were processed using the usual microbiological methods.10 The
CR-BSI rate was calculated by dividing the number of CR-BSI by 1000 device days.
Using a standard mycological protocol, fungal pathogens isolated from BSI were identified to species levels. Eight Candida isolates were classified using the germ tube test, colony shape, colour on chromogenic medium, and sugar assimilation test. We identified filamentous fungi using slide culture, lactophenol cotton blue (LPCB), and a cellophane tape mount.11,12 The antifungal susceptibility profile of fungal pathogens was studied using the disc diffusion method for Candida and the clinical and laboratory standard institute’s (CLSI) microbroth dilution technique for filamentous fungi.13,14 Candida spp. susceptibility to echinocandin was studied using the Epsilometer strip method.15
During the study period, intensive care units received a total of 68824 patients. ICU wise patient distribution is seen in Table 1.
Table (1):
ICU wise distribution of patients
ICU |
Frequency (%) |
|---|---|
Medical Intensive Care Unit (MICU) |
28722 (41.8) |
Surgical Intensive Care Unit (SICU) |
14371 (20.9) |
Pediatric Intensive Care Unit (PICU) |
10171 (14.8) |
Neurological Intensive Care Unit |
7807 (11.4) |
Neonatal Intensive Care Unit (NICU) |
6864 (9.9) |
Burn’s ICU |
889 (1.3) |
Total |
68824 |
Among various critical care areas, MICU (41.3%) followed by SICU (20.9%) and PICU (14.8%) had maximum admission. NICU had admission of 889 (1.3%) neonates. The mean length of stay (LOS) in ICU patients was 18.9 ± 10.5 days. Among various ICUs, LOS was significantly high in patients admitted to burn ICUs (T test, P value < 0.05*).
A total of 59,829 (87.1%) had indwelling medical devices (IMD). The use of IMD were significantly high in patients admitted to MICU as compared to other ICUs (Z test, P value < 0.00001*). Urinary catheter was the most common IMD.
A total of 3441 (5.1%) patients out of 68824 admitted to critical care areas developed HAI. The ICU wise rate of HAI is shown in Table 2. The rate of HAI in MICU was significantly high as compared to other ICUs (Z test, P < 0.05*).
Table (2):
ICU wise rate of HAI
ICU |
Patients admitted |
Patients developing HAI (%) |
|---|---|---|
MICU |
28722 |
2198 (7.6) |
SICU |
14371 |
642 (4.4) |
PICU |
10171 |
111 (1.1) |
Neurological Intensive Care Unit |
7807 |
83 (1.1) |
NICU |
6864 |
346 (5.1) |
Burn ICU |
889 |
61 (6.9) |
Total |
68824 |
3441 (5) |
A total of 823 developed nosocomial BSI. Therefore, the rate of nosocomial BSI was 1.2%. Out of these, 798 (97%) cases were of CRBSI whereas 25 (3%) were Non-CRBSI (Table 3). The rate of CRBSI (term used for the purpose of clinical diagnosis and therapy) was 5.5%. As per the surveillance formula, The blood stream infection rate (CLABSI) linked with central lines per 1000 central line days was 3.9.
Table (3):
Blood stream infections caused by bacteria and fungi
| CRBSI | Non-CRBSI | Total | ||
|---|---|---|---|---|
| Bacterial infection | Fungal isolates | Bacterial infection | Fungal Pathogens | |
| 757 | 41 | 20 | 05 | 823 |
In all, 46 fungal pathogens were identified from 823 nosocomial BSI patients. Therefore, the fungal BSI rate was 5.6%. Of 46 fungal pathogens from cases of hospital acquired BSI, 41 (88.1%) were from CRBSI/CLABSI cases whereas 05 (10.9%) were from Non-CRBSI/CLABSI cases.
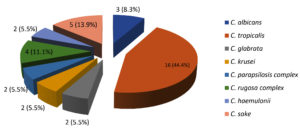
From 46 cases of nosocomial BSI, 39 (84.7%) isolates were of Candida spp., 06 (13.1%) were Trichosporon spp. whereas Aspergillus niger was isolated from 1 (2.2%) case of hospital acquired BSI. Isolation of Candida spp. from nosocomial fungal BSI was significantly high as compared to other fungal pathogens (Chi square test <0.00001*). As shown in the Figure, C. tropicalis was predominant cause of nosocomial candidemia.
When risk factors were analyzed using Chi square test male gender, advanced age, increased hospital stay and central venous catheterization were significantly associated with development of for nosocomial BSI (P value < 0.05*). Fluconazole prophylaxis/treatment was significantly high in patients with Non-albicans Candida (NAC) spp. BSI (Chi square test, P value < 0.00001**).
In Candida spp., fluconazole resistance was observed in 61.5% (n = 24), itraconazole resistance was seen in 25.6% (n = 10) whereas voriconazole and amphotericin B resistance was observed in 5 (12.8%) isolates. NAC spp. had substantially higher fluconazole resistance than C. albicans (Z test, P value < 0.05*). Among various Candida spp., C. parapsilosis demonstrated had high minimum inhibitory concentration to all three echinocandins (caspofungin, micafungin and anidulafungin). Aspergillus spp. demonstrated high MIC to amphotericin B compared to azoles.
HAI has several deleterious effects on patients and takes its tool in the form of increased morbidity, mortality, health care cost and LOS.16 once neglected in developing countries, incidence of HAI is now being published by various Indian researchers. Although many researchers have casted light on HAI in developing countries, the exact prevalence of HAI is still largely unknown mostly due to lack of National Surveillance System and multi-centric studies.16 In India very few Indian hospitals conduct regular HAI surveillance.
In developing countries like India, multitude of factors including resource poor infrastructure, poor hygiene, low patient to health care worker ratio, overcrowding and injudicious/irrational use of antimicrobials and IMD contribute both to development HAI and death due to HAI.17 Although compared to wards less number of patients is admitted to critical care areas, the incidence of HAI is more. As per available literature, incidence of HAI in ICU is 5-10 times more than general wards.18,19
In this research, the frequency of HAI in ICU patients was 5.1%. the rate of HAI was significantly high in patients admitted to MICU compared to other critical care areas. Similar observation was noted by Al-Tawfiq et al, where rate of HAI was high in patients admitted to MICU in comparison to other critical care areas like CCU and SICU.20 In their study, high rates of HAI in MICU as compared to other critical care areas was attributed to certain factors like high usage of medical devices and varied patient characteristics.20 Similar findings were noted in the current investigations.
In this research, the frequency of nosocomial BSI in ICU patients was 1.2% (n = 823). Nosocomial BSI is a serious and devastating infection reported across the world.21 ICU Patients are at a greater risk of hospital-acquired BSIs than those admitted to other kinds of units.22 Its incidence is reported to range between 1.3-18.4 episodes/1000 hospital admissions and is highly dependent varying with the type of study population, the number of admissions and location of the ward/critical care area, and LOS of patient in the hospital.23 The rate of mortality of hospital-acquired BSI varies markedly and is reported to range between 12% to 80%.24
97% of cases of nosocomial BSI were attributed to presence of central line (CRBSI/CLABSI). As per recent studies, the rate of CRBSI varies between 3% to 20% and is depended on type of study population.25 Numerous researchers, both domestically and internationally, have emphasised the significance of prompt diagnosis and treatment in lowering the morbidity and mortality rate related to CRBSI.
Health care providers should be taught and trained in the use of antiseptics containing 0.5% chlorhexidine and alcohol for skin preparation, avoid routinely replacing central venous catheters (CVCs) as a preventive measure, and use short-term CVCs that are antibiotic- and antiseptic-impregnated, as well as sponge dressings that are impregnated with chlorhexidine. These guidelines are crucial in preventing CRBSI.26
Over the last several decades, there has been a noticeable rise in both the incidence and severity of HAI caused by fungi. Most fungal HAI are due to AspergilIus spp. and Candida spp.27 These two fungal species accounts to majority of disseminated and life-threatening fungal infections. Research studies from various parts of the world have highlighted increased frequency of Candida infections. In the USA, Candida is the 4th leading cause of hospital acquired BSI.28 Candida is the number three leading cause of BSI in the ICU patients. Similarly, it is the 3rd among leading causes of CRBSI.28 From European countries, Candida spp. is reported as the 6th to 10th cause of HAI BSI.28 In consistent to findings of other authors, Candida spp. was the predominant cause of nosocomial BSI in this study.
Historically, C. albicans has been regarded as the most harmful member of the genus since it causes the bulk of infections.29 However, recent study have indicated emergence of NAC spp. as the prevalent of infections including HAI. Similar observation was noted in this study. The emergence of NAC spp. poses a clinical threat because, these NAC are intrinsically less susceptible/respond poorly or develop resistance during therapy with single or multiple classes of antifungal drugs.30 Among NAC spp., C. tropicalis was found to be significantly associated with HAI compared to other species Similar finding was noted in other Indian studies.31 According to Indian epidemiology, NAC spp. may be involved in 67-90% of Candida BSI infections.32
Six hospital-acquired BSI patients had Trichosporon spp. isolated from them. More and more reports of it are coming from all over the world, including India. In the study of Trichosporon spp. were reported from both immunocompetent and immunocompromised hospitalized patients.33 Trichosporon has historically been linked to significant mortality rates (60-80%) and disseminated infection in immunocompromised people.34 However better clinical outcomes have been reported with advancement of knowledge related to diagnosis (clinical and laboratory), treatment and prevention of Trichosporon infection. Trichosporon can be easily misidentified as Candida spp., however dry and wrinkled colonies on SDA, and presence in arthroconidia and blastoconidia in lactophenol cotton blue (LPCB) mount and positive urease test aid in identification Trichosporon.35
When risk factors for hospital acquired blood stream fungal infections were analyzed, male gender, advanced age and central venous catheterisation were substantially related with the development of hospital acquired blood stream fungal infections. Fluconazole prophylaxis/treatment was significantly high in patients with NAC spp. BSI. These risk factors are in consistent to that reported by other researchers. Many predisposing factors for candidemia have been found. These consist of major surgeries, central venous and urinary catheterisation, and complete parenteral feeding. Factors predisposing to candidemia are constant for past two decades. The National Epidemiology of Mycoses study group (NEMIS) also revealed a high prevalence of fungal HAI.36 Male predominance was also noted in the study of various national and international workers.37
The scenario of antifungal susceptibility pattern of Candida has changed with emergence of NAC spp., as NAC spp. exhibits varying degree of resistance to commonly prophylactic and therapeutic prescribed antifungal drugs.27 Hence antifungal susceptibility profile of infecting Candida spp. can’t be accurately and precisely seldom on the basis of identification.
According to the IDSA, the antifungal susceptibility testing is useful for both C. albicans and NAC spp.38 In C. albicans infection the result of susceptibility testing is often necessary important in patients with persistent candidemia or other form of systemic candidiasis whilst in case of NAC spp. infections, susceptibility testing is especially vital in patients who have prophylactically or therapeutically received dose of azole.39
Fluconazole resistance in Candida spp. was much higher than that of other antifungals. Since fluconazole is the most commonly prescribed first-line medication for treating and preventing practically all instances of candidiasis, resistance to it is concerning.39
The WHO published a list of fungal priority pathogens in 2022 to guide research, development, and public health action.40
Echinocandins, the most recent addition to antifungal arsenal, are increasingly utilized as first line drugs for treating and managing systemic/disseminated Candida infections. It is prescribed in conditions like terminal illness, clinically unstable patients, azole exposure history and are colonized/infected with fluconazole resistant Candida spp.15
All echinocandins are approved for treating BSI and other forms of systemic candidiasis.15 These antifungals have fungicidal action against most Candida spp. including NAC spp. and azole resistant isolates. However they demonstrate high MIC against C. parapsilosis.15 Similar observation was noted in this study. Aspergillus niger demonstrated high MIC to amphotericin B compared to azoles. Fakhim et al. reported the similar findings.41
Fungal pathogens have emerged as important cause of nosocomial BSI. From this study, it can be concluded that Hitherto, fungal isolates, once rarely encountered like Non-albicans Candida spp., Trichosporon spp. are now common in invasive mycosis. These pathogens often demonstrate less susceptibility to antifungal drugs, hence are associated with poor/no response to therapy and therefore may present as mayhem to patients. Finally, this emerging mayhem necessitates the importance of being vigilant about predisposing factors, stringent execution of infection prevention and control and the commencement of an antifungal stewardship program. All suspected blood culture samples for BSI should also be examined for fungal aetiology, since this would provide evidence of fungal infection.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethical Committee, Government Medical College, Kota, vide approval number 2021/59 dated July 07, 2021.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Djuric O, Markovic-Denic L, Jovanovic B, Bumbasirevic V. Agreement between CDC/NHSN surveillance definitions and ECDC criteria in diagnosis of healthcare-associated infections in Serbian trauma patients. Plos one. 2018;13(10):e0204893.
Crossref - Donaldson L, Philip P. Patient safety: a global priority. Bull World Health Organ. 2004;82(2):892.
- Girou E, Stephan F, Novara ANA, Safar M, Fagon JY. Risk factors and outcome of nosocomial infections: results of a matched case-control study of ICU patients. Am J Respirat Crit Care Med. 1998;157(4):1151-1158.
Crossref - Joshua P, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007;45(4):321-346.
Crossref - Oliveira M, Oliveira D, Lisboa C, Boechat JL, Delgado L. Clinical Manifestations of Human Exposure to Fungi. J Fungi. 2023;9(3):381.
Crossref - Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect. 2006;63(3):246-254.
Crossref - Vamvarian SE, Anaissie EJ, Bodey GP. Emerging fungal pathogens in immunocompromised pahents: classification, diagnosis, and management. Clin Infect Dis 1993;17(Suppl. 2):S487-91.
Crossref - Liu ZY, Sheng RY, Li XL, Li TS, Wang AX. Nosocomial fungal infections, analysis of 149 cases. Zhonghua Yi Xue Za Zhi. 2003;83(5):399-402.
- Singh S, Pandya Y, Patel R, Paliwal M, Wilson A, Trivedi S. Surveillance of device-associated infections at a teaching hospital in rural Gujarat-India. Indian J Med Microbiol. 2010;28(4):342-347.
Crossref - Deorukhkar SC, Saini S. Medical device-associated Candida infections in a rural tertiary care teaching hospital of India. Interdiscip Perspect Infect Dis. 2016;2016(1):1854673.
Crossref - Larone D. Media. In medically important fungi: A guide to identification. Harper and Row. Medical Department, London. 1976:127-140.
- Dolan CT. A Practical approach to identification of yeast-like organisms. Am J Clin Pathol. 1971;55(5):530-590.
Crossref - Clinical and Laboratory Standards Institute (CLSI), Reference Method For Antifungal Disk diffusion Susceptibility Testing of Yeasts, Approved standard M44-A, Clinical Laboratory Standard Institute,Wayne, Ind, USA, 2nd edition. 2004.
- Clinical and Laboratory Standards Institute (CLSI), Reference Method For Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved standard M27-A3, Clinical Laboratory Standard Institute,Wayne, Ind, USA, 2nd edition. 2008.
- Deorukhkar SC, Saini S. Echinocandin susceptibility profile of fluconazole resistant Candida species isolated from blood stream infections. Infect Disord Drug Targets. 2016;16(1):63-68.
Crossref - De M, Mukherjee D. A Study on Hospital Acquired Infections among Patients in a Tertiary Care Hospital of Darjeeling District, West Bengal. BJOHNS. 2020;26(3):197-206.
Crossref - Kumar AB, Latha M, Marriyamma KT, Reddy AK. Over view of various infection control activities conducted by hospital infection control committee at KIMS. J Med Sci Res. 2014;2(1):45-49.
Crossref - Mythri H, Kashinath K. Nosocomial infections in patients admitted in intensive care unit of a tertiary health center, India. Ann Med Health Sci Res. 2014;4(5):738-741.
Crossref - Ghanshani R, Gupta R, Gupta BS, Kalra S, Khedar RS, Sood S. Epidemiological study of prevalence, determinants, and outcomes of infections in medical ICU at a tertiary care hospital in India. Lung India. 2015;32(5):441-448.
Crossref - Al-Tawfiq JA, Abdrabalnabi R, Taher A, et al. Surveillance of device associated infections in intensive care units at a Saudi Arabian Hospital, 2017-2020. J Infect Public Health. 2023;16(6):917-921.
Crossref - Cortes JA, Reyes P, Gomez C, Buitrago G, Leal AL. Fungal bloodstream infections in tertiary care hospitals in Colombia. Rev Iberoam Micol. 2011;28(2):74-78.
Crossref - Rajni E, Garg VK, Bacchani D, et al. Prevalence of Bloodstream Infections and their Etiology in COVID-19 Patients Admitted in a Tertiary Care Hospital in Jaipur. Indian J Crit Care Med. 2021;25(4):369-373.
Crossref - Suljagic V, Cobeljic M, Jankovic S, et al. Nosocomial bloodstream infections in ICU and non-ICU patients. Am J Infect Control. 2005;33(6):333-340.
Crossref - Liu CY, Liao CH, Chen YC, Chang SC. Changing epidemiology of nosocomial bloodstream infections in 11 teaching hospitals in Taiwan between 1993 and 2006. J Microbiol Immunol Infect. 2010;43(5):416-429.
Crossref - Ahn HM, Kim JS, Park MG, Hwang J, Kim WY, Seo DW. Incidence and short-term outcomes of central line-related bloodstream infection in patients admitted to the emergency department: a single-center retrospective study. Sci Rep. 2023;13(1):3867.
Crossref - Hajjej Z, Nasri M, Sellami W, Gharsallah H, Labben I, Ferjani M. Incidence, risk factors and microbiology of central vascular catheter-related bloodstream infection in an intensive care unit. J Infect Chemother. 2014;20(3):163-168.
Crossref - Low CY, Rotstein C. Emerging fungal infections in immunocompromised patients. F1000 Med Rep. 2011;3:14.
Crossref - Ravikant, Kaur T, GuptA S, Kaur M. A review on emerging fungal infections and their significance. J Bacteriol Mycol Open Access. 2015;1(2):39-41.
Crossref - Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Giannini MJSM. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62(Pt 1):10-24.
Crossref - Deorukhkar S, Saini S, Mathew S. Non-albicans Candida Infection: An Emerging Threat. Interdiscip Perspect Infect Dis. 2014;2014(1):615958.
Crossref - Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp. among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010;14(11):e954-e966.
Crossref - Krcmery V, Barnes AJ. Non-albicans Candida spp. causing fungaemia: Pathogenicity and antifungal resistance. J Hosp Infect. 2002;50(4):243-260.
Crossref - Mehta V, Chander J, Gulati N, et al. Epidemiological profile and antifungal susceptibility pattern of Trichosporon species in a tertiary care hospital in Chandigarh, India. Curr Med Mycol. 2021;7(1):19-24.
Crossref - Erer B, Galimberti M, Lucarelli G, et al. Trichosporon beigelii: a lifethreatening pathogen in immunocompromised hosts. Bone Marrow Transplant.2000;25(7):745-749.
Crossref - de Almeida Junior JN, Hennequin C. Invasive Trichosporon Infection: a Systematic Review on a Re-emerging Fungal Pathogen. Front Microbiol. 2016;7:1629.
Crossref - Rangel-Frausto MS, Wiblin T, Blumberg HM, et al. National epidemiology of mycoses survey: (NEMIS): variation in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin Infect Dis. 1999;29(2):253-258.
Crossref - Chen L, Liao S, Kuo S, et al. Changes in the incidence of candidaemia during 2000-2008 in a tertiary medical centre in northern Taiwan. J Hosp Infect. 2011;78(1):50-53.
Crossref - Pappas PG, Kaufmann CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503-535.
Crossref - Rex JH, Pfaller MA, Walsh TJ, et al. Antifungal susceptibility testing: Practical aspects and current challenges. Clin Microbiol Rev. 2001;14(4):643-658.
Crossref - World Health Organization (WHO). WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization. 2022.
- Fakhim H, Badali H, Dannaoui E, et al. Trends in the prevalence of amphotericin B-resistance (AmBR) among clinical isolates of Aspergillus species. J Med Mycol. 2022;32(4):101310.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.