ISSN: 0973-7510
E-ISSN: 2581-690X
This study introduces a novel technique called Desiccation-Assisted Fabrication for fabricating antimicrobial cotton fabrics at the point of care. This method offers a simple, rapid, and cost-effective approach to impart antimicrobial properties to cotton fabrics, enabling their use in critical healthcare settings where infection control is paramount. Different concentrations of ZnO nanoparticles (2%, 2.5%, and 3% w/v) in alkaline water (pH 8.5) were prepared, drawn into a syringe, agitated for uniform dispersion, and precisely deposited onto cotton fabric. The fabric was placed on a natural desiccant powder (montmorillonite) to remove moisture, facilitating nanoparticle adhesion through physical adsorption. Subsequent heating thermofixed the nanoparticles onto the fabric. Characterization methods such as field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), and energy-dispersive X-ray spectroscopy (EDS) confirm the consistent dispersion of zinc oxide (ZnO) nanoparticles across the coated fabric. Antimicrobial activity testing against E. coli and Staphylococcus aureus demonstrated the effectiveness of the fabric in inhibiting bacterial growth. The ability to precisely control the amount of nanoparticle deposition ensures consistent and reproducible results. This novel coating technique offers a simple, efficient, and cost-effective approach for applying metal oxide nanoparticles to textiles, particularly for small-scale or prototyping applications. However, it also holds the potential for automation, paving the way for large-scale production.
Zinc Oxide Nanoparticles, Cotton Fabric, Desiccation, Coating
The use of textiles in hospital settings and patient care has drawn attention as one of the possible sources of infection transmission in the healthcare industry, increasing the risk of nosocomial infections.1,2 This is particularly concerning, as these fabrics often evade the regular and repeated chemical disinfection routines implemented in healthcare settings. Owing to their vast surface area and ability to retain moisture, textile fabrics make ideal growing environments for microbes, providing an optimal environment for microbial growth.3,4 The ability of fabrics to harbor and transmit microorganisms poses a significant challenge in infection control. Despite regular disinfection, bacteria and pathogens often persist on fabrics, leading to the spread of infections among patients and healthcare workers. This issue is further compounded by the fact that fabrics in healthcare settings are subjected to various bodily fluids, such as blood, saliva, and urine, which can provide a favorable environment for microbial growth.1,5,6 Additionally, the frequent handling and movement of fabrics, such as bed linens, gowns, and curtains, can facilitate the transfer of microorganisms from one surface to another.7,8 The persistence of microorganisms on fabrics in healthcare settings presents a significant challenge to patient safety and can contribute to the development of healthcare-associated infections (HAIs). These infections can result in increased morbidity and mortality and place significant financial strain on healthcare systems. Consequently, there is an urgent need for effective strategies to reduce the role of fabrics in the spread of infections within healthcare environments.2,9-11 This may involve the development of antimicrobial fabrics, improved disinfection protocols, and education and training for healthcare workers on the proper handling and care of fabrics.12,13
By addressing the issue of fabric-mediated infection transmission, healthcare facilities can increase patient safety, reduce the incidence of HAIs, and improve overall infection control practices. Thus, antimicrobial coatings on such materials undoubtedly help in the prevention of infections.13-15 As a result, the impact of antimicrobial metal and metal oxide nanoparticle coatings has become a highly researched area in the textile industry. This is because of their stability, heat resistance, and specific toxicity against pathogens such as bacteria, as well as their capacity to tolerate demanding and severe processing conditions.11,16 Owing to their potential applications, there has been increased emphasis on developing and applying nanometal oxide coatings on cotton substrates.12,17
Recently, various nanomaterials, including copper, gold, silver, aluminum, titanium dioxide, and zinc oxide, have been widely used in textiles as coating or embedding agents. Among these, zinc oxide nanoparticles (ZnO NPs) have shown significant promise because of their strong antibacterial and anti-inflammatory properties, safety, affordability, and biocompatibility.18 Their antibacterial properties make them particularly useful for medical devices, coatings for implants, medical textiles and wound dressings, and as ingredients in antibacterial ointments and creams. The coated textiles also offer protection from UV rays and do not cause skin irritation or sensitization. The high UV absorption of nanosized zinc oxide has led to its use in sunscreen formulations because of its UV blocking capabilities.17,19 Numerous studies have demonstrated that applying ZnO nanoparticles to cotton fabric can enhance its properties, including better UV protection, antimicrobial functionality, and increased durability. Additionally, the semiconductor properties of ZnO nanoparticles make them ideal for applications such as self-cleaning textiles and odor control.20-22 Characterization methods such as field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), and energy-dispersive X-ray spectroscopy (EDS) confirm the consistent dispersion of zinc oxide (ZnO) nanoparticles on the coated fabric. To achieve an effective coating, various methods, such as the sol gel process, electrochemical deposition, spin coating, dip coating, spray coating, layer-by-layer assembly, pad dry-cure method, and ultrasonic irradiation, have been employed. Each method offers distinct advantages in terms of coverage, uniformity, and adhesion of the nanoparticles to the fabric.22,23 Furthermore, to improve the stability of nanoparticles, binding agents, chemicals, surfactants or enzymes are used as activation tools for textiles. While these treatments offer potential benefits, they can also come with drawbacks. These drawbacks include changes to fabric properties like texture, tensile strength, breathability, durability, and wash resistance. Additionally, there may be health and environmental risks associated with certain treatments.24,25
In this study, we functionalized cotton fibers with ZnO NPs without the use of harsh chemicals, surfactants, enzymes, or binding agents. Instead, we used straightforward physical methods, such as mechanical agitation to stabilize and disperse ZnO NPs uniformly in alkaline water (pH 8.5 adjusted with 5% sodium bicarbonate), precise coating with a syringe, and desiccation with a natural desiccant (montmorillonite) to avoid nanoparticle agglomeration on the cotton surface. The type of desiccant used was carefully selected to ensure effective moisture removal without damaging the cotton fabric or interfering with or reacting with the ZnO nanoparticles. The morphology and chemical structure of the cotton coated with ZnO nanoparticles were analyzed via SEM, EDS, and XRD (Table 1). The ZnO nanoparticles were uniformly dispersed across the coated fabric, which also exhibited strong antibacterial activity and excellent wash durability (Figure 1).
Table (1):
Elemental composition of Zinc oxide coated cotton fabric (EDS Analysis)
Element |
Weight % |
Minimum detection limit (MDL) |
Atomic % |
|---|---|---|---|
C |
19.1 |
0.65 |
41.2 |
O |
21.7 |
0.15 |
35.3 |
Zn |
59.2 |
0.43 |
23.5 |
Chemicals and reagents
Zinc oxide NPs (<50 nm in size) were purchased from a commercial source (Sigma Aldrich, USA) and used for the experiment. Dehydrated nutrient agar powder, nutrient broth powder, EMB agar, mannitol salt agar, Muller-Hinton agar and blood agar were purchased from registered suppliers (HiMedia, India) and utilized for preparing culture media and broth.
Cotton material
For the experiments, gray cotton fabric with a plain weave structure was sourced from the local market. The fabric specifications are 100% cotton with 72 ends per inch (EPI), 67 picks per inch (PPI), and a weight of 152 grams per square meter (GSM). The cotton fabric was cut into 20 × 20 cm pieces for the experiment. The fabric requires pretreatment before use.
Pretreatment of cotton fabric
The cotton fabric was prewashed to eliminate wax and fat with water and detergent at 70 °C for 35 minutes. After multiple rinses with distilled water, the fabric was dried at 70 °C for 24 hours.
Desiccant
The natural desiccant montmorillonite was purchased as a dry fine powder from Iconic Naturals, India, and was used in sterilized form for the experiment. Montmorillonite is a naturally occurring clay mineral with a large surface area and high water absorption capacity, making it an effective desiccant. It is inexpensive, nontoxic and safe for use in biological applications.26 The montmorillonite powder was sterilized by dry heat before use.
Analytical methods
The characterization and chemical analysis of the ZnO-NP-coated cotton fabric were conducted via several techniques. Field emission scanning electron microscopy (SEM) was performed using a Gemini SEM 500 from Carl Zeiss. The Gemini SEM 500 offers remarkable resolutions of 0.5 nanometers at 15 kilovolts, 0.9 nanometers at 1 kilovolt, and 1.0 nanometers at 500 volts. X-ray diffraction (XRD) analysis was carried out via a Smart Lab 3 kW X-ray diffractometer from Rigaku, Tokyo, Japan. Energy dispersive spectroscopy (EDS) was conducted with a Hitachi 3600 N scanning electron microscope featuring a 5-axis motorized stage and an ultradry compact EDS detector from Thermo Fisher Scientific, Waltham, Massachusetts, USA.
Coating Cotton Fabric with Zinc Oxide Nanoparticles via a Syringe Desiccation Technique
- The dried cotton fabric (20 × 20 cm) in a square shape was spread on a sterilized tray atop a thin layer of montmorillonite desiccant compatible with the fabric and the nanoparticle solution. In this experiment, the desiccant was used in a controlled environment with low humidity to prevent it from absorbing moisture from the air and becoming saturated.
- Fifteen millilitres of freshly prepared 2% zinc oxide nanoparticle solution were drawn into a 20 mL syringe and agitated for 45 sec or for a sufficient amount of time to ensure that the nanoparticles were well dispersed. In the agar well diffusion test, the 2% ZnO nanosol exhibited the highest antibacterial activity. Therefore, the coating was performed using concentrations of 2% and above.
- Next, a precise volume of the sol (0.5 mL per square centimeter of fabric) was linearly deposited (slowly and evenly) onto the surface in the central area (approx. 15 cm × 15 cm square) to allow for handling of the fabric postcoating.
- To prevent the fabric from bending or folding during the process, the operation was carried out in a contained space with minimal fluctuations in air speed.
- The montmorillonite desiccant absorbed moisture, drying the fabric within approximately 7 minutes.
- The fabric was then removed and rinsed three times with distilled water to remove adhered desiccant and residual alkali.
- The coated fabric was then dried under vacuum at 65 °C overnight.
- Pieces of fabric of approximately one square centimeter were cut for characterization, analysis and antibacterial activity testing.
Wash durability of the coated fabric
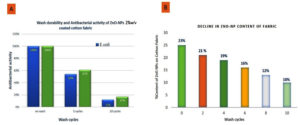
The coated fabric was washed with laundry detergent at 40 °C, and its antibacterial activity and ZnO content were determined.27-29 The results are shown in Figure 2 and Table 2. The ZnO content of the cotton fabric decreased from 23% to 10% after 10 washes, and the antimicrobial activity also decreased considerably to 61% and 54% for Staphylococcus aureus and Escherichia coli, respectively, after 5 washing cycles and then to 17% and 12%, respectively, after 10 washing cycles.
Table (2):
Percentage reduction in antimicrobial activity of cotton fabric with ZnO NPs against bacteria
| Test organism | Bacterial count (cfu/ml) | % Reduction after 24 hours | |
|---|---|---|---|
| Control | Test | ||
| S. aureus | 6 x 106 | 0.85 x 106 | 85.8% |
| E. coli | 6 x 106 | 0.93 x 106 | 84.5% |
Figure 2. (A): Bar chart showing antibacterial activity of ZnO-NPs (2% w/v) coated cotton fabric against E. coli (blue bar) and S. aureus (green bar); (B): Decline in ZnO nanoparticle content on fabric with increasing wash cycles
Antibacterial activity test
The antibacterial activity of the ZnO nanoparticles and ZnO nanoparticle-coated cotton fabric against gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) bacteria was assessed via agar well diffusion and agar disc diffusion methods (Table 3). These bacterial clinical isolates were generously provided by the Incharge Disease Investigation Lab, Pulwama, Kashmir. The isolates were cultured aerobically in nutrient broth for 24 hours at 37 °C until the bacterial suspensions reached a turbidity of
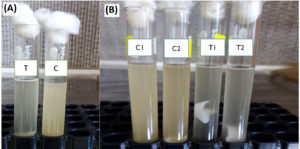
1.5 × 108 CFU/ml (Corresponding to a 0.5 McFarland standard tube) (Figure 1).30-32 The antibacterial activity was determined on Mueller-Hinton agar following standard guidelines (CLSI, 2021) as follows.
Table (3):
Zone of inhibition of Fabric treated with different concentrations of ZnO nanoparticles
| Cotton Fabric | Bacterium | Measured Zone of Inhibition (in cm) | ||||
|---|---|---|---|---|---|---|
| Trial no. 1 | Trial no.2 | Trial no.3 | Trial no. 4 | Mean value | ||
| Fabric treated with 2% ZnO NPs | Staphylococcus aureus | 2.0 | 1.9 | 2.0 | 1.9 | 1.95 |
| Escherichia coli | 1.8 | 1.9 | 1.8 | 1.9 | 1.85 | |
| Fabric treated with 2.5% ZnO NPs | Staphylococcus aureus | 1.6 | 1.5 | 1.4 | 1.5 | 1.5 |
| Escherichia coli | 1.5 | 1.4 | 1.3 | 1.4 | 1.4 | |
| Fabric treated with 3% ZnO NPs | Staphylococcus aureus | 1.3 | 1.3 | 1.4 | 1.4 | 1.35 |
| Escherichia coli | 1.3 | 1.2 | 1.3 | 1.2 | 1.25 | |
| Fabric without ZnO NPs (Control) | Staphylococcus aureus | 0 | 0 | 0 | 0 | 0 |
| Escherichia coli | 0 | 0 | 0 | 0 | 0 | |
In vitro antibacterial activity of the ZnO NPs
The antimicrobial efficacy of ZnO nanoparticles (ZnO NPs) was meticulously evaluated against pure clinical isolates of Staphylococcus aureus and Escherichia coli via a conventional agar well diffusion assay (Figure 3A). Freshly prepared Muller Hinton agar (MHA) Petri dishes were uniformly inoculated with isolated pure cultures of S. aureus and E. coli. Wells with a diameter of 5 mm were precisely created on the MHA using the base of a 1 mL microtip. In these wells, 100 µL of ZnO suspensions at various concentrations (0.5%, 1%, 1.5%, 2%, 2.5%, and 3% w/v) were carefully introduced. The inoculated plates were then incubated at a constant temperature of 37 °C for 24 hours.33,34
The extent of bacterial inhibition, indicated by the diameter of the zone of inhibition, was measured to assess the antibacterial potential of the ZnO NPs, adhering to the established methodology (Table 4). To ensure accuracy, multiple plates were employed to calculate the average zone of inhibition for each ZnO concentration, as presented in Table 4. For comparison, standard antibiotic agents, namely gentamicin and enrofloxacin, were used as positive controls in this experiment.
Table (4):
Agar well diffusion test of zinc oxide nanoparticles results
| Sample | Concentration % | Measured Zone of Inhibition (mm) | |||
|---|---|---|---|---|---|
| Trial no. 1 | Trial no. 2 | Trial no. 3 | Mean Value | ||
| Pure cotton | 0% | 0 | 0 | 0 | 0 |
| ZnO NPs | 0.5% | 11 | 9 | 10 | 10 |
| ZnO NPs | 1.0% | 16 | 18 | 14 | 16 |
| ZnO NPs | 1.5% | 19 | 21 | 20 | 20 |
| ZnO NPs | 2.0% | 26 | 24 | 22 | 24 |
| ZnO NPs | 2.5% | 21 | 22 | 20 | 21 |
| ZnO NPs | 3.0% | 19 | 20 | 19 | 19 |
| Positive Control | Gentamicin Disc | 22 mm (mean value) | |||
Similarly, the antibacterial efficacy of ZnO nanoparticle-coated cotton fabrics was scrutinized against both gram-negative (Escherichia coli) and gram-positive (Staphylococcus aureus) bacteria via the agar diffusion technique (Figure 3B). Fabric swatches, measuring approximately 8 to 10 mm, were evenly pressed onto the agar surface and subsequently incubated at 37 °C for 24 hours, alongside positive and negative controls. Postincubation, the presence of a clear zone-indicative of disrupted bacterial growth-beneath and around the edges of the fabric pieces served as evidence of the antibacterial properties of the coated samples, as depicted in Figure 3B & Figure 3C). The antibacterial impact of the fabrics was quantified by measuring the zones of inhibition surrounding the fabric pieces, with the results recorded in millimeters (mm) and detailed in Table 3.
Figure 3. (A): Zone of inhibition around ZnO-NP well on agar indicates antibacterial activity compared to the positive control (PC); (B): Agar plate comparing a ZnO-coated test cotton sample with negative (NC) and positive (PC) controls; (C): Antibacterial efficacy of cotton samples coated with ZnO-NPs at 1%, 2%, and 3% concentrations, compared to NC and PC
Quantitative analysis of antimicrobial activity
Test material samples were agitated in a bacterial suspension of known concentration, and the subsequent reduction in bacterial activity was monitored over a standardized time frame. The effectiveness of the antimicrobial treatment was determined by comparing the reduction in bacterial concentration of the treated samples to that of the control samples, with results expressed as a percentage reduction over the standard time, as illustrated in the Table 2.
The percentage reduction was calculated via the following formula: R = 100 (A – B)/A. Here, R represents the percentage reduction, A denotes the number of microbial cells recovered from the uncoated cotton material immediately after inoculation (at “0” contact time), and B is the number of bacteria recovered from the broth inoculated with the treated test fabric sample after the designated contact period of 24 hours, as shown in Table 2.
X-ray diffraction (XRD) analysis
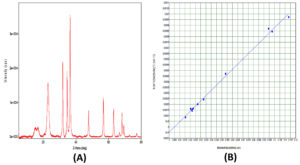
The XRD patterns of the ZnO-coated cotton fabric prepared via the syringe desiccation technique are presented in Figure 4. The analysis revealed the presence of crystalline metal oxides on the cotton fibers (Figure 4). The pattern corresponds to the hexagonal phase of ZnO; however, the peaks are somewhat broad due to the nanosized crystallites. All the observed diffraction peaks were accurately indexed to the hexagonal ZnO wurtzite structure. Notably, the three major peaks at 2θ = 31.8, 34.5, and 37 can be attributed to the 100, 002, and 101 lattice planes, respectively (Figure 4A). Peaks at diffraction angles below 30° are characteristic of the cellulosic cotton material.
No diffraction peaks corresponding to impurities were detected in the XRD patterns, indicating the high purity of the synthesized ZnO composites. The average crystallite size of the ZnO particles was calculated via Scherrer’s equation to be approximately 17.5 nm, which aligns with reported values for similar ZnO-coated cotton materials. The lattice strain, determined via the Williamson Hall method, was negligible, as shown in Figure 4B.
Figure 4. (A): X-Ray Diffraction (XRD) pattern of ZnO nanoparticles confirms their crystalline nature with sharp diffraction peaks (e.g., at 2θ ≈ 31.8°, 34.5°, 36.3°). (B): Williamson-Hall plot of ZnO nanoparticles indicates nanoscale crystal size and microstrain, based on the linear fit between β cos θ and sin θ
Field emission scanning electron microscopy (FESEM)
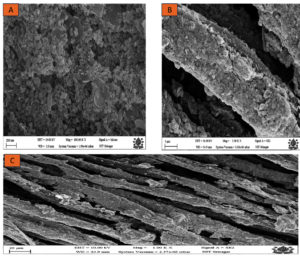
Field emission scanning electron microscopy (FESEM) is an advanced imaging technique that allows for high-resolution examination of material surfaces at the nanoscale. When applied to ZnO nanoparticle-coated cotton fabric, FESEM provides valuable insights into the size, shape, distribution, and agglomeration of ZnO nanoparticles on cotton fibers. These details are essential for assessing the effectiveness and potential applications of a coating. The morphology of the ZnO nanoparticles (ZnO-NPs) deposited onto cotton fibers was analyzed via FESEM, and the elemental composition was determined via energy dispersive spectroscopy (EDS). The FESEM analysis confirmed that the ZnO nanoparticles on the cotton fibers were within the nanoscale range. As illustrated in Figure 5, the cotton fibers appear as long, flat, ribbon-like structures with a rough surface texture (Figure 5A). The ZnO nanoparticles are predominantly spherical, although some exhibit slightly elongated or irregular shapes. These nanoparticles are generally smaller than the width of the cotton fibers.
The distribution of ZnO nanoparticles on the cotton fibers is relatively uniform, although certain areas exhibit a slightly higher concentration of particles. The ZnO nanoparticles within these aggregates are compact and densely packed. FESEM images revealed that the ZnO-NPs completely coated the fiber surface, as shown in Figure 5(A, B & C). This observation aligns with previous studies on similar materials. The SEM images, particularly those in Figure 5C, image B, clearly show that the ZnO nanoparticles thoroughly cover the fiber surface. Additionally, some agglomerates of ZnO-NPs appear as networked nanoflakes, further illustrating the complex morphology of these coated fibers. The compact and dense nature of these aggregates, as depicted in the SEM images, underscores the thoroughness of the ZnO nanoparticle coating on the cotton fibers.35-37
Figure 5. (A): High-magnification SEM image (100 KX) revealing the morphology of ZnO nanoparticles as a closely packed cluster; (B): Intermediate-magnification (5 KX) SEM image showing the surface of ZnO-coated fiber with rough particle aggregates; (C): Lower-magnification (1 KX) SEM micrograph illustrating the layered structure of cotton fibers and the distributed ZnO nanoparticle coating
Energy dispersive X-ray spectroscopy (EDS)
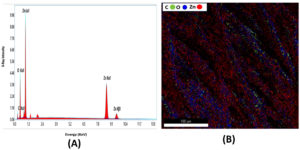
Energy dispersive spectroscopy (EDS) is a microanalysis technique used to determine the elemental composition of materials. When applied to ZnO nanoparticle-coated cotton fabric, EDS is particularly useful for confirming the presence of zinc (Zn) and oxygen (O), quantifying the elemental composition, and identifying any additional elements that may be present, such as impurities. The EDS spectra of the ZnO-coated cotton samples, shown in Figure 6 (A & B), reveal the uniform distribution of ZnO among the other elements in the sample. The spectra revealed the presence of key elements, including carbon (C), oxygen (O), and zinc (Zn), confirming the chemical composition of the ZnO-coated cotton samples, as detailed in Table 1. The EDS spectrum specifically highlights the Zn and O components, verifying that ZnO has been successfully deposited onto the cotton fibers. The presence of Zn atoms in the coated fibers further substantiates the formation of a ZnO coating on the fabric.
Figure 6. (A). Energy Dispersive X-Ray (EDX) spectrum of ZnO-coated cotton shows strong peaks for Zn (Zn La1, Zn Ka1, Zn Kβ1), O (Ka1), and C (C Ka1). (B). Elemental Map shows Spatial distribution of C (green), O (blue), and Zn (red) across the cotton fiber surface shows uniform ZnO presence along with the organic fiber matrix
In vitro antimicrobial activity of the ZnO NPs
The antimicrobial activity of ZnO nanoparticles (ZnO NPs) was evaluated against pure clinical isolates of Staphylococcus aureus and Escherichia coli via the standard agar well diffusion test. Freshly prepared Mueller-Hinton agar (MHA) Petri dishes were inoculated with pure cultures of these bacteria. The clinical isolates were resistant to enrofloxacin but sensitive to gentamicin and zinc oxide nanoparticles, as shown in Figure 3A. Multiple plates were used to calculate the average zone of inhibition for each concentration, as shown in Table 4. The 2% ZnONP concentration demonstrated the most potent antibacterial activity, producing a substantial zone of inhibition against Staphylococcus aureus. The 2.5% and 3% ZnO-coated fabrics exhibited progressively lower zones of inhibition and tended to settle in the well, indicating that 2% is the optimal concentration for achieving the maximum antibacterial effect against this particular bacterium. Similar trends were observed for Escherichia coli, further corroborating the concentration-dependent antibacterial properties of the ZnO-coated fabrics. These findings align with those of previous studies, confirming the potential of ZnO-coated cotton fabrics as effective antimicrobial agents.26,38-41
Antibacterial efficacy of ZnO NP-coated cotton fabric
The antibacterial effectiveness of zinc oxide (ZnO)-coated cotton fabrics was tested against two commonly found bacterial strains: Staphylococcus aureus (a gram-positive bacterium) and Escherichia coli (a gram-negative bacterium). Pure cotton had no inhibitory effect on Staphylococcus aureus, which underscores the significant antibacterial capability of the ZnO coating.
Among the ZnO-coated fabrics, those with a 2% ZnO concentration exhibited the most effective antibacterial activity, creating a considerable zone of inhibition against both Staphylococcus aureus and E. coli. These findings suggest that the antibacterial activity of ZnO-coated cotton fabrics increases as the ZnO concentration increases, reaching an optimal level at 2.0%. However, further increases in the ZnO concentration to 2.5% and 3.0% led to reduced antibacterial effectiveness, likely due to the agglomeration of the ZnO nanoparticles. These results align with findings from earlier studies.36,42,43 Specifically, the average zone of inhibition was the largest for the fabrics coated with 2% ZnO nanosol, measuring 24 mm for Staphylococcus aureus and 19.5 mm for E. coli.
In addition to the ZnO concentration in the coating, other factors can influence the antibacterial activity of ZnO-coated cotton fabrics. These factors include the size and morphology of the ZnO particles, the surface properties of the ZnO particles, the properties of the cotton fabric, the type of bacteria, and the environmental conditions.29,44-46
The antimicrobial efficacy of ZnO nanoparticles is governed by multiple factors, such as their size, morphology, surface characteristics, and concentration. Smaller nanoparticles with a high surface area tend to exhibit enhanced antimicrobial activity.32,43 Additionally, surface modifications or coatings can modulate the antimicrobial properties of ZnO NPs.26,38 Zinc oxide nanoparticles (ZnO NPs) have been integrated into a wide array of antimicrobial solutions, finding their place in coatings for medical instruments, wound care products, fabrics, and food packaging materials. The broad-spectrum antimicrobial activity and low toxicity of these nanoparticles make them promising candidates for combating antimicrobial resistance and preventing the spread of infections.42,43,47,48
Cotton fabric acquires specific functional attributes through a ZnO nanocoating, as revealed through a meticulous quality evaluation of the treated versus untreated fabric. These findings indicate that the treated fabric has significant antibacterial properties. All the coated samples were effective against gram-positive Staphylococcus aureus and gram-negative Escherichia coli bacteria. Notably, the sample coated with 2% ZnO nanoparticles had the highest antibacterial activity, eliminating 85.8% of the S. aureus bacteria and 84.5% of the E. coli bacteria. These outcomes align with earlier research findings.36,49-53
The characterization of the Zno nanoparticle-coated cotton fabric was meticulously carried out via three advanced analytical techniques: X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), and energy-dispersive X-ray spectroscopy (EDS). XRD analysis verified the presence of crystalline ZnO nanoparticles on the cotton fibers, with the diffraction pattern showing distinct peaks corresponding to the hexagonal wurtzite structure of ZnO. The average crystallite size, which was calculated via Scherrer’s equation, was determined to be 17.5 nm, which aligns with previous studies. The lattice strain, as calculated via the Williamson Hall method, was found to be negligible. FESEM, a high-resolution imaging technique, was employed to scrutinize the surface morphology of the ZnO nanoparticle-coated cotton fabric at the nanoscale. FESEM provided detailed insights into the size, shape, distribution, and aggregation of the ZnO nanoparticles on the cotton fibers, which is vital for evaluating the effectiveness and potential applications of the coating. EDS analysis offers elemental confirmation and quantification, which are essential for assessing the uniformity and quality of a coating. The characterization results provide a comprehensive understanding of the structural and morphological properties of ZnO-coated cotton fabric, which directly influence its functional performance.43 XRD confirmed the crystalline nature of the ZnO nanoparticles, ensuring the desired crystal structure for optimal functionality. FESEM images provided a detailed view of the morphological characteristics of the coated fabric, highlighting the ZnO nanoparticles and their distribution.42,54,47
Fabrics used in healthcare environments can become reservoirs for pathogens, contributing to the spread of nosocomial infections, which is a significant challenge for infection control. Despite rigorous disinfection efforts, microbes persist on these fabrics, facilitating the transmission of infections through contact with bodily fluids or handling by health care workers.41,55 This underscores the urgent need to develop antimicrobial fabrics, enhance disinfection protocols, and improve staff training on fabric handling to mitigate the transmission of infections and reduce healthcare-associated infections.41,56,57
The applications of ZnO-coated cotton fabrics are diverse and promising, including the following: i) Healthcare textiles: hospital gowns, bed sheets, and wound dressings can benefit from their antibacterial properties ii) food packaging: ZnO NP-coated fabrics can prevent microbial spoilage and extend the shelf life of food items; iii) hygiene products: towels, wipes, and face masks; iv) water filtration membranes: ZnO nanoparticles can be incorporated into membranes to purify water and remove harmful bacteria; and v) protective clothing: uniforms for healthcare workers and first responders.43 However, certain challenges remain, including A) washing and durability-maintaining the coating’s effectiveness over multiple washing cycles remains an area of research40,58,44; B) the production of high-quality ZnO nanoparticles and the coating process can be expensive; and C) the potential environmental effects of ZnO nanoparticles need further investigation.18,37,59-61
The syringe desiccation technique offers several advantages for coating cotton fabric with ZnO nanoparticles, including improved moisture control, uniform coating, good adhesion, controllable thickness, and scalability. The coating method using a syringe and desiccant eliminates the need for stabilizing agents in the application of ZnO nanoparticles to cotton fabric. The coated fabric exhibited uniform nanoparticle distribution, strong antibacterial efficacy against E. coli and Staphylococcus aureus, and wash durability. For small-scale or prototyping applications, this technique offers a rapid and precise approach to coating textiles with metal oxide nanoparticles. The ability to precisely control the amount of nanoparticle deposition ensures consistent and reproducible results.
Moreover, the potential for automation opens up the possibility of scaling up the process for large-scale manufacturing. By integrating automated systems for nanoparticle dispersion, deposition, and drying, high-throughput coating of textiles with metal oxide nanoparticles can be achieved. This automation not only increases production efficiency but also enhances the consistency and quality of the coated textiles. Automated processes allow for precise control over various parameters, such as the nanoparticle concentration, deposition rate, and drying conditions, resulting in uniform and reproducible coatings. Overall, the combination of simplicity, efficiency, and scalability makes this novel coating technique a promising approach for both small-scale and large-scale applications of metal oxide nanoparticles on textiles.
The syringe desiccation technique is an innovative method for coating zinc oxide nanoparticles onto cotton fabric, offering improved uniformity, enhanced adhesion, simplicity, and cost-effectiveness. By controlling the release of a nanoparticle suspension through a syringe, this method is feasible and scalable. The coated fabric exhibited good antibacterial effects, making it suitable for healthcare applications. With potential for automation, this technique could enable large-scale production of antibacterial, self-cleaning, and UV-protective textiles. However, challenges such as environmental impact and durability require further investigation. Collaborative efforts will advance this technique, integrating it into mainstream textile manufacturing for hygienic and durable fabrics.
ACKNOWLEDGMENTS
The authors are thankful to the financial assistance provided by NAHEP which played a crucial role in facilitating the successful execution of this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All the authors listed have made substantial, direct and intellectual contributions to the work and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Belkin N. Hospital Textiles: Are They a Possible Vehicle for Healthcare-Associated Infections? Int J Environ Res Public Health. 2009;9(8):3330-3343.
Crossref - Lee K, Browning LM, Nallathamby PD, Desai T, Cherukuri PK, Xu XH. Investigations on the Antimicrobial Effect of Silver and Zinc Oxide Nanoparticles on Cotton and Polyester Fabrics. Text Res J. 2012;82(13):1293-1304.
- Nerandzic MM, Cadnum JL, Pultz MJ, Donskey CJ. Evaluation of the Potential for Secondary Transmission of Infections from Textiles in Healthcare Environments. Infect Control Hosp Epidemiol. 2010;31(8):898-905.
- Wiegand C, Hipler UC, Boldt S, Strehle J, Wollina U. Skin Protective Effects of a Zinc Oxide Functionalized Textile and Its Relevance for Atopic Dermatitis. Clin Cosmet Investig Dermatol. 2013;6:115-121.
Crossref - Belay A, Mekuria M, Adam G. Incorporation of Zinc Oxide Nanoparticles in Cotton Textiles for Ultraviolet Protection and Antibacterial Activities. Nanomaterials and Nanotechnology. 2020;10:1-8.
Crossref - Neely AN, Maley MP. Survival of Enterococci and Staphylococci on Hospital Fabrics and Plastic. J Clin Microbiol. 2000;38(2):724-726.
Crossref - Patel R, McManus SB, Collins PK, Morgan RD, Wood PC, O’Neill F. Antimicrobial Activity of Nanoparticle-Coated Textiles for the Reduction of Nosocomial Infections. J Hosp Infect. 2013;85(1):56-62.
- Rutala WA, Gergen MF, Weber DJ. Microbial contamination on used surgical instruments. Infect Control Hosp Epidemiol. 2014;35(8):1068-70.
Crossref - Scott E, Bloomfield SF. The Survival and Transfer of Microbial Contamination via Cloths, Hands, and Utensils. J Appl Bacteriol. 1990;68(3):271-278.
Crossref - Smith J, Jones M. Role of Nanotechnology in Textiles. ACS Nano. 2011;5(4):3203-3210.
- Walker SL, Redman R, Howell DL. Potential of Textile Materials to Transmit Infectious Pathogens. Am J Infect Control. 2007;35(6):417-422.
- Wilson M, Caul JER, McGuigan RSG, James LH. The Potential for Hospital Textiles to Serve as a Reservoir for Nosocomial Pathogens. Healthc Infect. 2007;12(3):107-113.
- Kolodziejczak-Radzimska A, Jesionowski T. Zinc Oxide-From Synthesis to Application: A Review. Materials. 2014;7(4):2833-2881.
Crossref - Applerot G, Lipovsky A, Dror R, et al. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv Funct Mater. 2009;19(6):842-852.
Crossref - Sawai J. Quantitative Evaluation of Antibacterial Activities of Metallic Oxide Powders (ZnO, MgO, and CaO) by Conductimetric Assay. J Microbiol Methods. 2003;54(2):177-182.
Crossref - Solano R, Patin-Ruiz D, Herrera A. Preparation of Modified Paints with Nano-Structured Additives and Its Potential Applications. Nanomater Nanotechnol. 2020;10:1-17.
Crossref - Xiao XF, Zhang B, Lyu P, et al. Controllable Coating of Zinc Oxide on Protein-Based Fibers/Fabrics for Superior Antibacterial Performance Preserving Wearable Abilities. Appl Surf Sci. 2023;610:155487.
Crossref - CDC. Guidelines for Environmental Infection Control in Health-Care Facilities: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-10):1-42.
- Gholizadeh SS, Dezhampanah S, Vasheghani-Farahani E. Synthesis, Characterization, and In Vitro Antibacterial Activity of ZnO Nanoparticles Against Gram-Positive and Gram-Negative Bacteria. BioNanoScience. 2019;9:78-85.
- Pushpalatha C, Suresh J, Gayathri VS. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front Bioeng Biotechnol. 2022;10:917-990.
Crossref - El-Nahhal IM, Elmanama AA , El-Ashgar NM, Amara N, Selmane M, Chehimi MM. Stabilization of Nano-Structured ZnO Particles onto the Surface of Cotton Fibers Using Different Surfactants and Their Antimicrobial Activity. Ultrason Sonochem. 2017;38:478-487.
Crossref - Ibrahim NA, Ameen HA, Eid BM. Green Synthesized Chitosan and ZnO Nanoparticles for Sustainable Use in Multifunctionalization of Cellulosic Fabrics. Polym Bull. 2023;81(4):1-20.
Crossref - Cheng WN, Han SG. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments – A Review. Asian-Australas J Anim Sci. 2020;33(11):1699-1713.
Crossref - Becheri A, Durr M, NOstro PL, Baglioni P. Synthesis and Characterizations of Zinc Oxide Nanoparticles: Applications to Textiles as UV-Absorbers. J Nanopart Res. 2008;10:679-689.
Crossref - Tamburro M, Rippa M, Ditaranto N, Chiriacò MS, Lorusso V. Antibacterial Properties of Nanosilver-Coated Textiles and Their Safety Assessment in a Human Skin Model. J Nanopart Res. 2012;14(4):966.
- Carretero MI, Pozo M. Clay and non-clay minerals in the pharmaceutical industry: Part I. Excipients and medical applications. Applied Clay Science. 2009; 46(1):73-80.
- Sexton T, Clarke P, O’Neill E, Dillane T, Humphreys H. Environmental Reservoirs of Methicillin-Resistant Staphylococcus aureus in Isolation Rooms: Correlation with Patient Isolates and Implications for Hospital Hygiene. J Hosp Infect. 2006;63(2):187-194.
- Zhang L, Jiang Y, Ding Y, et al. Mechanistic Investigation into Antibacterial Behaviour of Suspensions of ZnO Nanoparticles Against E. coli. J Nanopart Res. 2010;12(5):1625-1636.
Crossref - Yetisen AK, Qu H, Manbachi A, et al. Nanotechnology in Textiles. ACS Nano. 2016;10(3):3042-3068.
Crossref - Yadav A, Kathe A, Raj S, Yadav D, Sundaramoorthy C, Vigneshwaran N. Functional Finishing in Cotton Fabrics Using Zinc Oxide Nanoparticles. Bull Mater Sci. 2006;29:641-645.
Crossref - El-Nahhal IM, Salem J, Anbar R, Kodeh FS, Elmanama A. Preparation and Antimicrobial Activity of ZnO-NPs Coated Cotton/Starch and Their Functionalized ZnO-Ag/Cotton and Zn(II) Curcumin/Cotton Materials. Sci Rep. 2020;10:5410.
Crossref - Tam KH, Djurisic AB, Chan CMN, et al. Antibacterial Activity of ZnO Nanorods Prepared by a Hydrothermal Method. Thin Solid Films. 2008;516(18):6253-6258.
Crossref - Algharib SA, Dawood A, Xie S. Nanoparticles for Treatment of Bovine Staphylococcus aureus Mastitis. Drug Deliv. 2020;27(1):292-308.
Crossref - Kasahun M, Yadate A, Belay A, Belay Z, Ramalingam M. Antimicrobial Activity of Chemical, Thermal, and Green Route-Derived Zinc Oxide Nanoparticles: A Comparative Analysis. Nano Biomed Eng. 2020;12(1):47-56.
Crossref - Shateri-Khalilabad M, Yazanshenas EM. Biofunctionalization of Cotton Textiles by ZnO Nanostructures: Antimicrobial Activity and Ultraviolet Protection. Text Res J. 2013;83(10):993-1004.
Crossref - Fouda A, Hassan SED, Salem SS, Shaheen TI. In-Vitro Cytotoxicity, Antibacterial, and UVProtection Properties of the Biosynthesized Zinc Oxide Nanoparticles for Medical Textile Applications. Microb Pathog. 2018;125:252-261.
Crossref - Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D, Bolzinger MA. The Contribution of Zinc Ions to the Antimicrobial Activity of Zinc Oxide. Colloids Surf A Physicochem Eng Asp. 2014;457:263-274.
Crossref - Sirelkhatim A, Mahmud S, Seeni A, et al. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015;7(3):219-242.
Crossref - Pasquet J, Chevalier Y, Couval E, et al. Antimicrobial Activity of Zinc Oxide Particles on Five Micro-Organisms of the Challenge Tests Related to Their Physicochemical Properties. Int J Pharm. 2014;460(1-2):92-100.
Crossref - Petkova P, Francesko A, Perelshtein I, Gedanken A, Tzanov T. Simultaneous Sonochemical-Enzymatic Coating of Medical Textiles with Antibacterial ZnO Nanoparticles. Ultrason Sonochem. 2016;29:244-250.
Crossref - Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial Activity of ZnO Nanoparticle Suspensions on a Broad Spectrum of Microorganisms. FEMS Microbiol Lett. 2008;279(1):71-76.
Crossref - Brown R, Smith S. Enhancement of Antibacterial Properties of Cotton Fibers Through Nanotechnology. J Nanomater. 2013;2013:892671.
- Kim D, Lee P. ZnO Nanoparticles and Microbial Inhibition: The Role of ROS Mediated Cell Injury. Nanotechnol Sci Appl. 2015;8:19-29.
- Thompson R. Characterization of Nano-Treated Textiles on Microbial Activity and Comfort. Text Res J. 2013;83(14):1459-1469.
- Nomicisio C, Ruggeri M, Bianchi E, et al. Natural and Synthetic Clay Minerals in the Pharmaceutical and Biomedical Fields. Pharmaceutics. 2023;15(5):1368.
Crossref - Sivakumar A, Murugan R, Sundaresan K. Certain investigations on the effect of nano metal oxide finishes on the multifunctional characteristics of cotton fabrics. J Ind Tex. 2013;43(2):155-173.
Crossref - Wang X, Sun T, Zhu H, Han T, Wang J, Dai H. Roles of pH, cation valence, and ionic strength in the stability and aggregation behavior of zinc oxide nanoparticles. J Environ Manag. 2020;267:110656 .
Crossref - El-Nahhal IZ, Zourab S, Kodeh FS, Elmanama A, Selmane M, Genois I, Babonneau F. Nano-structured zinc oxide-cotton fibers: synthesis, characterization and applications. J Mater Sci: Mater Electron. 2013;24(11):3970-3975.
Crossref - Ghayempour S, Montazer M. Ultrasound irradiation based in-situ synthesis of star-like Tragacanth gum/zinc oxide nanoparticles on cotton fabric. Ultrason Sonochem. 2017;34:458-465.
Crossref - Ran J, He M, Li W, Cheng D, Wang X. Growing ZnO Nanoparticles on Polydopamine-Templated Cotton Fabrics for Durable Antimicrobial Activity and UV Protection. Polymers. 2018;10(5):495.
Crossref - Zhu C, Shi J, Xu S, Ishimori M, Sui J, Morikawa H. Design and characterization of self-cleaning cotton fabrics exploiting zinc oxide nanoparticle-triggered photocatalytic degradation. Cellulose. 2017;24(6):2657-2667.
Crossref - El-Naggar ME, Shaarawy S, Hebeish A. In-situ synthesis of zinc oxide nanoparticles onto cotton fabrics for UV protection and antibacterial properties using date seed extract as a capping agent. Int J Biol Macromol. 2018;108:1010-1016.
- Sivakumar V, Murugan R, Sundaresan S. Durable antibacterial and UV-protection finishing of cotton fabrics using zinc oxide nanoparticles. Indian J Fibre Tex Res. 2013;38(3):285-292.
- Butola BS, Garg A, Chauhan P. Functional finishing of cotton fabric with ZnO nanoparticles for improved UV protection and antibacterial properties. J Ind Tex. 2018;47(4):695-710.
- Rajendra A, Balakumar C, Ahammed HAM, Jayakumar S, Vaideki K, Rajesh EM. Use of zinc oxide nano particles for production of antimicrobial textiles. Int J Eng Sci Technol. 2010;2(1):202-208.
Crossref - Mulchandani SN, Karnad S. Antibacterial properties and durability of ZnO nanoparticles on cotton fabric: Impact of polymeric binders. Tex Res J. 2021;91(1-2):3-15.
- Babu B, Dubey A, Sharma S. Antimicrobial finishing of cotton fabric using zinc oxide nanoparticles synthesized from medicinal plant leaf extracts. J Environ Chem Eng. 2021;9(2):104717.
- ASTM International. ASTM E2149-20: Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions. West Conshohocken, PA: ASTM International. 2020.
Crossref - Liu C, Lu Y, Hu C. Effects of Anions and pH on the Stability of ZnO Nanorods for Photoelectrochemical Water Splitting. ACS Omega. 2018;3(3):3429-3439.
Crossref - Fardad S, Massudi R, Manteghi A, Amini M. Synthesis, size and colloidal stability of ZnO nanoparticles in ionic solutions. 2007 7th IEEE Conference on Nanotechnology (IEEE NANO). 2007:925-929.
Crossref - Cullity BD, Stock SR. Elements of X-ray Diffraction (3rd ed.). Prentice Hall. 2001.
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.