ISSN: 0973-7510
E-ISSN: 2581-690X
Production of bioethanol from renewable carbohydrates materials has become worldwide interest. Floral wastes are easily, freely and abundantly available bio resource which has distinct advantages such as high fermentable sugars and zero investment. In this paper the production of ethanol from golden trumpet flower (Allamanda schottii L) by fermentation using yeast Saccharomyces Cerevisiea was studied. The optimization of various parameters for the production of ethanol and emission testing with the blend of petrol and ethanol has been studied. The flower of this study allamamda contains 65% total sugars. The yeast strain Saccharomyces Cerevisiea was purchased from local super market as baker’s yeast. The ethanol obtained was maximum 18.75 ml for 100 ml slurry composition by optimizing various parameters like slurry composition as 1: 8, pH as 5.5, inoculum age as 72 hours, inoculum level as 3.75 g 100 ml-1, temperature at 35p C and fermentation period as 5 days. Maximum production of ethanol is obtained by the addition of urea. This method can be tried for large scale ethanol production from Allamanda Schottii flowers.
Allamanda Schottii, ethanol, Saccharomyces Cerevisiea, fermentation.
The tremendous amounts fossil fuel usage poses pollution threat to the atmosphere. In near future the industries may depend upon bio fuels as an alternative source to petroleum based fuel. The best fuel which can supplement petrol is ethanol.Bio ethanol is most widely used used bio fuel for transportation(Balat, 2010). Ethanol is a clean fuel which can be obtained from renewable energy source and can play important role to solve the oil shortage to occur in near future. (Zhang and Feng, 2012). Demand for ethanol occurred world wide due to drastic in crease in population and industrialization. Ethanol produced from corn and sugarcane were not able to meet the world’s demand since they are consumed as food and feed (Khan and Dwivedi, 2013). Ethanol produced from cereal crops will pose threat on food prices and food security (Seag et al., 2013).
In order to cut down the production costs of fuel the source should be available freely and abundantly in nature. In the developing countries like India a balance between energy and food security is possible only if ethanol production is from non-food crops and biomass (Ragaukas et al., 2006; Lin and Tanaka, 2006)
Keenan and ASCE discussed the potential for bio mass utilization as a source of fuel, petro chemicals and petroleum sparing substances. The following plant families Euphorbiaceae, Asclepiadaceae, Apocyanaceae, Urticaceae ,Concvolvulasceae, Sapotaceae, were studied for their suitability as petro crops by various workers(Dipul kalita, 2008). At present world wide researchers show their interest in finding various bioresources for ethanol production..
The world energy crisis can be solved in producing hanol by fermentation (Ward O.P. and Singh A. 2006). The floriculture industry of India is largest among other countries in the world. The sugars present in flowers can be easily converted in to ethanol. The production of ethanol from mahua flower (Madhuca indica) through submerged fermentation (smf) was studied recently by D.S.N.Benarji et al (2010) using Scharacomyces Cerevisiae -3090.
Madhuca latifolia flowers are very rich in fermentable sugars (28.1-36.3g kgL-1 ) . The batch fermentation of fresh and 12 month stored flowers yields 193 and 148g kg-1 by using free cells and 205 and 152g kg-1 by using immobilized cells of Saccharomyces cerevisiae was studied by Swain et al (2007)
Sujit et al (2009) found Madhuka latifolia L. flowers in solid state fermentation by Saccharomyces cerevisiae can yield maximum ethanol after 72 hours of fermentation (225.0±4.0 g kg-1 flower) with optimized parameters as moisture level 70%, pH 6.0 and temperature 30p C.
In the present study the substrate chosen for ethanol extraction was withered waste flowers. Thorough study was made on the Allamanda species as a source for ethanol production by Saccharomyces cerevisiae. Extensive research was done on screening and selecting the plant species of Allamanda schotti (family Apocyanaceae) as substrate for the production of ethanol. The micro organism Saccharomyces cerevisiae is efficient in converting hexose sugars in to ethanol and carbon di oxide (Potphode Arati and Agarwal Seema, 2015). The application of activated dry yeast for production of ethanol quickens fermentation and prevents any contamination which can occur due to bacteria. (Daoqiong et al, 2013).
Allamanda schottii is abundantly found as hedge crop in tropical countries. Hence these flowers can be used to produce ethanol through fermentation which may be an economic advantage in the Indian context. This study also paves way for the production of ethanol from nonfood crop i.e. second generation bio fuels. Allamanda schottii has rich source of carbohydrate 69%. The physio chemical properties of Allamanda schottii were studied and the analysis revealed that allamanda contains total sugars 60-65%, protein 2.40 %, ash 1.4% and moisture content 79.40%.
Substrate
Flowers of Allamanda schotti collected from APEI campus, G.B.Nagar, kalavai, Vellore district, Tamil Nadu during the month of March- August and authenticated at department of floriculture, Adhiparasakthi Horticultural College, G.B.Nagar, kalavai. The withered flowers were collected by engaging women labourer and manually cleaned by them. The flowers were then shade dried The yeast .Saccharomyces cerevisiae was purchased from local super market as commercial activated dry yeast (ADY) and it was used for fermentation.
Sterilization
The withered flowers of Allamanda were manually collected and cleaned. The allamanda flowers are sterilized in an autoclave at a pressure of about 10 lb / inch2. The sterilization was carried out for 25 minutes. For quick drying the flowers were kept in a hot air oven (HASTHAS) at 65p C for 5-6 hours.The dried flower was powdered in Willey mill (SECOR, 220 Volts, 0.37 Kw, 0.5 Hp, rpm 1440). The flower powder was sealed in polythene cover and used for analysis.
Estimation of sugar
The anthrone method was followed to find out the sugar content in the allamanda flowers and it was estimated to be 60-65%.
Medium for seed culture
Yeast Saccharomyces Cerevisiae was obtained from local super market in the form of activated dry yeast. Yeast Saccharomyces cerevisiae was maintained on the yeast extract on potato dextrose broth at pH 5.5 (2.4g in 100ml distilled water). The inoculated medium was incubated for 3 days at 35°C. The inoculum size was assessed by serial dilution and plating technique and the samples were adjusted to cell concentration of 109/ml of sample using sterilized medium.
Ethanol Estimation
Potassium di chromate oxidation method and Spectro photometric method was followed to estimate ethanol. The ethanol production was tested by LCMS (Liquid Chromatography Mass Spectrometer).
Experimental
About 10 g of allamanda flower powder was mixed with 80 ml distilled water and made into a slurry. The slurry was thoroughly mixed in an autoclave at 120°C for 25 minutes and then the contents were cooled to room temperature. The slurry was pretreated with H2 SO4 at a concentration of 0.05N and NaOH 0.5% alkalinity. The yeast strain Saccharomyces Cerevisiae were prepared and added at room temperature (30±2°C) to the suspension. The slurry was centrifuged at 1000 rpm for 15min. The slurry was kept at room temperature for fermentation. Optimization of various parameters like slurry composition as 1: 8, pH as 5.5, inoculum age as 72 hours, inoculum level as 3.75 g 100 ml-1, temperature at 35p C and urea 0.05g L-1were maintained in the The fermentation medium to get best results. The process was done in triplicates. The ethanol obtained was maximum 18.75 ml for 100 ml slurry composition.
Distillation
The fermented broth was removed after 5 days and the contents were analyzed for ethanol. The filtration was done with whatman no 1 filter paper to collect the supernatant from the fermented slurry. The simple distillation unit consists of a round bottom flask, a condenser and a distillate. The filtered sample was transferred to round bottom flask heated by heating mantle (ILECO- 300 watt- Capacity 1000ml). Ethanol was separated at a temperature of 78.5°C (boiling point of ethanol). The vapours of ethanol was condensed by the circulating water around the condenser and received in the distillate. Moisture free ethanol was obtained by keeping the distillate under refrigerated condition. The water present in the sample freezed at 0°C and 99.50% pure ethanol (freezing point -117°C) was collected and stored.
Confirmation of Ethanol
Potassium dichromate method was adopted to estimate ethanol followed by confirmation with LCMS (Liquid Chromatograph Mass Spectroscope). The distillate contained 1.5 ml of ethanol. The volatile and semi volatile compounds in the distillate can be found using the above analytical technique. LCMS was done at Shiva Analyticals (India) Private Limited, Bangalore (NABL (ISO / IEC 17025: 2005) Accredited & ISO 9001: 2008 Certified Laboratory)
The appearance of blue green colour in Jones reagent test shows that the fermented sample has ethanol (Tripti Agarwal et al. 2013). Oxidation of ethanol to acetic acid with an excess of potassium dichromate in the presence of sulphuric acid showed off a blue green colour (Brooks et al 2008).
Quantitative estimation of ethanol as volume of ethanol per volume of fermented liquid from Allamanda flowers was found to be 15 ml/100 ml slurry, i.e 18.75ml 100 gm-1 of dry flower. Behera et al., 2010 studied fermentation of mahula flowers produced ethanol (154.5gm kg-1 flowers) by immobilized cells of Saccharomyces cerevisiae in calcium alginate beads. After 96 hrs fermentation Swain et al., (2007) studied fermentation of Madhuca latifolia L. using free cells of yeast Saccharomyces cerevisiae produced ethanol 193 and 148gm kg-1 from fresh and 12 month mahula flowers. Recently Mandal et al., studied fermentation of mahulla flowers madhuca latifolia L. using Saccharomyces cerevisiae -3040 gives maximum yield of ethanol (38ml @1.5 kg L-1) ratio of slurry for 48 hrs. Arati et al., (2015) studied submerged fermentation of fresh rangoon creeper (Quisqualis indica) flowers with Saccharomyces cerevisiae yield ethanol 1.41gm% with fermentation efficiency of 36.29%. Geetha et al., (2013) studied the potential of degrained sunflower head waste as substrate for ethanol production and found that ethanol yield was maximum in the treatment with acid hydrolysis(20.528gm L-1).
The effect of slurry composition
The effect of slurry composition on the production of ethanol using Saccharomyces Cerevisiae was carried out by varying slurry composition of 1:4 to 1:10 ratios (w/v). The various parameters such as inoculum age 72 hours, inoculum level 3.75g/100ml, agitation 100 rpm, urea 0.5g w/v, pH 5.5 at room temperature of 35p C was maintained.
It can be seen from fig 1 the slurry composition 1:8 showed the maximum yield of ethanol (15ml/10g). The fermentation time was observed as 5 days and it can be seen from fig, that the production of ethanol was decreased with increase in slurry composition. It was confirmed that the substrate Allamnada flower powder slurry of 1:8 is an optimum slurry composition for maximum ethanol production. Taking this in view, slurry composition1:8 were taken as optimum and the effect of pH, Temperature, Inoculum level, inoculum age, agitation, addition of urea were studied. (Banerji D.S.N. et al, 2010)
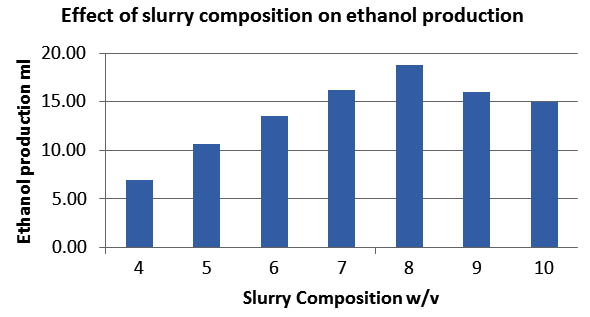
Fig. 1. Effect of slurry composition on ethanol production
Effect of pH
It has been seen from the fig 2 that the rate of ethanol production was maximum at pH of 5.5. This is due to the fact that proteins function in an environment that reflects this pH (Berg, 2007). A of pH 2 had the lowest carbon dioxide production presumably because the low pH encourages the production of acid instead of alcohol (Jennings, 1995).The value of pH during fermentation period changed from 5.5 to 4.5. The change in pH was adjusted by the addition of NaOH and H2SO4 at every 5 hours.
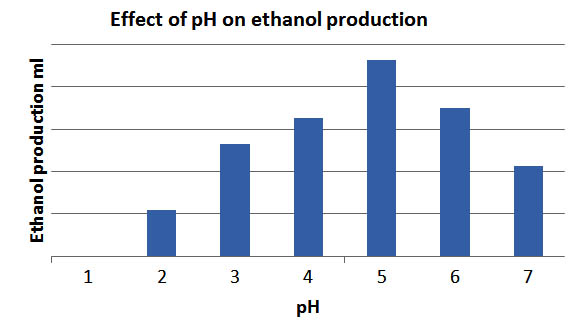
Fig. 2. Effect of pH on ethanol production
Effect of temperature
The previously reported studies show that fermentation temperature is an important parameter which affects the production of ethanol. It was found that maximum ethanol yield was obtained when the fermentation medium was 30 – 32°C. The further increase in temperature affects microbial growth and decreases the production of ethanol.(Fig 3)
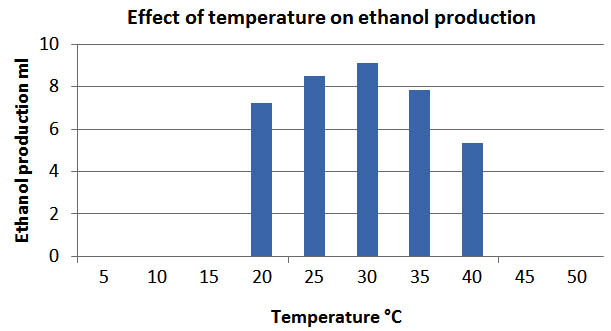
Fig. 3. Effect of temperature
Effect of inoculum age
Inoculum age was investigated to determine the potentiality of the yeast Saccharomyces Cerevisiae. The process of fermentation was carried out for a period of 6 days and ethanol yield was calculated after every 24 hours. It was found that the optimum yield of 8.23 ml was found after 72 hours. The fermentation was carried out for all six days but it was noted that the ethanol production dropped drastically to 2.80 ml after 6th day. The results are reported in fig 4

Fig. 4. Effect of inoculum age
Effect of inoculum level
The inoculum level was studied to determine te percentage of inoculums in the media for optimum fermentation. The range selected was from 1 mg to 5mg . It was found that 3 mg of inoculum was effective yield of ethanol as 9.1ml. The results obtained are reported in fig 5.
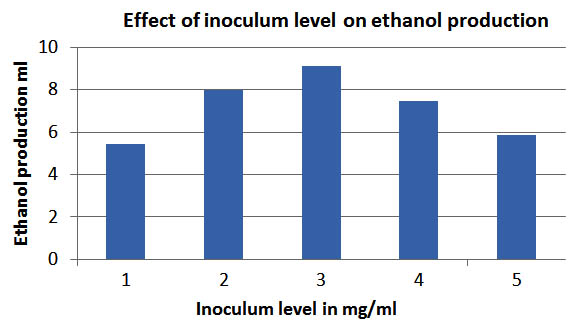
Fig. 5. Effect of inoculum level
Effect of nitrogen
Most microbes utilize nitrogen to metabolize nitrogenous substances for the growth and their activity, effect of nitrogen source (Beltran et al., 2007). Urea was added to the slurry so that yeast Saccharomyces Cerevisiae can be metabolized the nitrogenous substances for their growth and activity. It was observed that maximum production was obtained at 0.05g/L concentration of urea. From the Fig.6 it can be concluded that the maximum production was obtained at .05 gm/litre concentration of urea and it has been seen that the ethanol production increased from .02gm/litre to .05 gm/litre, and further increase in urea decreases the ethanol production. The addition of urea had a significant effect on the rate of production of ethanol and it can be concluded that addition of 0.05gm / litre urea is desirable for maximum ethanol production.
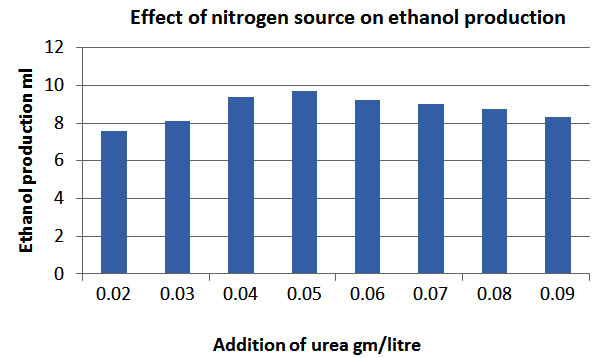
Fig. 6. Effect of nitrogen source
Effect of agitation
Agitation at optimum rpm plays an important role in utilizing maximum sugars for the growth of Saccharomyces cerevisiae and for ethanol production through fermentation. By maintaining the optimized parameters, agitation was studied. In this experiment the rpm was varied from50 to 250 rpm with an increment of 50 rpm. It is seen from the fig.7 the ethanol production increased with increase in agitation up to 100 rpm, but further increase in rpm shows the decrease in ethanol yield and hence 100 rpm was found as optimum agitation for further studies.
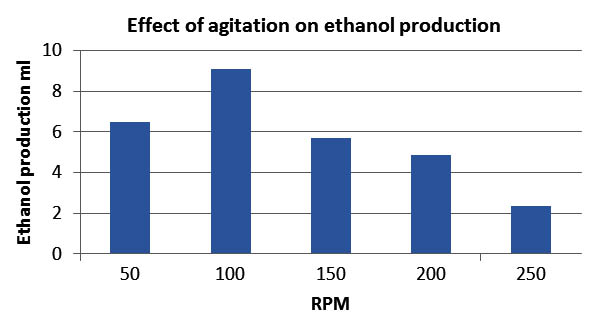
Fig. 7. Effect of agitation

Fig. 8. Withered flower

Fig. 9. Shade dried flower

Fig. 10. Oven dried flower

Fig. 11. Dry flower powder

Fig. 12. Flower slurry

Fig. 13. Distillation unit

Fig. 14. Willey mill
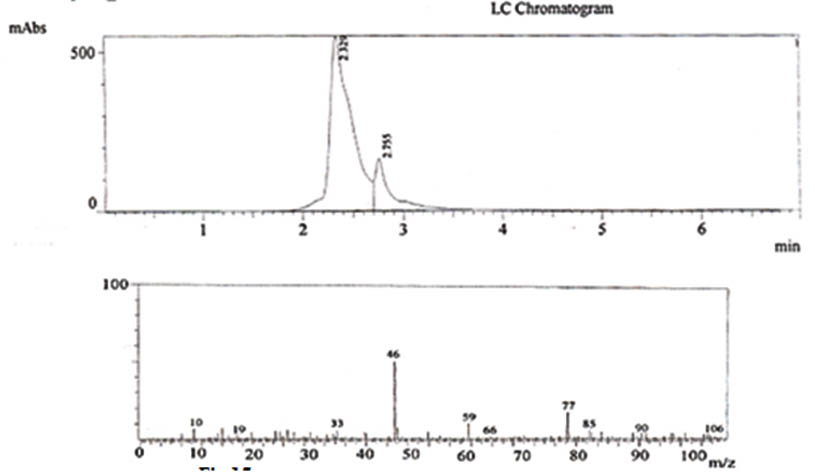
Fig. 15. LCMS reports showing the presence of ethanol
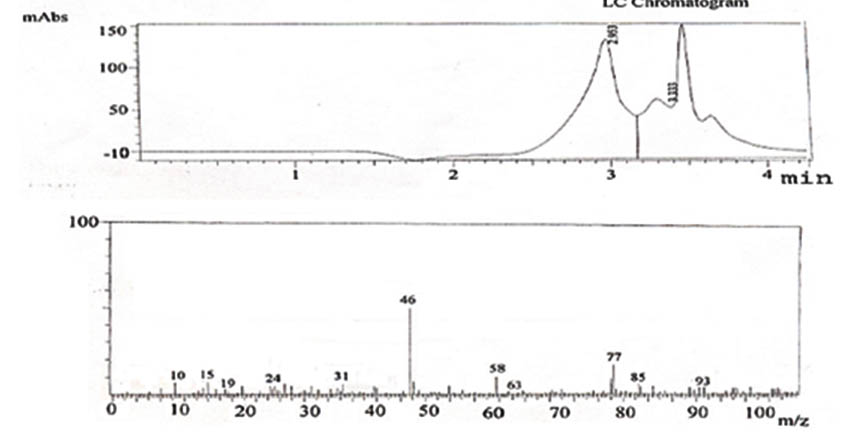
Fig. 16. LCMS reports showing the presence of ethanol
The changes in various parameters such as slurry composition, pH, Temperature, inoculum age, inoculums level, addition of chelating agents during the course of fermentation of allamada flower slurry were studied. The confirmation of presence of ethanol in the distillate was done by LCMS. The LC analysis confirms the presence of ethanol in the distillate (Fig.11 and Fig.12) and result for MS shows that along with Ethanol, Dimethyl ether, Propanol, Iso butane, Methyl nitrate, Hexane, Acetaldoxime, Malononitrile are also present in the distillate.
Bio ethanol is a viable transportation fuel which can replace petrol and extend its availability for a longer run. The bio ethanol produced from non food crop provides energy security to satisfy the energy needs of growing population. Withered waste flowers, a low cost substrate available freely and abundantly in nature could be a best alternative to produce bio ethanol than producing it from food crops. The withered waste flowers of Allamanda is a potential unutilized agriculture residue contains considerable amount of which can form an ideal medium for ethanol production. Therefore the substrate was evaluated for ethanol production by optimizing various parameters such as slurry composition, pH, Temperature, Inoculum level, Inoculum age, Fermentation time and nutrient supplementation.
- The Wealth of India, A Dictionary of Indian Raw Materials and Industrial Products-Raw Materials Series, Publications and Information Directorate. Council of Scientific & Industrial Research VI: 1962: 207–16.
- Hydrocarbon plant—New source of energy for future Dipul Kalita_ Renewable and Sustainable Energy Reviews12 (2008) 455–471-www.elsevier.com/locate/rser
- Benerji D.S.N., Rajini K., B. SrinivasaRao, Banerjee D.R.N., K. Swaroopa Rani, Rajkumar G., and Ayyanna C., Studies on physic-chemical and nutritional parameters for the production of ethanol from mahua flower (Madhuca indica) using Saccharomyces Cerevisiae –3090 through submerged fermentation (smf), J. Microbial Biochem. Technol. 2010; 2: 46–50.
- Ethanol production from degrained sunflower head waste by Zymomonas mobilisand saccharomyces cerevisiae–Geetha.S, kumar.A & Deiveekasundaram.M- International Journal of AgriculturalScience and Research (IJASR)ISSN 2250-0057. 3(4), Oct 2013, 93-102.
- Potphode Arati and Agarwal Seema ; Bioethanol production from Flowers of Quisqualis indica, International Journal of Science, Engineering and Technological Research, 2015; 4(4).
- Zuber Khan and Anjani K.Dwivedi; Fermentation of Biomass for production of Ethanol-A Review, Universal journal of Environmental Research & Technology, 3(1):1-13
- Graeme M. Walker & Venus; Bioethanol-Science and technology of fuel alcohol, 2010 Publishing ApS ISBN 978-87-7681-681-0
- Experimental Determination of Fuel Properties of Ethanol/ Gasoline Blends as Bio fuel for SI Engines; A.F.Kheriralla, Mohamed M. El-Awad, MathaniY.Hassan, Mohammed A.Hussen and Hind I.International Conference on Mechanical, Automobile and Robotic Engineering(ICMAR’2012) Panang, Malaysia.
- Doelle, H. W., & Greenfield, P. F. The production of ethanol from sucrose using Zymomonasmobilis. Applied microbiology and biotechnology, 1985; 22(6): 405-410.
- Schwald, W., Breuil, C., Brownell, H. H., Chan, M., & Saddler, J. M. Assessment of pre treatment conditions to obtain fast complete hydrolysis on high substrate concentrations. Applied Biochemistry and biotechnology, 1989; 20(1), 29-44.
- Torget, R., Hatzis, C., Hayward, T. K., Hsu, T. A., & Philippidis, G. (1996, January).Optimization of reverse flow, two-temperature, dilute-acid pre treatment to enhance biomass conversion to ethanol.In Seventeenth Symposium on Biotechnology for Fuels and Chemicals(pp. 85-101). Humana Press.
- Sutaria P.B. and Magar N.G., Preparation of sugar analysis of flowers from various districts. Indian Chem. Soc., 1955; 18: 75–80.
- Production of Ethanol from Mahua Flower (Madhuca Latifolia L.) Using Saccharomyces Cerevisiae – 3044 And Study Of Parameters While Fermentation- PranavMandal And Niren Kathale ; Abhinav National Monthly Refereed Journal Of Research In Science &Technology- 1(9)
- Comparative study of bioethanol production from mahula (MadhucalatifoliaL.) flowers by immobilized cells of Saccharomyces cerevisiae and Zymomonas mobilisin calcium alginate beads-Shuvashish Behera, Ramesh C Ray and Rama C Mohanty- Journal of Scientific & Industrial Research, 2010; 69; 472-475
- Ethanol determination by distillation Protocol Copyright © 1978 D. B. Fankhauser, June 19, 2011, Background and additional information Copyright © 1993 J. L. Stein Carter
- Behera S, Kar S, Mohanty R C & Ray R C, Comparative studyof bio-ethanol production from mahula (Madhuca latifoliaL.)flowers by Saccharomyces cerevisiaecells immobilized in agar agar and Ca-alginate matrices, Appl Energy, 2010; 87; 96-100.
- Swain M R, Kar S, Sahoo A K & Ray R C, Ethanol fermentationof mahula (MadhucalatifoliaL.) flowers using free and immobilized yeast Saccharomyces cerevisiae, Microbiol Res, 2007; 162: 93-98
- Ethanol Production from Mahua (MadhucaindicaJ. F. Gmel) Flowers by Soil Bacteria TriptiAgrawal, Afaque Quraish2, KishanLal Tiwari and Shailesh Kumar Jadhav*- Researcher 2013;5(12)
- Yield and Properties of Ethanol Biofuel Produced from Different Whole Cassava Flours, F. T. Ademiluyi and H. D. MepbaB. C. Akin-Osanaiye, H. C. Nzelibe, and A. S. Agbaji, “Production of ethanol from Carica papaya(pawpaw) agro waste: effect of saccharification and different treatments on ethanol yield,” African Journal of Biotechnology, 2005; 4(7): 657–659.
- Sulfahri, S. Mushlihah, E. Sunarto, M. Yusuf Irvansyah, R. S. Utami, and S. Mangkoedihardjo, “Ethanol production from algae Spirogyra with fermentation by Zymomonasmobilis and Saccharomyces cerevisiae,” Journal of Basic and Applied Scientific Research, 2011; 1(7): pp. 589–593.
- P. Atthasampunna, P. Somchai, A. Eur-aree, and S. Artjariyasripong, “Production of fuel ethanol from cassava,” World Journal of Microbiology and Biotechnology, 1987; 3(2): pp. 135–142.
- Ethanol, Wikipedia Encyclopedia, 2012, http://en.wikipedia.org/wiki/Ethanol.
- S. A. Ado, G. B. Olukotun, J. B. Ameh, and A. Yabaya, “Bioconversion of cassava starch to ethanol in a simultaneous saccharification and fermentation process by co-cultures of Aspergillus niger and Saccharomyces cerevisiae,” Journal of Pure and Applied Microbiology, 2010; 4(1): 55–61.
- Experimental Determination of Fuel Properties of Ethanol/Gasoline Blends as Bio-fuel for SIEngines. A.F. Kheiralla, Mohamed M. El-Awad, Mathani Y. Hassan, Mohammed A. Hussen, and Hind I.International Conference on Mechanical, Automobile and Robotics Engineering (ICMAR’2012) Penang. Malaysia
- Jadhav SK, Tiwari KL, Renjini. Production of bioethanol from Mahua flower, Bioethanol a bioenergy resource. LAP Lambert academic publishing 2011;1-76.
- Rice, R.W., Sanyal, A.K., Elrod, A.C., and Bata, R.M., Exhaust gas emissions of butanol, ethanol and methanol–gasoline blends. Journal of Engineering for Gas Turbine and Power. 1991; 113: 337–381.
- Mustafa K, Yakup S, Talga T, Huseyin. The effects of ethanol unleaded gasoline blends on engine performance and exhaust emissions in spark ignition engine, Review Energy, 2009; 34(10): 2101-2106.
- Bioethanol Production Using Saccharomyces cerevisiaewith Different Perspectives: Substrates, Growth Variables, Inhibitor Reduction and Immobilization. Bhartiand Madhulika Chauhan -Fermentation Technology, 2016; 5(2): 1000131.
- Mussatto SI, Dragone G, Guimarães PM, Silva JP, Carneiro LM, et al. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv, 2010; 28: 817-830.
- Lin Y, Zhang W, Li C, Sakakibara K, Tanaka S. Factors affectingethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass and Bioenergy, 2012; 47: 395–401.
- Aiba S, Nagai S, Nishizagma Y. Fed batch culture of S. cerevisiae:a perspective of computer control to enhance the productivity in Baker’sYeast cultivation. Bio technology Bio engineering, 1976; 18: 1001-1016.
- Association of Official Agricultural Chemistry. Determination of moisture content, Official and tentative Methods of analysis 9th Ed.Washinton, D.C, USA 685; 1960.
- Karagoz P, Ozkan M. Ethanol production from wheat straw by Saccharomyces cerevisiae and Scheffersomyces stipitis co-culture in batch and continuous system. Bio resoTechnol, 2014; 158: 286–293.
- Caputi AJ, Ueda M, Brown T. Spectrophotometric determination of ethanol in wine. Am J EnolViti, 1968; 19: 160.
- Shahsavarani D, Hasegawa D. Enhanced bio-ethanol production from cellulosic materials by semi-simultaneous saccharification and fermentation using high temperature resistant Saccharomyces cerevisiaeTJ14. J Biosci Bioeng, 2013; 115: 20–23.
- Singh A, Bajar S, Bishnoi NR. Enzymatic hydrolysis of microwave alkali pre treated rice husk for ethanol production by Saccharomyces cerevisiae, Schefferso mycesstipitis and their co-culture. Fuel, 2014; 116: 699-702.
- Bansal R & Singh R S, A comparative study on ethanol production from molasses using Saccharomyces cerevisiae and Zymomonasmobilis, Ind J Microbiol, 2003; 43; 261-264.
- Akaracharanya A, Kesornsit J, Leepipatpiboon N, Srino-rakutara T,Kitpreechavanich V, et al. Evaluation of the waste from cassava starch production as a substrate for ethanol fermentation by Saccharomyces cerevisiae. Ann Microbiol, 2011; 61: 431-436.
- Razmovski R, Vucurovi V. Ethanol production from sugar bee tmolasses by S. cerevisiae entrapped in an alginate-maize stem ground tissue matrix. Enzy Micro Technol, 2011; 48: 378-385.
- Ratnam BVV, Rao MN, Rao MD, Rao SS, Ayyanna C. Optimization of fermentation conditions for the production of ethanol from sago starch using response surface method. World Journal of Microbiology &Biotechnology, 2003; 19: 523-526
- Ballesterose, I., Ballesterose, M., Cabanas, A., Carrasco, J., Martin, C.,Negro, M.J., Saez, F., Saez, R., Selection of thermo tolerant yeasts for simultaneous saccharification and fermentation (SSF) for cellulose to ethanol. Applied Biochemistry and Biotechnology, 1991; 307–315.
- Banat, I.M., Nigam, P., Singh, D., Marchant, R., McHale, A.P., Review: Ethanol production at elevated temperatures and alcohol concentrations: Part I – Yeasts in general. World Journal of Microbiology and Biotechnology, 1998; 14: 809–821.
- Nikita Naik, Rupashree Salvi, Arati Potphode, International Journal of Science, Engineering and Technology Research (IJSETR) Volume 5, Issue 5, May 2016, Successful production of Bioethanol from Canna Indica ISSN: 2278 – 779.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


