ISSN: 0973-7510
E-ISSN: 2581-690X
Cystic echinococcosis (CE) is a cosmopolitan disease especially endemic in temperate areas. Human acts as an intermediate host, and there is no implementable non-invasive technique for definitive diagnosis of this disease. This study aimed to evaluate the diagnostic value of serum concentration of three cytokines: interleukin-4 (IL-4), interleukn-10 (IL-10) and interleukin-12 (IL-12) for detection of CE. A total of 42 patients with hepatic CE and other 30 age- and sex-matched apparently healthy subjects were recruited for this case/control study. Sera were obtained for each subjects, and anti-echinococcal IgG antibodies and serum concentration of IL-4, IL-10 and IL-12 were measured by enzyme linked immunosorbent assay (ELISA). Receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of these cytokines. All cases as well as two control subjects were positive for anti-echinococcal IgG antibodies. Serum concentration of IL-4 and IL-10 were higher in cases than controls with significant differences, while there was no such difference in IL-12 concentration. Discrete ROC analysis revealed a moderate diagnostic value for each of IL-4 and IL-10. However, the combined ROC analysis for these two cytokines resulted in a very good discriminative value in which the area under the curve (AUC) was 0.803, 95%CI=0.702-0.905, P<0.001. The sensitivity and specificity of the test at 7.45 pg/mL and 764.5 pg/ml cut-off values (for IL-4 and IL-10 respectively) were 0.70 and 0.71 respectively, Serum concentrations of IL-4 and IL-10 but not IL-12 could be utilized as an additional diagnostic tool for definitive diagnosis of suspected CE. More studies on other Th2-related cytokines or chemokines in CE patients are required to further develop this diagnostic assay.
Cystic echinococcus, Th1 Interleukins, Receiver Operating Characteristic Curve
Cystic echinococcosis (CE) is a zoonotic disease with a cosmopolitan distribution. It is caused by the larval stage of the cestode parasite Echinococcus granulosus. The disease is particularly endemic in temperate zone including Iraq1. Globally, there are approximately 2-3 million infections with CE2. One recent study even linked CE with the liver carcinoma3. Human acts as an intermediate host for the parasite harboring the larval stage. As such, there is no shedding for any developmental stages of the parasite outside the human body, a situation which complicate the diagnosis.
Accordingly, the clinical diagnosis primarily based on indirect methods rather than direct detection of the parasite. Of these, imaging modalities (mainly ultrasonography (US)) is considered the foremost approach. To less extent, serological tests, especially enzyme-linked immunoassay (ELISA), are also widely used based on E. granulosus hydatid cyst fluid antigen4. Other techniques, such as molecular diagnosis and molecular markers like recP29 and recB2t5,6 are mainly used for research purposes because their clinical setting is not yet proven7.
In all these diagnostic techniques, there are many limitations which render considering a particular technique as golden standard for diagnosis of CE a hard task. Serology, for instance, has low sensitivity with up to 25% false negative results8, apart from cross reaction with the other parasites9. Thus, there a is a persistence need for the improvement of diagnostic approach for CE.
It is well-known that CE provokes both humoral and cell-mediated immune response10. When it is active, a hydatid cyst secretes several compounds that interfere with host’s immune response. Of particular interest is the antigen B (AgB). This is a highly immunogenic protein which can skew the immune response towards non-protective Th2 arm11. As such, it is reasonable to suppose relatively high levels of Th2-associated cytokines like IL-4 and IL-10. Measuring these cytokines can reflect the disease status and help in the diagnosis of CE. Thus, this study aimed to assess value of two Th2-cytoknes (IL-4, IL-10) and one Th1-cytokine (IL-12) in the diagnosis of CE using ROC analysis.
The Study Population
This is a case/control study which included 42 patients with hepatic CE who were attending Baaquba General Hospital/Diyala/Iraq during the period from April 2016 to March 2017. Physical and utrasonography were achieved for all patients under the supervision of experienced clinician in US. Inclusion criteria were the presence of at least one hydatid cyst in the liver as detected by US, no previous surgery or albendazole treatment for at least 6 month before sampling. Other 30 age- and sex-matched health donors were recruited to represent the control group. A consent form including demographic data such as age, sex, residence and educational level was obtained from each subject.
Samples
Five milliliter of peripheral blood were obtained from each participant in a plain tube. Sera were separated and kept at -20. A commercial ready kit (GmbH/Germany) was used for estimation of serum levels of anti-echinococcus IgG antibodies in patients and controls using ELISA technique following the manufacturer’s instruction. ELISA was considered positive when IgG concentration is greater than 1.2 U/ml.
Cytokine Assays
Serum concentration of IL-4, IL-10 and IL-12 was measured by ELISA commercial kits (Cusabio/China) following the manufacturer’s protocols. The detection ranges of these kits were 6.5-400 pg/ml, 12.5-800 pg/ml and 4.7-300 pg/ml respectively. Some samples required dilution with distilled water because the concentration of IL-10 was greater than the kit capacity.
Statistical Analysis
All data were analyzed with SPSS software for Windows (version 19). Continuous variables were expressed as median and range while dichotomous variables were expressed as frequency and percentage. Data were subjected for normality test and found to be abnormally distributed according Shapiro-Wilk test. Therefore, Mann Whitney U test was used to find the statistical significant between pairwise comparisons, while chi-square test was used to analyze the dichotomous variables. The ROC curve was used to find the area under the curve (AUC), sensitivity, specificity and cut-off value for the cytokines. The level of significance was set at P<0.05.
Demographic Characteristics of the Study Population
In all studied demographic data, hydatid cyst-infected group did not differ significantly from controls (Table 1).
Table (1):
Demographic characteristics of cases and controls
Variables |
Cases (42) |
Controls (30) |
P-value |
|---|---|---|---|
Age, years (Mean±SD) |
42.98±14.46 |
39.18±11.52 |
0.832 |
Gender, No (%) Male Female |
11 (26.19%) 31 (73.81%) |
9 (30%) 21 (70%) |
0.723 |
Smoking, No (%) Non-smokers Ex/ current smokers |
31 (73.81%) 11 (26.19%) |
22 (73.33%) 8 (26.67%) |
0.512 |
Occupation, No (%) House-keeping Farmers Butchers Others |
21 (50%) 9 (21.42%) 7 (16.67%) 5 (11.9%) |
14 (46.67%) 6 (20%) 3 (10%) 7 (23.33%) |
0.588 0.275 0.346 0.191 |
Educational level, No (%) Primary and less Secondary and above |
29 (69.05%) 13 (30.95%) |
17 (56.67%) 13 (43.33%) |
0.282 |
IgG Antibody Titer
According to the kit manufacturer’s protocol, all CE patients were positive for anti-echinococcal IgG test (100%), while only two (5.7%) of controls had such a result.
Serum Levels of Cytokines
Table 2 shows serum levels of different cytokines in cases and controls. Median levels of IL-4 and IL-10 in cases (8.2 pg/mL and 810.3 pg/mL respectively) were higher than that in controls (5.5 pg/mL and 689.1 pg/mL respectively) with significant differences (P=0.035 and P= 0.018 respectively). In contrast median level of IL-12 did not differ significantly between cases and controls (155.65 pg/ml vs 153.2 pg/ml).
Table (2):
Median serum levels of different cytokines in cases and controls
Cytokines |
Cases |
Controls |
P-value |
|---|---|---|---|
IL-4 |
8.2(1.8-18.1) |
5.5 (0-17.9) |
0.035 |
IL-10 |
810.3(225-969.6) |
689.1(466.2-1057) |
0.018 |
IL-12 |
155.65(47.2-221) |
153.2(74-222.6) |
0.742 |
Diagnostic Values of IL-4 and IL-10 in Detection of Hydatid Cyst
Figure 2 (A and B) shows ROC analysis for IL-4 and IL-8 separately. For IL-4 (Figure 1-A), AUC was 0.647 (95%CI=0.512-0.782, P=0.035). The sensitivity and specificity of this cytokine in detection of CE at cut-off value of 7.45 pg/mL were 0.619 and 0.633 respectively which indicate a moderate discriminative value.
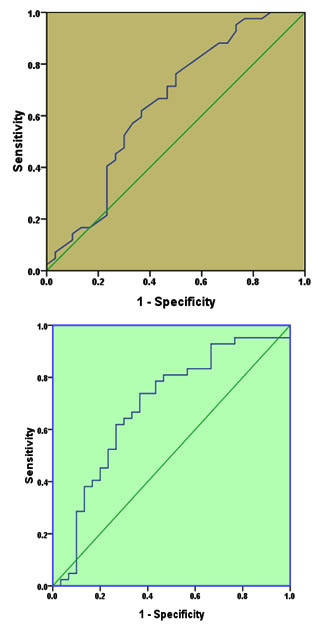
Fig. 1. Receiver operating characteristic for IL-4 (A) and IL-10 (B) for diagnosis of cystic echinococcosis
The ROC for IL-10 test (Figure 1-B) revealed that AUC was 0.69, 95%CI= 0.561-0.819, p=0.006. The sensitivity and specificity of the test at cut off value of 764.5 pg/ml were 0.643 and 0.70 respectively, indicating a moderate discriminative value too.
As neither IL-4 nor IL-10 has good discriminative value for CE, ROC analysis was performed for the combination of the two cytokines (Figure 2). As it is expected, the AUC increased to 0.803, 95%CI=0.702-0.905, P<0.001. The sensitivity and specificity of the test at 7.45 pg/mL and 764.5 pg/ml cut-off values (for IL-4 and IL-10 respectively) were 0.70 and 0.71 respectively, indicating a very good discriminative value.
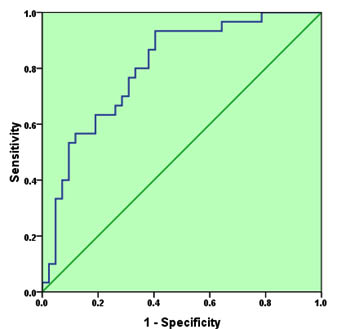
Fig. 2. Receiver operating characteristic for the combination of IL-4 and IL-10 as a diagnostic tool for cystic echinococcosis
Diagnosis of CE, especially in early stages, remains a challenging goal because of inadequate sensitivity or lack of the proper facilities. Thus, investigations for new methods for CE diagnosis becomes a prerequisite demand.
Although serum concentration of different cytokines is sensitive for large numbers of internal and external factors, cytokine concentration can be utilized either alone or in combination with other clinical and lab markers for the diagnosis of some infectious and non-infectious diseases. For example, a breakthrough in diagnosis of latent tuberculosis was performed recently via assessment of serum levels of interferon-gamma (IFN-³)12,13. The current study showed a significantly higher serum levels of IL-4 and IL-10 but not IL-12 in CE patients compared with the healthy controls. Accordingly, these two cytokines (IL-4 and IL-10) are good candidates for the detection CE. Similar results were previously reported in more than one study. Rigano et al14 for example showed that patients with active CE are characterized by a prominent Th2 profile with high levels of IL-4. Such humoral environment will promote the parasite survival by reducing proto-scoleces killing15. These observations were further evidenced by a molecular study which found that reciprocity of IL-4 and IL-10 impairs the protective Th1 response and favors the CE survival16. In the same context, Rigano et al17 investigated the cytokine profile in CE patients receiving chemotherapy. The study revealed relatively high concentrations of IL-4 and IL-10 in unresponsive patients, while Th1-associated cytokines (particularly IFN-³) were predominant in responsive patients.
On the other hand, there are many evidence which indicated a non-significant change or even low concentrations of IL-12 in CE patients. In a retrospective study, Tamarozzi et al18 recruited 27 Italian patients with different stage of CE and found no alteration in IL-12 in patients compared with controls in contrast to IL-4 concentration which was significantly higher in patients. However, in an Algerian study including 51 patients and 12 healthy indiviuals, Amri et al15 found both IL-12 and IL-8 concentration were significantly elevated in patients compared with controls.This disparity in the results may reflect the differences in cystic stage, viability, chemical therapy and ethnicity between the two studies.
The two cytokines (IL-4 and IL-10) whose concentrations were significantly higher in CE patients than controls were subjected either separately or in combination to ROC analysis. For separated analysis, the results indicate a similar diagnostic value of these two cytokines (moderate discrimination). In the combined ROC model, the AUC has increased indicating a very good diagnostic value of the two cytokines with good sensitivity and specificity (0.70 and 0.71 respectively) at a cut-off values 7.45 pg/mL and 764.5 pg/ml respectively. To the best of our knowledge, this is the first study which addressed the diagnostic value of three cytokines (IL-4, IL-10 and IL-12) in the diagnosis of CE using ROC analysis. Of course, the results of this study are not sufficient to recommend adaptation of this method for clinical CE diagnosis, but serum levels of these cytokines each with the corresponding cut–off values can add very useful information to the other diagnostic methods. In order to develop the cytokine-dependent assay into a reliable method, more studies on other Th2-related cytokines or chemokines in CE patients are required.
ACKNOWLEDGMENTS
The authors highly appreciate the great efforts of all staff in Medical Research Unit/ College of Medicine/ Al-Nahrain University in sample processing.
- Grosso G, Gruttadauria S, Biondi A, Marvenano S, Mistretta A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol 2012; 18(13):1425-1437.
- Petrone L, Vanini V, Petruccioli E, et al. IL-4 specific-response in whole blood associates with human cystic echinococcosis and cyst activity. J Infect 2015; 70(3):299-306.
- Kamaes ES, Al-Bayati NY, Al-Mayah QS, Al-Bashier NM. Infection with hydatid cyst can predispose to liver and lung cancers. Am J Res Communication 2015; 3(10):83-90.
- Brunetti E, Kern P, Vuitton DA, Writing Panel WI. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans.Acta Trop 2010; 114:1–16.
- Ben Nouir N, Gianinazzi C, et al. Isolation and molecular characterization of recombinant Echinococcus granulosus P29 protein (recP29) and its assessment for the post-surgical serological follow-up of human cystic echinococcosis in young patients. Trans R Soc Trop Med Hyg 2009; 103:355–364
- Hernandez-Gonzalez A, Muro A, Barrera I, Ramos G, Orduna A, Siles-Lucas M. Usefulness of four different Echinococcus granulosus recombinant antigens for serodiagnosis of unilocular hydatid disease (UHD) and postsurgical follow-up of patients treated for UHD. Clin Vaccine Immunol 2008; 15:147–153.
- Banes TS, Deplazes P, Gottstein B et al. Challenges for diagnosis and control of cystic hydatid disease. Acta Tropica 2012; 123:1-7.
- Ortona E, Rigano R, Margutti P, et al. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol 2000; 22:553-559.
- Brunetti E, Junghanss T. Update on cystic hydatid disease. Curr Opin Infect 2009; 22:497-502.
- Zhang W, Ross AG, McManus DP. Mechanisms of immunity in hydatid disease: implications for vaccine development. J Immunol 2008; 181(10): 6679–6685.
- Siracusano A, Delunardo F, Teggi A, Ortona E. Host-parasite relationship in cystic echinococcosis: an evolving story. Cli Dev Immunol 2012; 2012: 639362.
- Teklu T, Kwon K, Wondale B, et al. Potential immunological biomarkers for detection of Mycobacterium tuberculosis infection in a setting where M. tuberculosis is endemic, Ethiopia. Infec Immun 2018; 86(4):pii:e00759-17.
- Ilievska-Poposka B, Metodieva M, Zakoska M, Vragoterova C, Trajkov D. Latent tuberculosis infection: diagnosis and treatment. Macedonian J Med Sci 2018; 6(4):651-655.
- Rigano R, Buttari B, Profumo E, Ortona E, Delunardo F, Margutti P, et al. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect Immun 2007; 75:1667e78.
- Amri M, Mezioug D, Touil-Boukoffa C. Involvement of IL-10 and IL-4 in evasion strategies of Echinococcus granulosus to host immune response. Eur Cytokine Netw 2009; 20:63e8.
- Jankovic D, Liu Z, Gause WC. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 2001; 22:450–457.
- Rigano R, Profumo E, Ioppolo S. et al. immunological marker indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol 1995; 102:281-285.
- Tamarozzi F, Meroni V, Genco F, et al. Ex vivo assessment of serum cytokines in patients with cystic echinococcosis of the liver. Parasite Immunol 2010; 32:696-700.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


