ISSN: 0973-7510
E-ISSN: 2581-690X
Soil microorganisms play a central role in decomposition, nutrient mineralization, and nearly all ecological processes in a soil ecosystem. A number of soil microbiological parameters, notably microbial biomass carbon and basal respiration, have been suggested as the promising indicators of soil quality. Therefore, this study aims to investigate microbial respiration as an indicator of microbial activity under different ages of rubber tree in different season. Three treatments were selected, categorized by tree age included: rubber trees 4-5 years old, 12 years old, and 22-23 years old. Soil samples were taken from a randomly selected location in each plot, at 0-10 cm, in rainy and dry season. The physical and chemical properties of the soil were also observed. The result showed that soil microbial biomass carbon, activity and MicroResp-CLPP (community level physiological profile) were influenced by rubber ages and season. The highest carbon dioxide emission, which was related to microbial activity, was found in the 4-5 years old rubber tree, during the rainy season. However, contrary results emerged during the dry season. In addition, the more aged rubber trees exhibited a higher microbial biomass carbon. The results further demonstrated that bacterial biomass and soil activity were higher than that of the fungi. In rainy season, MicroResp-CLPP showed the highest catabolic responses in young age (4-5 years old) of rubber tree followed by tapped rubber tree (12 and 22-23 years old). Contrary, the highest catabolic responses in dry season was observed in tapped rubber tree (12 and 22-23 years old). These findings suggested that microbial biomass and relative activity were directly correlated with the rubber tree age.
Rubber, MicroResp-CLPP (community level physiological profile), plant age
Natural rubber (Hevea brasiliensis), originating from the Amazon watershed of South America, is an important worldwide commodity1. Today, the newer plantations of South East Asia have experienced rapid expansion in the past few decades. Thailand, in particular, has enjoyed rapid expansion of rubber tree plantations in the traditional areas of the southern most regions, extending to the northeast.
The production and growth of rubber trees vary due to the influence of many environmental factors such as the amount of rainfall, soil moisture, soil characteristics, and biological properties. However, mostly studies investigating rubber tree plantations impact on soil focused on the physical and chemical parameters.
Importantly, soil microorganisms also represent as an essential component within the rubber ecosystem. In fact, changes in soil microbial community composition may be among the earliest indicators of soil quality in many ecosystem processes2, 3, 4. Microbial activity, including microbial respiration, also acts as the main biological indicators of soil quality, and responds to changes resulting from varied agronomic practices5. Soil microbial biomass, the living part of soil organic matter, function as a transient nutrient sink and is responsible for releasing nutrient from organic matter which is used by plant6. In addition, soil microorganisms play a central role in decomposition, nutrient mineralization, the sustainability of soil nutrients, and nearly all soil-based ecological processes within the functions of the forest ecosystem7, 8. Several study reported that large scale environment perturbations, such as plantation conversion, can cause large shifts in the soil microbial community. Moreover, soil microbial properties are more sensitive to land use change than soil chemical and physical properties9, 10. Interestingly, previous studies showed different age group of rubber trees affected available soil nutrient and soil carbon stock11, 12 and that could be consequently influence to soil microbial biomass and activity.
Therefore, the purpose of this study was to investigate the impact of different ages of rubber tree plantation on soil microbial activity based on the MicroResp-CLPP (community level physiological profile) analysis which provides a direct measurement of microbial communities catabolic profile and reflects the actual size of the microbial population13 and can determines the immediate response of active microorganisms in a short-term period, providing physiological information. Moreover, using CLPP to estimate soil functional diversity was able to highlight the activity of living microorganisms. The finding of this study should be beneficial to recognize the microbial activity to indicate soil quality for the purpose of soil and environment conservation.
Study site and sampling
The experimental site is located in the Kranuan district, Khon Kaen province, Thailand (16° 44´-16° 75´ N; 103° 07´ – 103° 08´ E). This area is characterized by a tropical climate with a rainy season and dry season. The mean rainfall was 168.7 mm, the mean annual temperature was 32°C, and the highest and lowest average monthly temperatures were 37°C in June and 18°C in February. Sampling sites for our study were selected in 4-5, 12, and 22-23 years old rubber tree plantations (clone RRIM 600). Each site was sampled once in rainy season (June, 2015) and once again in dry season (February, 2016). At each site, we established a sampling spot for our trees, measuring 1×1 m2. Soil samples were taken from randomly selected locations in each plot, at depths of 0-10 cm. The litter layer was removed prior to sampling, and three core samples were taken at the same depth in each plot, and were combined to a single composition sample.
Physical and chemical soil analyses
Soil moisture was determined by oven-drying fresh soil at 105°C to constant weight. Soil pH was measured from soil – water suspension (1:2.5: soil: H2O) with a digital pH meter. Soil organic carbon (SOC) was determined through dichromate oxidation. Soil microbial biomass carbon (MBC) was measured in fresh soil immediately after sampling, by the chloroform fumigation extraction technique14. Briefly, 20g of soil was extracted with 100 ml of 0.5 M K2SO4. We determined the amount of microbial biomass carbon in the extracts after oxidation, with K2Cr2O7. Calculations were made to determine the differences between fumigated (36 hours) and un-fumigated values, employing a KEC factor of 0.3315.
The MicroResp-CLPP (community level physiological profile) analysis
Analysis of CLPP was performed using the MicroResp soil respiration system (MicroRespTM, Macaulay Scientific Consulting Ltd., Aberdeen, UK) as described in Campbell et al13 and Lagomarsino et al16 to determine microbial activity via the basal respiration (BR) and the ability of the soil microbial community to metabolise a wide range of carbon sources, i.e. community level physiological profiles. The soil was air-dried, sieved at 2 mm and stored at room temperature before analysis. The MicroResp-CLPP method consisted of two 96-well microplate placed face-to-face13. One of the plates, with a capacity of 300 µl/well, holds 150 µl/well of an agar gel (10 g/L) enriched in KCl (0.15 mol/L), NaHCO3 (2.5 mmol/L), and cresol red dye (32.7 µmol/L); in which to estimate the CO2 air fraction based on gel absorbance at 570 nm. After preparation, this plate was conserved for seven days in a closed environment with soda lime and water, with and without a protective parafilm, during the first through the sixth subsequent days. The second plate, with a capacity of 1.2 mL/well, held roughly 0.45 g/well of moisture soil, with or without carbon substrate. The substrates were adapted from the MicroResp technical manual17, 18, 19, and were partly chosen based on availability in the laboratory. Stock solutions for 15 substrates were prepared using distilled water with antibiotic (Bronopol) and further diluted accordingly for each soil based on the soil moisture content. Fifteen types of carbon sources were selected from the previously described carbon sources based on carbon sources that are ecologically relevant to soil (specifically as plant root exudates) and that can be dissolved in water. The substrates used in this study were reported in Table 1. Just before incubation, the plate containing the gel was read with an absorbance microplate reader (VMAX; Molecular Devices, Wokingham, United Kingdom). The two plates were then sealed together with a silicone rubber gasket with interconnecting holes. After six hours of incubation at 25°C, the plates were separated, and the plate containing the gel was immediately re-read. The absorbance after 6 h was normalized for any differences recorded at zero time before exposure and then converted to the headspace CO2 concentration by using the calibration curve. The average amount of CO2 that evolved per sample was calculated and used to normalize individual carbon source concentrations before the multivariate analysis.
Table (1):
Substrate used for MicroResp-CLPP analysis.
Substrate |
Type |
|---|---|
None (no carbon source) |
|
Glucose |
carbohydrate |
Cellulose |
carbohydrate |
Glycine |
amino acid |
Glutamine |
amino acid |
Malic acid |
carboxylic acid |
Oxalic acid |
carboxylic acid |
Vanil acid |
phenolic acid |
Urea (N source) |
amino acid |
Ferulic acid |
aromatic compound |
Catechol |
aromatic compound |
Cellobiose |
carbohydrate |
Glucosamine |
amide |
Phytate (P source) |
phytic acid |
Phytate-glucose |
phytic acid – carbohydrate |
Casein |
phosphoprotein |
Microbial respiration (CO2 emission) and metabolic quotient analyses
Alkali trap method was used to measure field CO2 emission, a small glass jar (5.5 cm height, 6 cm diameter) containing 20 ml of 1 M NaOH was placed in a closed metal chamber (16 cm diameter and 29 cm height) and left for 24 h. The evolved CO2 trapped was subsequently determined by back titration with 0.5 M HCl after precipitating the carbonate with excess 0.5 M BaCl2. Microbial respiration, i.e., CO2 emission, was computed according to the equation described by
Anderson20. The metabolic quotient (qCO2), the ratio of microbial respiration to microbial biomass carbon was also determined 21, 22.
Statistical analysis
The data were subject to analysis of variance SPSS statistical program (SPSS for Windows, version 17.0; SPSS Inc., Chicago, IL, USA.). The difference among various treatments mean was compared using the least significant difference test (LSD) at 5% (p < 0.05) probability level.
Soil properties
The soil within our sample rubber tree plantations was characterized as Chum Phuang (Cpg) soil series which was classified as a coarse-loamy, Siliceous, Isohyperthermic, Typic Kandiustuls based on soil taxonomy classification23.
The soil pH, soil moisture, and SOC were given in Table 2. Soil texture of all plot sites was loamy sand. The soil pH in the rainy and dry seasons was found in the range of 5.13-5.55 and 5.37-5.76, respectively; which indicated light soil acidity.
Table (2):
Soil physical and chemical properties analyses.
Season |
Ages (years) |
pH |
Moisture (%) |
SOC (%) |
|---|---|---|---|---|
Rainy |
4-5 years |
5.31±4.50 |
13.15±10.0ab |
0.24±0.03b |
12 years |
5.31±4.80 |
15.71±14.4a |
0.32±0.45b |
|
22-23 years |
5.55±4.60 |
12.79±11.5b |
0.41±0.46a |
|
p-values |
ns |
* |
* |
|
Dry |
4-5 years |
5.38±4.70b |
2.77±1.90b |
0.20±0.15b |
12 years |
5.37±4.60b |
2.42±1.70b |
0.31±0.28a |
|
22-23 years |
5.76±4.50a |
2.93±2.40a |
0.28±0.19a |
|
p-values |
* |
* |
* |
The soil moisture in the rainy and dry seasons ranged from 13.15-15.71% and 2.42-2.93%, respectively. The bulk densities of the soil samples in both seasons ranged between 1.69-1.76 g.cm-3 (data not shown).
The SOC was highest in rubber trees 22-23 years of age, and lowest in early stages of growth, or unproductive rubber trees, 4-5 years old. Seasonal comparisons further determined a higher overall SOC (0.24-0.41%) in the rainy season, than those of the dry season (0.20-0.31%).
Microbial biomass carbon and microbial respiration
Differences in MBC and microbial respiration are shown in Table 3. Microbial respiration was significantly different between the two seasons, in which the rainy season yielded higher respiration, especially in rubber trees 4-5 years of age.
Table (3):
Soil biological properties analyses.
Season |
Ages |
Microbial respiration |
Microbial biomass carbon |
Metabolic quotient |
|---|---|---|---|---|
(years) |
(µgCO2/kg/h) |
(µgCO2/kg) |
(qCO2) |
|
Rainy |
4-5 |
326.90±289a |
1541.97±647c |
0.212±0.15a |
12 |
240.51±228a |
2939.65±2384a |
0.081±0.05b |
|
22-23 |
166.61±142b |
2476.87±364b |
0.067±0.03b |
|
p-values |
* |
* |
* |
|
Dry |
4-5 |
80.57±79.43b |
507.24±53 |
0.158±0.07 |
12 |
97.38±89.45a |
843.34±369 |
0.115±0.07 |
|
22-23 |
114.26±110.23a |
763.73±19.3 |
0.149±0.05 |
|
p-values |
* |
ns |
ns |
The greater moisture and higher microbial activity of the rainy season were also responsible for an increase in MBC; which was also influenced by the age of the rubber tree. The highest MBC was exhibited in rubber trees 12 years of age. Similar with the report according to Puttaso et al24 that showed the greatest MBC was found in old age of trees (17 – 27 years old) in rainy season. The greatest metabolic quotient-C (qCO2), were found in 4-5 year-old trees; which further demonstrates the efficiency of heterotrophic microorganisms in converting organic carbon into microbial biomass.
The MicroResp-CLPP
Community level physiological profile (CLPP) is a useful, sensitive and rapid method to assess functional diversity of microbial community as results of measured potential metabolic activity25.
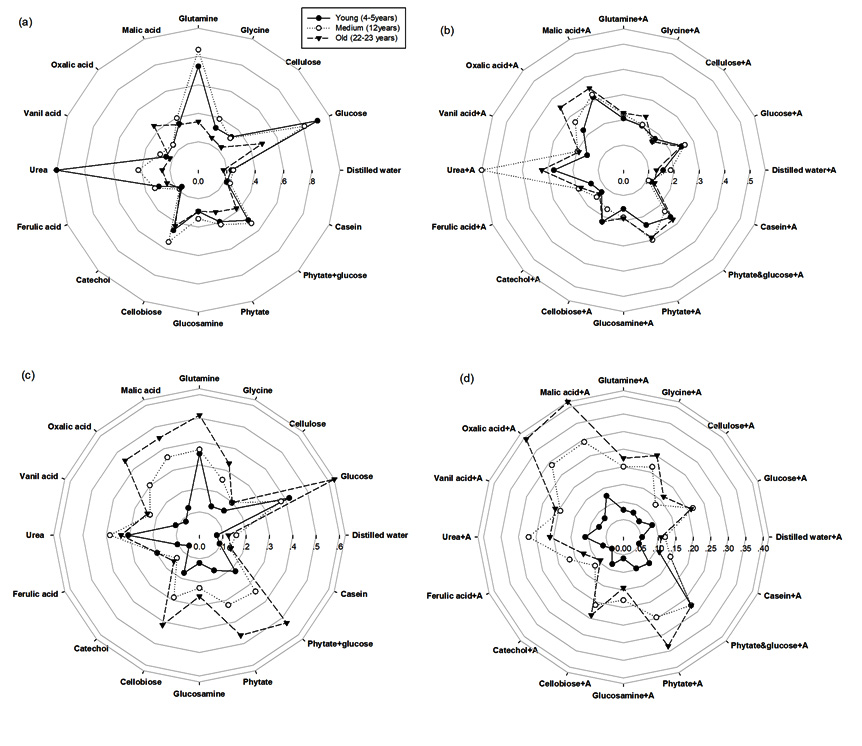
The differences were found in the Microresp-CLPP of rubber plantations at different ages and seasons as shown in Fig. 1. The proportional CLPP of bacteria+fungi (Fig. 1a and 1c) and fungi (Fig. 1b and 1d) were significantly affected by season and plantation age. In rainy season, CLPP showed the highest catabolic responses in the 4-5 years old rubber tree plantation and the lowest in the 12 years old rubber as well as in the 22-23 years old. Contrary in dry season, CLPP showed the highest catabolic responses in the 22-23 years old rubber tree plantation and the lowest in the 12 years old rubber as well as in the 4-5 years old.
Fig. 1. The relative soil microbial activity from MicroResp-CLPP analysis (substrate induced respiration (SIR): µgC-CO2/g/h) under different ages of rubber tree (a) bacteria in rainy season, (b) fungi in rainy season, (c) bacteria in dry season and (d) fungi in dry season. The solid line with black circle represents young age of rubber tree (4-5 years), dashed line with hollow circle represents medium age of rubber tree (12 years), and dashed line with black triangle represents old age of rubber tree (22-23 years)
We also observed that the bacterial and fungal population increased with the application of organic sources. The rainy season proved to be more active than the dry season, in which the quantity of CO2 released, due to different substrates and activity with glucose, glutamine, cellobiose and urea (N source), especially in the 4-5 year old rubber trees. This may be due to the relatively abundant rainfall in the region, and the effect of moisture on soil respiration; which was often covered by the effects of temperature. In addition, Wu et al26 found that from June to August, water and heat conditions improve root and microbial activities; and respiratory metabolism will lead to an increasing trend in soil respiration. Heightened catabolic responses in the 4-5 year old trees may be a result of intercropping, in which cover canopies do not exist, and sunlight may penetrate through the foliage.
In contrast, the dry season’s lower catabolic response was highest in the 22-23 year old trees, in which the glucose and urea of the older trees deposited a litter over the age of rubber tree. Similarly, Skujins27 reported that the catabolic responses of urease, phosphatase, protease, invertase, and catalase can be used as fertility indices of soil to complement soil chemical analyses, in order to predict the nutrient availability and crop yield. In such cases, a high level activity was observed with both malic and oxalic acid.
The rubber tree plantations exhibited the greatest microbial biomass carbon within the 12 years old trees during the rainy season. The highest carbon dioxide emission, which was related to microbial activity, was found in the 4-5 years old rubber tree, during the rainy season. On the other hand, 22-23 years old rubber tree showed the highest carbon dioxide emission. MicroResp-CLPP showed the highest catabolic responses in young age (4-5 years old) of rubber tree followed by tapped rubber tree (12 and 22-23 years old). Contrary, the highest catabolic responses in dry season was observed in tapped rubber tree (12 and 22-23 years old). Additionally, the greater biomass of bacteria over fungi was found in both seasons. These correlating microbiological activities seemed to significant changes of rubber tree ages suggested that microbial activity was responsive to the age of the rubber tree plantations.
ACKNOWLEDGMENTS
This work was supported by grant from Jeunes Equipes Associées à l’IRD program (JEAI), Knowledge Development for Rubber Tree in Northeast (KDRN-KKU) research group, Khon Kaen University. Authors are also grateful to Land Development Department, LMI LUSES and IRD for their technical support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Food and Agriculture Organization of the United Nations. FAOSTAT. www.fao.org/faostat/en/. 2018. Retrieved 26 January 2018.
- Aanderud, Z.T., Schoolmaster, D.R., Lennon, J.T. Plants mediate the sensitivity of soil respiration to rainfall variability. Ecosystems., 2011; 14: 156-167.
- Zornoza, R., Guerrero, C., Mataix-Solera, J., Scow, K. M., Arcenegui, V., Mataix-Beneyto, J. Changes in soil microbial community structure following the abandonment of agricultural terraces in mountainous areas of Eastern Spain. Appl. Soil Ecol., 2009; 42: 315-323.
- Rajaniemi, T.K., Allison, V.J., Goldberg, D.E. Root competition can cause a decline in diversity with increased productivity. J. Ecol., 2003; 91: 407-416.
- Araújo, A.S.F., Santos, V.B., Monteiro, R.T.R. Responses of soil microbial biomass and activity for practices of organic and conventional farming systems in Piauí state, Brazil. Eur. J. Soil Biol., 2008; 44: 225-230.
- Smith, L., Paul, E.A. The significance of soil microbial biomass estimations. In: Bollag JM, Stotzky G, editors. Soil biochemistry. New York: Dekker, 1990; pp 357-396.
- Burton, J., Chen, C.R., Xu, Z.H., Ghadiri, H. Soil microbial biomass, activity and community composition in adjacent native and plantation forests of subtropical Australia. J. Soils Sediments., 2010; 10: 1267–1277.
- Xu, Z. H., Ward, S., Chen, C. R., Blumfield, T., Prasolova, N., Liu, J.X. Soil carbon and nutrient pools, microbial properties and gross nitrogen transformations in adjacent natural forest and hoop pine plantations of subtropical Australia. J. Soils Sediments., 2008; 8: 99-105.
- Peerawat, M., Blaud, A., Trap, J., Chevallier, T., Alonso, P., Gay, F., Thaler, P., Spor, A., Sebag, D., Choosai, C., Suvannang, N., Sajjaphan, K., Brauman, A. Rubber plantation ageing controls soil biodiversity after land conversion from cassava. Agr. Ecosyst. Environ., 2018; 257: 92–102.
- Romaniuk, R., Giuffre, L., Costantini, A., Bartoloni, N., Nannipieri, P. A comparison of indexing methods to evaluate quality of soils: the role of soil microbiological properties. Soil Res., 2011; 49: 733-741.
- Promruksa, W., Smakgahn, K. Carbon stock in soil rubber plantation. J. Appl. Phytotechnol. Environ. Sanit., 2014; 3(3): 101-107.
- Saengruksawong, C., Khamyong, S., Anongrak, N., Pinthong, J. Growths and carbon stocks of para rubber plantations on phonpisai soil series in northeastern Thailand. Rubb. thai J., 2012; 1: 1-18.
- Campbell, C.D., Chapman, S.J., Cameron, C.M., Davidson, M.S., Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol., 2003; 69: 3593–3599.
- Amato, M., Ladd, J.N. Assay for microbial biomass based on ninhydrin reactive nitrogen in extracts of fumigated soil. Soil Biol. Biochem., 1988; 20: 107–114.
- Sparling, G.P. West, A.W. A direct extraction method to estimate soil microbial C: calibration in situ using microbial respiration and 14C labeled cells. Soi lBiol Biochem., 1988; 20: 337–343.
- Lagomarsino, A., Knapp, B., Moscatelli, M., De Angelis, P., Grego, S., Insam, H. Structural and functional diversity of soil microbes is affected by elevated CO2 and N addition in a poplar plantation. J. Soils Sediments., 2007; 7: 399–405.
- Yao, H., He, Z., Wilson, M.J., Campbell, C.D. Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb. Ecol., 2000; 40: 223-237.
- Cameron, C. MicroResp™ Technical Manual — A Versatile Soil Respiration System Macaulay Institute, Craigiebuckler, Aberdeen, Scotland, UK: 2007.
- Campbell, C., Grayston, S., Hirst, D. Use of rhizosphere carbon sources in sole carbon source tests to discriminate soil microbial communities. J. Microbiol. Methods., 1997; 30: 33–41.
- Anderson, J.P.E. Agronomy monograph number 9, part II. Chemical and biological properties. Wisconsin, USA: Madison: American Society of Agronomy and Soil Science Society of America: 1982.
- Franchini, J.C., Crispino, C.C., Souza, R.A., Torres, E., Hungria, M. Microbiological parameters as indicators of soil quality under various tillage and crop-rotation systems in southern Brazil. Soil Till. Res., 2007; 92: 18–29.
- Anderson, J.M, Domasch, K.H. Application of ecophysiological quotiens (qCO2 and qD) on microbial biomass from soils of different cropping histories. Soil Biol. biochem., 1990; 22: 251-255.
- USDA, Keys to Soil Taxonomy, USDA-Natural Resources Conservation Service. Washington, USA, 2014.
- Puttaso, P., Kaewjampa, N., Lawongsa, P. Carbon stock assessment under different ages of rubber tree plantation. Asia Pac. J. Sci. Technol., 2006; 21(4): 74-81.
- Vidican, R., Albu_, A., ^andor, M. Community level physiological profile: a tool to assess functional microbial diversity in soil. ProEnvironment., 2012; 5: 231 – 233.
- Wu, Z., Guan, L., Chen, B., Yang, C., Lan, G., Xie, G., Zhou, Z. Components of soil respiration and its monthly dynamics in rubber plantation ecosystems. Res. J. Appl. Sci. Eng. Technol., 2014; 7: 1040-1048.
- Skujins, J. Enzymes in soil. Eds. McLaren A. D. and Peterson G. H. Marcel Dekker, New York Chapter 15 in Soil Biochemistry, 1: 1967.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.